Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

It is clear from the situation just described that the latter situation is ideal for a
stable chemical modification of the metal substrate and that molecules should be
chosen that are able to interact strongly with the metallic substrate. However, there
are some characteristic differences between corrosion inhibitors and molecular
adhesion promoters: whereas for corrosion inhibition the composition and structure
of the metal surface are defined by the corrosive medium, the surface properties can
be changed and adjusted to the structure of the adhesion promoter. Inhibitors must be
soluble in the electrolyte (e.g., water) and can be applied only for well-defined
reaction conditions. Adhesion promoters, however, may be applied from aqueous or
nonaqueous solvents or even from the gas phase and the reaction conditions can be
optimized for the given substrate. Therefore, some characteristic molecular features
of inhibitors such as heteroatoms S, P, and O should be incorporated into the
structure of the adhesion promoter; however, the molecule itself should show
minimum solubility in water and the possibility to bind a polymer onto the adhesion
promoter.
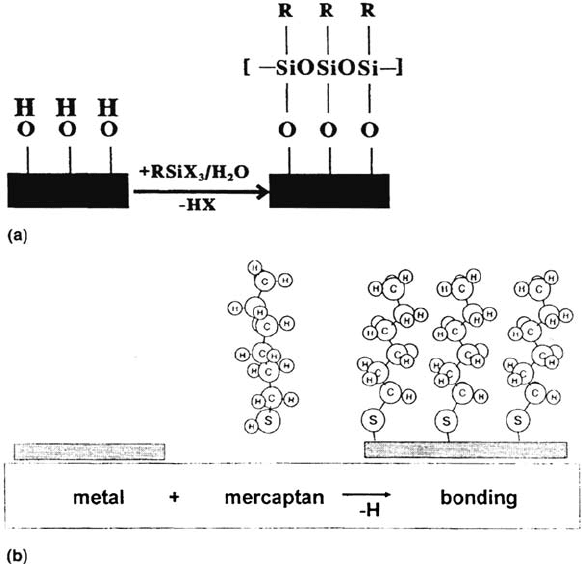
In this chapter, only very simple molecules will be discussed. They are
composed of one reactive center such as —SH, —Si(OCH
3
)
3
, or PO(OH)
2
, which
should be able to bind to the metal surface [20,21], and a long aliphatic chain (e.g.,
—C
18
H
37
), which allows ordering of the individual molecules and the formation of a
dense packing on top of the substrate (self-organization) (see Fig. 2). We will discuss
482 Rohwerder et al.
Figure 2 (a) Reaction of silanes with hydroxylated surface in humid atmospheres.
(b) Reaction of mercaptans with metal surfaces.
Copyright © 2002 Marcel Dekker, Inc.
how these modified surfaces may be prepared, what kind of structure is observed,
and how stable the modification is in aggressive electrolytes.
The preparation technique must fulfill certain requirements: the surface
properties of the substrate, e.g., the density of chemisorbed OH groups, must be
well defined and the organic monomer must be allowed to bind to the reactive
centers of the surface without destroying the defined surface structure; the
monomer itself should form a dense structure with a high degree of ordering so
that the substrate surface is not accessible to water molecules.
Another method for the modification of metal surfaces by ultrathin organic
films is plasma polymerization. Plasma polymerization, as a process technology
for corrosion-resistant thin-film deposition, has been explored during the last 20
years. Plasma polymers can be deposited from an electric discharge containing
organic or metal-organic molecules [44,45]. A glow discharge is formed by expos-
ing a gaseous monomer at reduced pressure to an electric field. The monomer is
fragmented in the discharge and the reactive intermediates generated polymerize
on a substrate according to a special reaction mechanism [46,47]. The resulting
films can be highly cross-linked and, depending on process parameters, show
more inorganic or more organic properties; moreover, adhesion is excellent to
most metal surfaces, i.e., the process is less specific to certain metals than is the
case with molecular self-assembly, and deposition of ultrathin films is fast. The
main disadvantage is that up to recently the process required a very low residual
pressure; i.e., vacuum equipment was needed. Lately, plasma polymers have also
been prepared under atmospheric pressure conditions.
