Markovchick Vincent J., Pons Peter T., Bakes Katherine M.(ed.) Emergency medicine secrets. 5th ed
Подождите немного. Документ загружается.


Chapter 54 SKIN DISEASES378
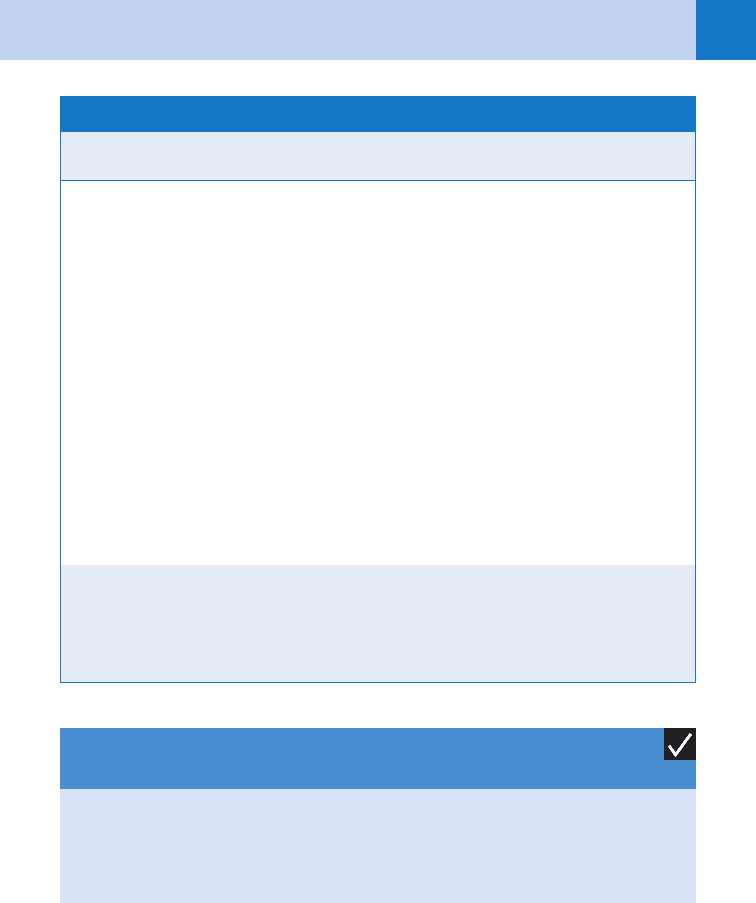
Causes
Clinical Signs/
Symptoms
Mortality
Rate Treatment
SJS Drugs
(70%–90%):
antibacterial
sulfon-
amides,
anticonvul-
sants,
NSAIDs,
allopurinol
Mycoplasma
pneumoniae
infection
Prodrome 1–14 days
before the onset of
mucosal involvement is
common (e.g., fever,
malaise, headache,
sore throat, rhinorrhea
and cough)
Acute eruption with red
to dusky, tender papules
at times targetoid with
potential for blistering/
sloughing (usually ,10%
of the BSA involved)
Extensive, hemorrhagic
necrosis of two or more
mucous membranes
(mouth and eye most
common)
5% Prompt withdrawal
of drug
Supportive care
(wound care in an ICU
or bum unit setting if
sloughing of skin
prominent, enteral nu-
trition, fluid/electrolyte
replacement, pain
management, moni-
toring for infection)
Steroid use is contro-
versial; some evidence
that steroid use re-
sults in worse progno-
sis; if part of treat-
ment should be
started very early in
the course
TEN Drugs
(95%):
antibacterial
sulfon-
amides,
anticonvul-
sants
NSAIDs,
allopurinol
Onset with fever and
tender skin several days
before blistering/slough-
ing begins
Painful, dusky red
macules appear, become
dusky, then confluent
with islands of sparing,
then blisters/sloughing of
the skin begins (usually
.30% BSA involved)
Mucous membrane
involvement in 90%
of cases
Poor prognostic factors
include increasing age,
delay in withdrawal of
offending agent, and
greater extent of epider-
mal attachment
30%–35% Prompt withdrawal of
drug*
Supportive care in an
intensive care burn
unit setting (see SJS
section)
The following are used
in treatment of TEN,
but efficacy is contro-
versial: intravenous
immunoglobulin,
systemic steroids
(see SJS section)
TABLE 54-2. SIGNS AND SYMPTOMS OF THE MOST SERIOUS DRUG ERUPTIONS
Chapter 54 SKIN DISEASES 379
BSA, body surface area; DRESS, drug reaction with eosinophilia and systemic symptoms; ICU, intensive care
unit; NSAIDs, nonsteroidal anti-inflammatory drugs; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal
necrolysis.