ORGANIC MONOLAYER FILMS
Corrosion Protection by Self-Assembled Films
As outlined above, in order to improve the stability of the polymer/metal interface it
is of utmost importance to find ways to prepare interfaces that have better ability to
inhibit oxygen reduction and are less vulnerable to the products of oxygen reduction.
One way is to use monolayers of bifunctional molecules as adhesion promoters.
Ideally, such a molecule should form a tight chemical bond to the metal or metal
oxide surface with its head group and to the polymer with its tails groups. The
monolayer should be as dense as possible with as few defects as possible, for
optimum stability and inhibition capability. Also, for technical application the
formation of such monolayers should be quick, i.e., be finished within a few seconds.
The following paragraph will focus on electrochemical aspects of the self-
organization and the resulting effect on the final defect structure. The discussion will
distinguish between oxide-covered and oxide-free surfaces. The protective impact of
the films will also be discussed.
In recent years the process of molecular self-assembly on solid surfaces, i.e.,
the adsorption and self-organized formation of highly ordered monolayers from
monomers in solution, has received increasing interest, especially the self-assembly
of thiols on gold [48]. As could be shown, thiol monolayers proved to be excellent
inhibitors of oxygen reduction and moreover are not easily destroyed by the
radicals set free during the oxygen reduction [49]. First tests on iron also gave
promising results [50,51] although the preparation is not easy on this substrate.
Corrosion Prevention by Adsorbed Monolayers 483
Copyright © 2002 Marcel Dekker, Inc.
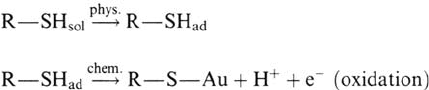
Because thiol molecules do not adsorb on iron oxide, the iron has to be electrochem-
ically polarized to cathodic potentials in order to get rid of the oxide layer and then
to keep it free of oxide during self-organization. The SA of BTA on copper was
investigated by Magnussen and Behm [52]. Now, even though self-organization is
the subject of hundreds of publications, up to very recently nothing has been known
about the influence of the electrode potential and surface charge on the self-assembly
process. For bare metal surfaces the surface charge is controlled by the electrode
potential, for oxide-covered samples by the pH of the solution.
Self-Assembly on Oxide-Free Metal Surfaces