*Garcia-Doval I, LeCleach L, Bocquet H, et al: Toxic epidermal necrolysis and Stevens-Johnson syndrome:
does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 136: 323–327, 2000.
Causes
Clinical Signs/
Symptoms
Mortality
Rate Treatment
DRESS Drugs:
anticonvul-
sants, sul-
fonamides,
allopurinol,
minocycline,
dapsone,
gold salts
Fever and a morbilliform
skin eruption are the
most common presenting
features. The eruption
then becomes edematous
with follicular
accentuation.
Facial edema is a
hallmark of DRESS.
Lymphadenopathy,
eosinophilia, and atypical
lymphocytosis are
common features.
Visceral inflammation
(hepatitis most common)
10% Prompt withdrawal
of the drug
Systemic steroids
(severe cases with
visceral inflammation,
although efficacy
unproven in controlled
clinical trials)
Topical steroids
(milder cases for cuta-
neous manifestations)
TABLE 54-2. SIGNS AND SYMPTOMS OF THE MOST SERIOUS DRUG ERUPTIONS—cont’d
14. How do I recognize a melanoma?
Melanoma requires urgent diagnosis and treatment. Recognition of the potential and referral
for surgical removal of melanoma before it has metastasized can be life-saving. Findings
suggestive of melanoma are irregular pigment; irregular borders; and presence of red, white,
or blue-black color. An underemphasized finding that can be extremely helpful is a difference
in appearance between the lesion in question and the patient’s other moles. A brown macule
on a fair-skinned person should be viewed with suspicion if the person does not have other
similarly pigmented lesions, even if the brown macule is regularly pigmented, small, and
perfectly round. A history of change in a mole is a risk factor, as is personal or family history
of melanoma.
KEY POINTS: USUAL TIME TO ONSET FOR CUTANEOUS
DRUG ERUPTIONS
1. DRESS syndrome begins 2 to 6 weeks after the responsible drug is started.
2. One to 3 weeks are required for the development of SJS and TEN.
3. Four to 14 days are required for exanthematous drug eruptions.

Chapter 54 SKIN DISEASES380
15. Describe common benign skin conditions that mimic melanoma.
n
Seborrheic keratoses are extremely common benign lesions that usually first appear in
middle age. They are benign growths of keratinocytes and can look alarmingly dark or
irregularly pigmented. The scaling produced by the seborrheic keratosis may be so
compact that it is difficult to discern, but detection of this rough scaling helps considerably
in distinguishing this lesion from melanoma.
n
Venous lakes are vascular growths that often appear on the helix of the ears and on the
lips of older persons with sun damage. The purple color may mimic that of a melanoma.
Pressing firmly on the lesion drains much of the blood from the lesion and reveals it as a
vascular growth.
16. Which spider bites can cause a necrotic reaction?
Only a few species of spiders in North America produce bites that may lead to skin necrosis.
Of these, bites of the brown recluse (Loxosceles reclusa) and the hobo spider (Tegenaria
agrestis) are important because they may be fatal. The range of the brown recluse is centered
in Arkansas, Tennessee, and Missouri. Outside endemic areas, brown recluse bites are
uncommon, although they may occur because the spider may be transported on clothing or in
boxes. The range of the hobo spider is the northwest United States and western Canada. Other
Loxosceles species may cause necrotic reactions, and many of these spiders live in the
deserts of the southwest United States. If one is outside an endemic area, the diagnosis of
necrotic reaction to spider bite should be made with caution.
17. What skin lesions may be confused with a necrotic reaction to a spider bite?
Necrotizing fasciitis, ecthyma, pyoderma gangrenosum, vasculitis, and clotting disorders.
Erythematous reactions to stings or bites, such as bee stings or tick bites, occasionally may
be confused with early reactions to spider bites. Most recently, methicillin-resistant
Staphylococcus aureus (MRSA) skin infection is often confused with spider bites.
18. What are the cutaneous findings of skin and soft-tissue infections (SSTI) with
MRSA?
SSTIs are the most common clinical manifestation of community-acquired MRSA (CA-MRSA).
This presents most frequently as a skin abscess or furunculosis. Often the patient will give a
history of an “infected spider bite.” There is no evidence to conclude that MRSA SSTIs are
clinically distinguishable from infection with susceptible strains of S. aureus, but several risk
factors are more common with MRSA such as a history of a spider bite, antibiotics in the past
month, history of previous MRSA, close living quarters, and activities with skin-to-skin
contact (athletes). Primary treatment is incision and drainage of abscesses. Adjunctive therapy
of purulent skin infections with antibiotics has not been proven to be necessary in several
studies in healthy, immunocompetent patients. Antibiotics are indicated if there is fever or
KEY POINTS: CUTANEOUS FEATURES CONCERNING
FOR MELANOMA
1. Different from patient’s other pigmented lesions
2. Recent changes in size, shape, and color
3. Markedly irregular borders
4. Markedly irregular pigmentation with colors of red, white, or blue
5. Areas of pigment regression
6. Any one of these may be an indicator of melanoma without the other features being present.

Chapter 54 SKIN DISEASES 381
surrounding cellulitis. For further recommendations for the treatment of CA-MRSA infections,
see: www.cdc.gov/ncidod/dhqp/pdf/ar/CAMRSA_ExpMtgStrategies.pdf
19. Describe common benign skin conditions that mimic purpura resulting from
systemic disease.
n
Solar purpura is common on the forearms and backs of hands of people who have chronic
sun damage. Large areas of purpura may be evident and may have occurred with minimal,
sometimes unnoticeable trauma. Solar purpura is restricted to chronically sun-damaged
skin and is particularly common in patients on long-term systemic steroid therapy.
n
Schamberg’s purpura is a benign condition characterized by petechiae primarily on the
lower legs. The lesions tend to be pinpoint, nonpalpable, and extremely numerous. By
contrast, purpuric lesions of leukocytoclastic (hypersensitivity) vasculitis tend to be slightly
larger in diameter (often 2–4 mm), and frequently some of the lesions are palpable.
(Although leukocytoclastic vasculitis sometimes is referred to as palpable purpura, it is
common to find that most lesions are flat and only a few are palpable.)
20. Describe common skin conditions that mimic cellulitis.
n
A kerion caused by fungal infection in the scalp (tinea capitis) may be so intensely inflamed
that it is mistaken for cellulitis. Because a kerion may produce permanent, scarring
alopecia, it is important to recognize and institute therapy early. Kerions occur almost
exclusively in children.
n
Stasis dermatitis sometimes may be confused with cellulitis. Stasis dermatitis is usually
bilateral, whereas leg cellulitis is more often unilateral. Stasis dermatitis is characterized by
scaling and mild-to-moderate erythema. If the erythema is fiery red, the redness is rapidly
progressive, the patient is systemically ill, or there is a leukocytosis, cellulitis may be the
presumptive diagnosis.
n
Allergic contact dermatitis, such as poison ivy dermatitis, may result in lesions that are
intensely inflamed. The distribution of the lesions often suggests an exogenous cause. In
plant dermatitis, linear erythema, often with blisters, is an indicator of where the plant has
brushed against the skin. Antibiotic creams containing neomycin are another relatively
common cause of allergic contact dermatitis. Because antibiotic creams often are used on
wounds, allergic contact dermatitis caused by the cream may be mistaken for a resistant or
on-going wound infection.
21. In which disease associated with leg ulceration is débridement generally
contraindicated?
Pyoderma gangrenosum.
22. When should patients with dermatitis (eczema) be treated with systemic
steroids?
Systemic steroids generally should not be given to patients with chronic dermatitis. Topical
steroids should be used to avoid systemic side effects. Topical ointments and creams target
one of the primary problems in chronic atopic dermatitis, which is a skin barrier defect.