Thiol Self-Assembly on Gold The well-studied system thiol/Au is ideal for
investigating the effect of the electrode potential on the kinetics of self-assembly
and on the resulting defect structure.
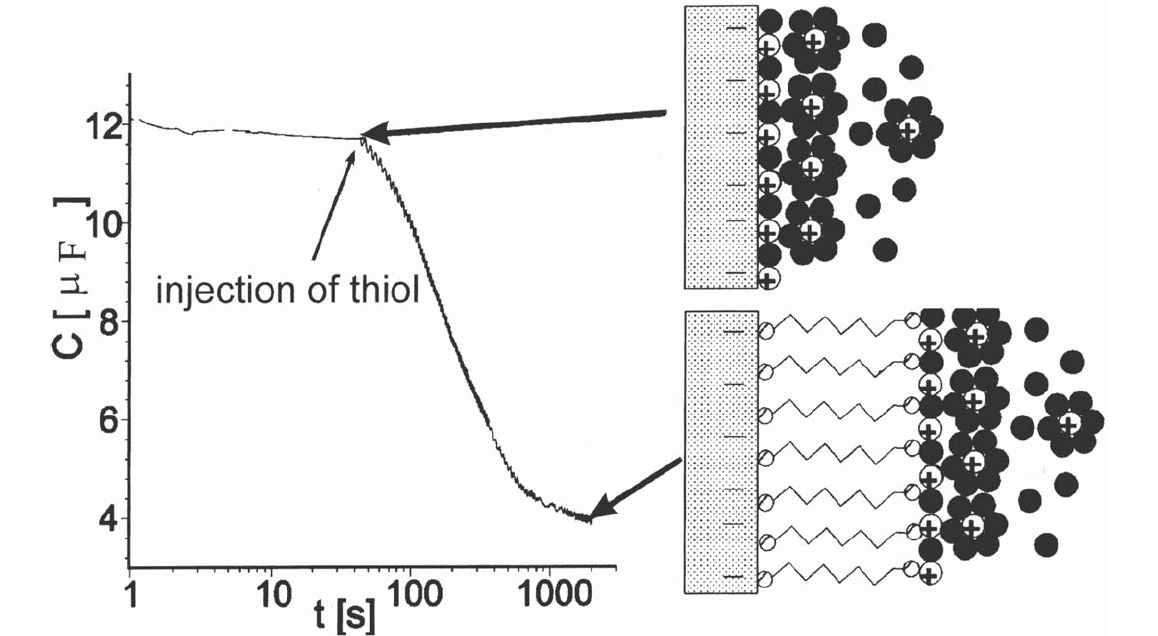
A direct way to monitor the self-assembly in situ is to measure the decrease
in capacitance of the immersed sample (see Fig. 3). The double layer at the
metal/electrolyte interface is pushed apart by the growing monolayer, which
causes a decrease of capacitance. Because an electrode’s capacitance depends on
the electrode potential, it is useful to normalize curves obtained for adsorption at
different potentials, e.g., by referring the change in capacitance ΔC at the time t to
the final capacity change ΔC
max
. Such normalized curves for the adsorption at –400
and –800 mV versus a special Ag/AgCl reference electrode [19,53] in ethanol
and typical nanoscopic structures of the films at different stages of the thiol
self-assembly are shown in Figure 4. A detailed analysis of the curves yields the
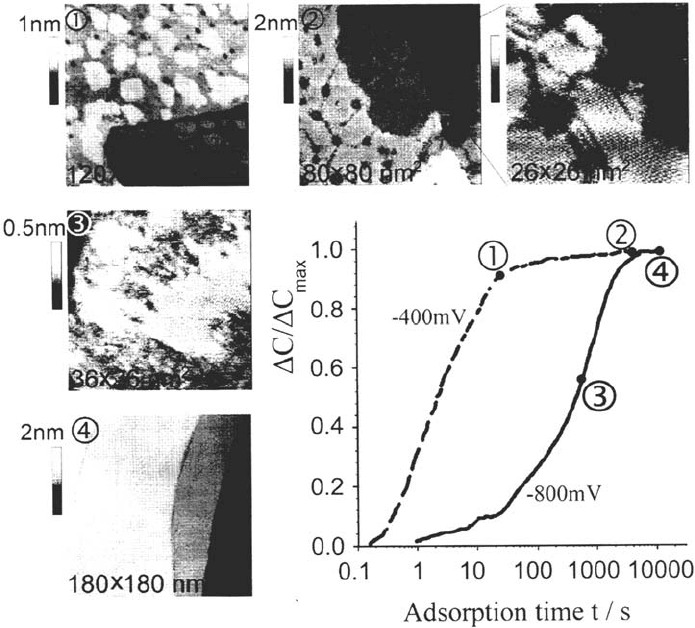
distinction of three characteristic potential ranges for thiol self-assembly [19,53].
An intermediate potential range from –400 to + 200 mV versus the Ag/AgCl
reference electrode where the rate of self-assembly shows only a
comparatively weak dependence on the electrode potential; scanning
tunneling microscopy (STM) images of the completed films show basically
the same features already known from the literature [54–62].
A cathodic potential range where the rate of self-assembly decreases significantly
with increasingly cathodic potentials; STM images of the completed films
show that the average domain size also increases, so that for adsorption at
–800 or –900 mV the thiol domains fill whole terraces, even if the lateral size
of these exceeds 100 or even 200 nm (Fig. 4).
An anodic potential range where there is a slight decrease in the rate of
self-assembly with increasingly anodic potentials.
Whereas the film is finished to 95% within 10 s at potentials of –400 to
+200 mV, yielding a film composed of domains with a lateral size of 10–20 nm,
it takes thousands of seconds at very cathodic potentials for the domains to cover
the surface, but the domains are much larger. A detailed analysis shows that the
thiol adsorption is at least a two-step process: physisorption followed by
chemisorption [53a]:
484 Rohwerder et al.
Copyright © 2002 Marcel Dekker, Inc.
Corrosion Prevention by Adsorbed Monolayers 485
Figure 3 If the capacity of the gold surface is measured during thiol adsorption, a decrease in capacitance can be observed due to the
pushing apart of the double layer by the adsorbing thiol.
Copyright © 2002 Marcel Dekker, Inc.
It is the electrode potential dependence of the latter step that is responsible for the
extreme slow-down in adsorption velocity at cathodic potentials.
Assuming a symmetry factor β = 0.5 (because of the symmetry of the system)
and a complete charge transfer, it can be shown from the measured capacity curves
that about 36% of the overall potential difference between the electrode and the
solution occurs over the alkane chains; i.e., the longer the alkane chain, the smaller
the slowdown effect at cathodic potentials. This could be shown for the adsorption
of octadecylthiol as compared with decylthiol [53b].
Thiol on Iron For technical application this proves to be a problem, because
adsorption times on the order of thousands of seconds are out of the question. In
order to circumvent this problem, another coating technique has to be applied: the
sample (the working electrode in the figure) is polarized to cathodic potentials to
486 Rohwerder et al.
Figure 4 The normalized capacity curves clearly show that thiol (here decanethiol)
self-assembly is much faster at intermediate potentials (here –400 mV) than at cathodic
potentials (–800 mV). Typical STM images of the film structure are shown for different
stages of self-assembly at the two potentials. At –400 mV, thiol self-assembly is fast and
occurs via small individual domains (1), which sets the foundation for the final domain
structure (2). At –800 mV, self-assembly is very slow and occurs via domains that are
interconnected by molecular rows of chemisorbed thiol (3), and thus very large domains
result in the final film (4).
Copyright © 2002 Marcel Dekker, Inc.
reduce the oxide layer and to prevent oxide formation during the coating process
and then pulled through a thiol film, floating on top of the aqueous electrolyte
[50,51]. In this way about 10-nm-thick multilayer films can be prepared, which
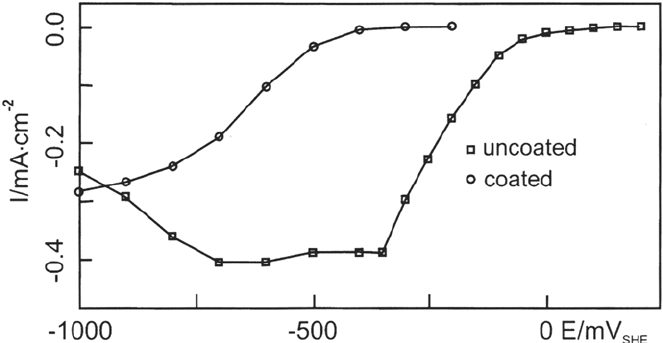
show excellent blocking of oxygen reduction (see Fig. 5). It is quite obvious that
the oxygen reduction is drastically limited on the modified surface and a much
higher cathodic overpotential is necessary to reduce oxygen at significant rates. If
oxygen is reduced, however, the film is destroyed by the radicals formed during
oxygen reduction.
For adhesion promotion only the first monolayer will be effective. Because it
proved not to be possible to prevent iron surface reoxidation completely while
pulling the sample through the floating thiol film, and thus parts of the sample
are not covered by chemisorbed thiols but by oxide, it is necessary to get rid of
such defects in a second preparation step in order to improve the quality of the
chemisorbed monolayer beneath the multilayer film. Immersing the as-prepared
sample in aqueous solution and cycling the potential from the cathodic limit,
where hydrogen evolution is beginning, through the potential range where iron
is oxidized (peak in curves 1 and 2), and back for several times results in a
healing of the disordered film (see Fig. 6), as can be seen from the decrease of
the oxidation peak with increasing number of cycles. With each sweep into the
potential range where iron oxide is reduced, bare iron is exposed at the defect
sites to the thiol in the multilayer, and in the subsequent anodic sweep the thiol
can at least partly be chemisorbed before reoxidation of the remaining bare iron
surface sets in. Finally, all the surface is covered by chemisorbed thiol mole-
cules. Then the modified sample is pulled out of the solution and the polymer
coating is applied. Alternatively, the sample may be polarized at negative –800 mV.