Patients taking systemic steroids also may exhibit a rebound of disease when the steroids are
tapered. Patients with acute dermatitis, such as severe poison ivy dermatitis, that is expected
to be self-limited may be given systemic steroids if the severity of disease merits and there
are no contraindications.
23. Should steroids be used for psoriasis?
Systemic steroids are generally considered to be contraindicated for the treatment of psoriasis
because severe rebound with generalized pustular psoriasis may occur following withdrawal of
the steroid.
Chapter 54 SKIN DISEASES382
24. What are the divisions of the classes of topical corticosteroids? On which
areas of the skin are they appropriately applied?
n
Low-potency topical corticosteroids (class 6 or 7 topical steroids, such as 1% and 2.5%
hydrocortisone, 0.05% desonide) are appropriate to use on the face, axillae, groin, breasts,
and genitalia, where the skin is thinner and more prone to cutaneous side effects.
n
Moderate-potency topical corticosteroids (class 4 or 5 topical steroids, such as 0.025%
fluocinolone, 0.1% triamcinolone, 0.2% hydrocortisone valerate) are useful on the neck and
body, avoiding the more sensitive areas mentioned previously. This class is given most
appropriately as first-line therapy for skin conditions diagnosed in the ED.
n
High-potency topical corticosteroids (class 2 or 3 topical steroids, such as 0.05%
fluocinonide, 0.1% halcinonide, 0.25% desoximetasone) should not be applied to the face,
breasts, genitalia, axillae, or groin. Topicals in this class are more likely to produce
cutaneous side effects if used diffusely or for long periods (.2 weeks). This class should
be prescribed only if moderate-potency topical corticosteroids have not been effective or if
the condition is limited to the particularly thick skin of the palms and soles.
n
Superpotent topical corticosteroids (class 1 topical steroids, such as 0.05% clobetasol,
0.05% betamethasone dipropionate in optimized vehicle, 0.05% halobetasol, 0.05%
diflorasone) are usually reserved for chronic, recalcitrant conditions, often of the palms and
soles. The risk of cutaneous side effects is greatest with this class, and this class of steroid
should be dispensed in a continuity-of-care rather than an ED setting.
25. Does the vehicle of the topical corticosteroid affect potency?
Yes. The same corticosteroid may be significantly more or less potent, depending on the
vehicle. In general, ointments are most potent, followed by gels, emollients, creams, lotions,
solutions, and sprays.
BIBLIOGRAPHY
1. Dyer JA: Childhood Viral Exanthems. Pediatr Ann 36(1):21–29, 2007.
2. French LE, Prins C: Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In
Bolognia JL, Jorizzo JL, Rapini RP, editors: Dermatology ed 2, London, 2008, Mosby, pp 287–300.
3. Frigas E, Park MA: Acute urticaria and angioedema: diagnostic and treatment considerations. Am J Clin
Dermatol 10(4):239–250, 2009.
4. Ghislain PD, Roujeau JC: Treatment of severe drug reactions: Stevens-Johnson syndrome, toxic epidermal
necrolysis and hypersensitivity syndrome. Dermatol Online J 8(1):5, 2002.
5. May TJ, Safranek S: Clinical inquiries. When should you suspect community-acquired MRSA? How should you
treat it? J Fam Pract 58(5):276, 278, 2009.
6. Patel M: Community acquired methicillin-resistant Staphylococcus aureus: epidemiology, recognition, and
management. Drugs 69(6):693–716, 2009.
7. Revuz J, Valeyrie-Allanore L: Drug reactions. In: Bolognia JL, Jorizzo JL, Rapini RP, editors: Dermatology ed 2,
London, 2008, Mosby, pp 301–320.
8. Roujeau JC, Kelly JP, Naldi L, et al: Medication use and the risk of Stevens-Johnson syndrome or toxic
epidermal necrolysis. N Engl J Med 333:1600–1607, 1995.
9. Sams HH, Dunnick CA, Smith ML, et al: Necrotic arachnidism. J Am Acad Dermatol 44:561–573, 2001.
WEBSITE
www.cdc.gov/ncidod/dhqp/pdf/ar/CAMRSA_ExpMtgStrategies.pdf
383
LIGHTNING AND ELECTRICAL INJURIES
CHAPTER 55
Gabrielle A. Jacquet, MD, and Timothy R. Hurtado, DO, FACEP
XII. ENVIRONMENTAL EMERGENCIES
LIGHTNING INJURIES
1. What causes lightning?
The etiology of lightning is a highly complex science involving physics and meteorology. First
of all, a large charge separation must occur within a cloud, turning it into a giant battery or
capacitor. This occurs from hailstones and raindrops settling at various rates in a convectively
active cloud. Charged particles get stripped off, and the cloud separates into a dipole or tripole
(two or three, respectively, equal and opposite electric charges or magnetic poles separated by
a small distance). Most often, the cloud base becomes negatively charged. In response, an
induced shadow of positive charge forms on the ground. The potential between the ground
and the cloud can be as great as 7,500 volts per inch. Lightning is initiated by the formation of
a stepped leader, a zigzag, short, stepped, downward series of branching ionized plasma
channels. At the same time, a positively charged pilot stroke arises from the ground, usually
from the tops of tall objects. When the two meet at about 50 to 100 m above the ground, the
return stroke is initiated. This is what we think of as the lightning bolt. It is a high-voltage,
high-current, high-velocity discharge that travels up the plasma channel. There is an average
of four to five return strokes. Lightning only sees objects that are in a radius of 30 to 50 m
from the tip. Thus, tall objects (such as a tall tree on a ridge) that are greater than 50 m from
the stepped leader may not offer protection from a strike.
2. How about thunder?
Thunder is an acoustic wave caused by the sudden heating of the air from lightning. It is the
result of explosive shock waves from instantaneous superheated ionized air. This can cause a
pressure rise up to 10 atm. The sound of thunder is rarely heard over 10 miles away and has
a lower pitch at greater distances. In addition, atmospheric turbulence may decrease the
distance the sound travels.
3. What are the mechanisms of lightning injury?
Lightning causes injuries in three basic ways:
a. Its electrical effects
b. The heat it produces
c. The concussive forces it creates.
Another way to consider lightning mechanisms is as follows:
n
Direct strike or contact injury: This occurs when the victim is in direct contact with the
lightning or an object or structure that is struck by lightning. It usually results in the
highest mortality and morbidity.
n
Splash or side flash: Lightning arcs from an object to a nearby person. Current seeks a
path of least resistance and can “jump” from its primary target to others.
n
Step or stride voltage: The lightning current strikes the ground, then spreads out. Current
moving underground can cause an induced current, up one leg and down another. This is a
reason for multiple victims of a single strike. Humans offer less resistance than the ground
thus lightning frequently spreads into their bodies after the nearby ground is struck.

Chapter 55 LIGHTNING AND ELECTRICAL INJURIES384
n
Rising upward streamer: This newly described mechanism happens with an injury from
the rising positively charged streamer, which does not connect with a pilot stroke to create
the plasma channel that allows return strokes, what we think of as a lightning bolt.
n
Blunt trauma and blast trauma: Blast trauma may occur from the thunderclap. Common
findings from this blast include pulmonary contusions, tympanic membrane rupture, and
conductive hearing loss. A victim may also be “thrown” by diffuse muscle contractions.
n
Secondary trauma and burns from fires: Trauma from falls or falling objects and thermal
burns from ignited clothing, heated metal, and burning surroundings can also complicate
lightning injuries.
4. True or false: During a thunderstorm, get into a car because the rubber tires
will insulate it?
False. You are safer inside a car than out during a thunderstorm but not because of the tires.
Think about it: The lightning bolt just passed several kilometers through the air to get to the
ground. It won’t be intimidated by 6 inches of rubber (or the rubber soles of your tennis
shoes, for that matter). The real reason that the car is safer is the metal skin of the body. It
acts as a Faraday cage (Table 55-1) allowing electromagnetic flow over the outer surface,
isolating the occupants. Now, of course, that may not protect the occupants from the flash,
thunderclap, splash current, or induced electromagnetic currents through the interior.