But then the healing process takes much longer (see Fig. 7a) because the thiol
chemisorption at cathodic potentials is very slow (see earlier). Most of the
multilayer thiol is dissolved into the polymer so that the first chemisorbed
monolayer should perform its function as an adhesion-promoting layer.
Corrosion Prevention by Adsorbed Monolayers 487
Figure 5 Rate of oxygen reduction on iron (c) and iron modified by one monolayer of
n-decylmercaptan ().
Copyright © 2002 Marcel Dekker, Inc.
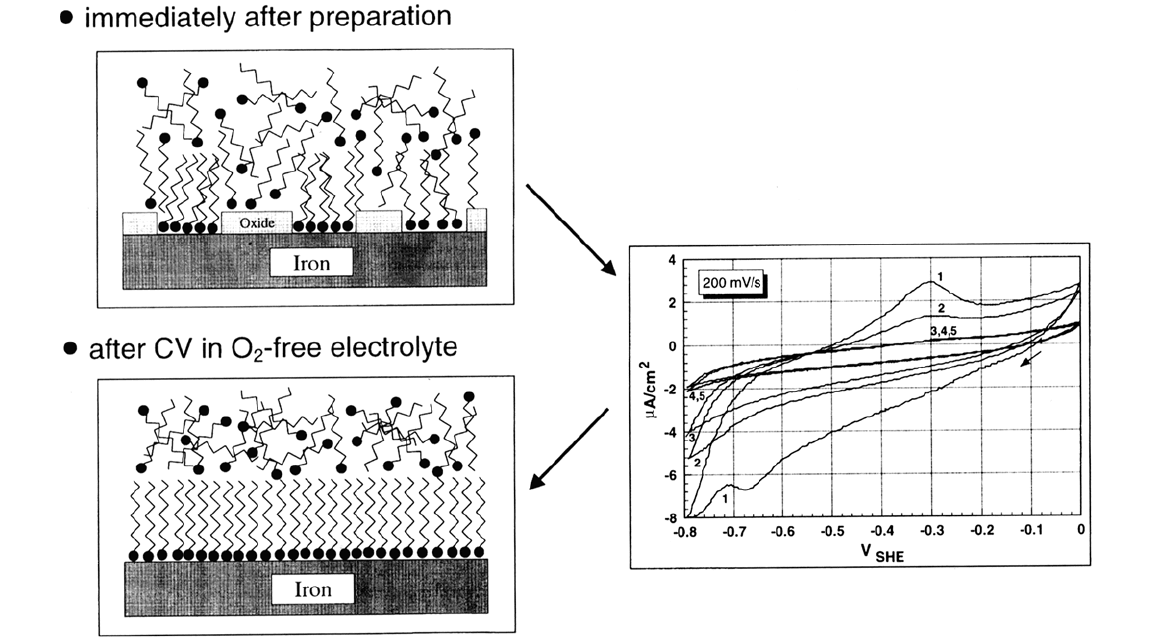
488 Rohwerder et al.
Figure 6 (Upper left) Model of a thiol multilayer on iron with remnants of oxide. After several cycles of the electrode potential
(CV) in O
2
-free electrolyte (right), the interface between thiol and iron is healed (lower left).
Copyright © 2002 Marcel Dekker, Inc.
Scanning Kelvin probe mappings of a thus modified iron sample coated with a
polymer show more than three times slower delamination kinetics. Still, a more
pronounced effect is desirable for future applications.
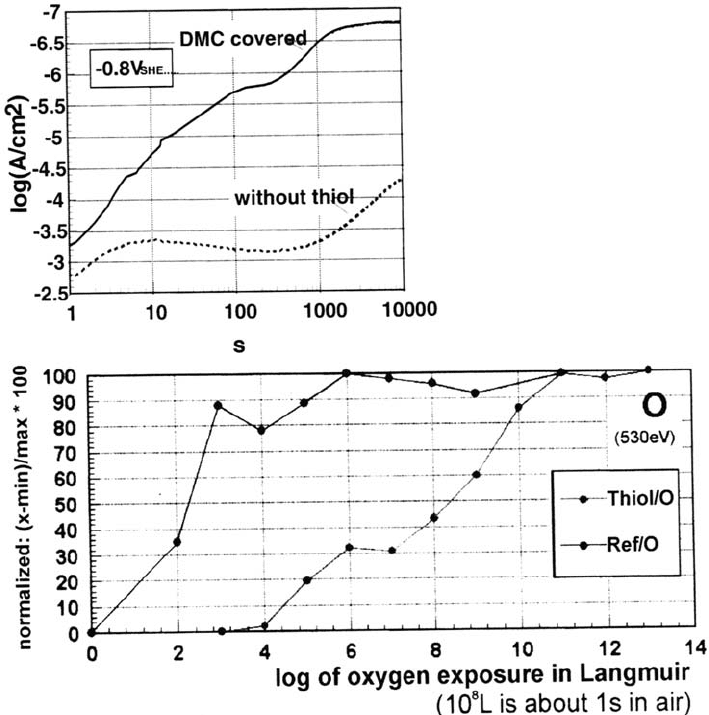
Closer investigation shows that the bond between the first thiol monolayer
and the metal surface is destroyed by reoxidation of the iron surface beneath the
multilayer film within tens of seconds. Of course, this results in diminished ability
of the layers to promote adhesion compared with what would be expected from an
intact monolayer. This reoxidation is faster the thinner the multilayer film. For
a monolayer film the reoxidation takes about 10s. That is an impressive factor of
10
6
to 10
7
slower than for bare iron, achieved by an only 1-nm-thick monolayer
film, as can be seen from Figure 7b.
Corrosion Prevention by Adsorbed Monolayers 489
Figure 7 (a) Current observed during healing at –800 mV of a decanethiol monolayer
compared with bare iron. (b) Normalized oxygen peak vs. oxygen exposure for
thiol-covered iron in comparison with bare iron.
Copyright © 2002 Marcel Dekker, Inc.
A first step to find ways to improve this technique is a understanding of
the reoxidation process, which is at the center of current research. Important
parameters are chain length and functional groups of the thiol molecules. If the
choice of more suitable thiols together with a more refined modification technique
resulted in a thiol layer that survives even one order of magnitude longer in air,
this could be an important breakthrough, because then the polymer coating could
be applied before most of the thiol film is destroyed.
Self-Assembly on Stable Oxide Surface
Self-Assembly of Phosphonate Films Whereas in the case of iron the adhesion
layers have to be adsorbed directly on the metal because the oxides are unstable, the
situation is different for metals that form stable oxides. Here it is better to have the
films formed on the stable oxide. On aluminum oxides, for example, phosphonates,
X—R—PO
3
2–
, form stable and well-ordered monolayers. In X—R—PO
3
2–
, X
designates the functional end group, R the alkane chain, and PO
3
2–
the head group.
With technical samples, high-resolution STM is not applicable to obtain
information about the molecular order. Other methods such as Fourier transform
infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) have
to be applied.
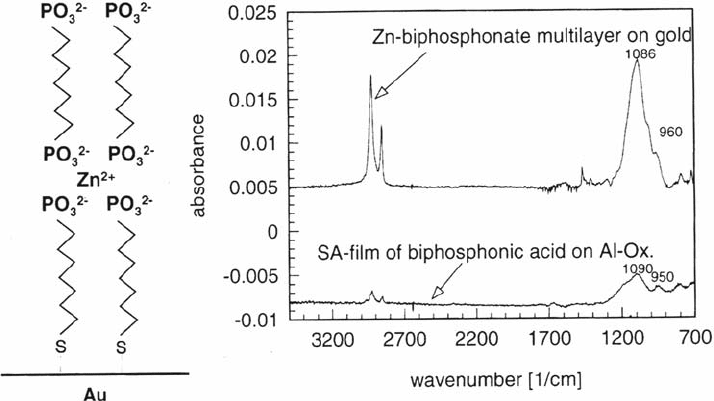
The adsorption of phosphonates on aluminum surfaces is an acid-base reaction.
The driving force is the formation of a surface salt [63]. Figure 8 shows FTIR
spectra supporting this theory. The similarity in the spectra of a Zn-biphosphonate
multilayer on gold and of an self-assembly (SA) film of biphosphonic acid on
aluminum oxide clearly supports this theory. The peaks at wave numbers 1100 and
950 cm
–1
are attributed to vibrational modes of the RPO
3
2–
anion.
490 Rohwerder et al.
Figure 8 Infrared adsorbance spectra of a phosphonate film on an aluminum oxide
surface and of Zn-biphosphonate multilayer on gold (see figure on the left).
Copyright © 2002 Marcel Dekker, Inc.
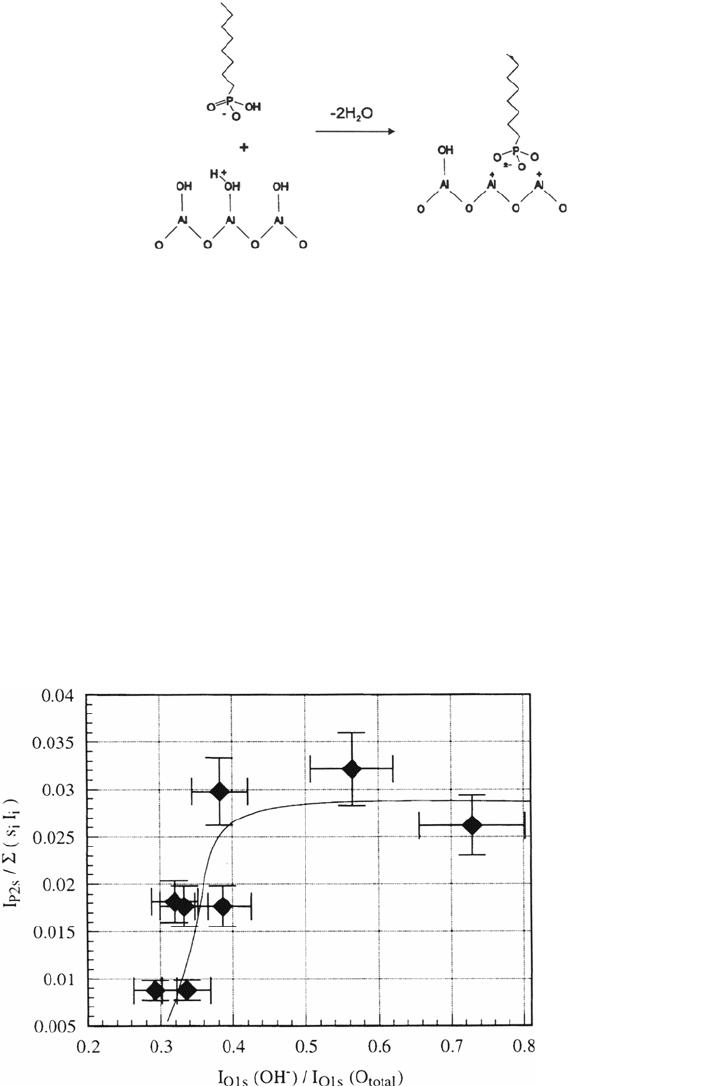
The following adsorption scheme is supposed:
Corrosion Prevention by Adsorbed Monolayers 491
This requires the presence of OH groups on the aluminum oxide surface. Different
levels of OH on the oxide surface can be adjusted by gas-phase adsorption of
different amounts of oxygen and water on aluminum in a special ultrahigh vacuum
(UHV) chamber, which also allows electrochemical experiments and adsorption
from solution without exposing the samples to air. After the OH ratio in the oxide
layer was determined with XPS, the sample was exposed to the phosphonate
solution and then the amount of adsorbed phosphonates was determined with XPS.
Figure 9 shows that for low OH content in the oxide surface no phosphonate
adsorption occurs.
Because aluminum is only rarely used as the pure material, it is of crucial
importance for a successful application of the protecting and adhesion-promoting
monolayer films that not only the aluminum surface is covered by the molecules but
also the inclusions, such as Al
2
Fe, as they are typical of most aluminum alloys. Even
Figure 9 Intensity of the phosphorus 2s photoelectron signal as a function of the OH
–
content in the oxide surface.
Copyright © 2002 Marcel Dekker, Inc.