5. True or false: People are safe from lightning if they are indoors?
False. Maybe safer, but a significant number of lightning injuries occur to people who are
inside buildings. One mechanism for this is a side flash through plumbing, telephone wires,
and electrical appliances connected to the outside of the building by metal conductors.
6. True or false: Lightning never strikes twice in the same place?
False. The Empire State Building in New York is struck about 23 times a year; once, it was
struck eight times in 24 minutes.
7. Is a lightning bolt an alternating current (AC) or a direct current (DC)?
It is neither. It is a unidirectional current impulse (technically neither AC nor DC, although the
closest model would be a large DC discharge). It is very high voltage (100 million to 2 billion
volts), very large current (20,000–300,000 peak amps), and very high energy (1 billion joules
or 280 kilowatt hours). The bolt is also very hot: 8000°C to 25,000°C (the surface of the sun
is only 6000°C). The good news is that it’s a very short duration phenomenon (0.1–1 msec).
This ultrashort duration of exposure is the most important factor that separates lightning
injuries from other high voltage electrical injuries. The energy released in a lightning bolt is
more electrical energy than is produced by all of the electrical generators in the United States
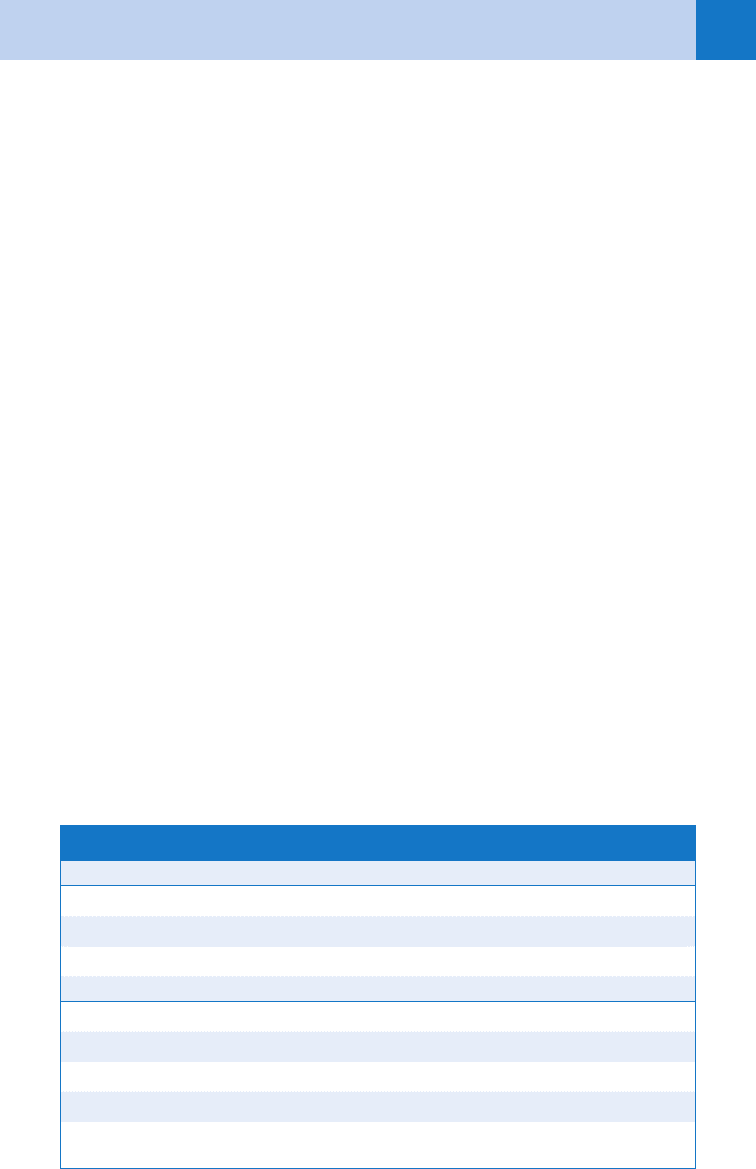
Fulgurite The word is from Latin, meaning lightning stone. When lightning strikes the
ground, it can spread out underground for up to 60 ft in radial horizontal arc-
ing. Depending on soil’s characteristics, there can be enough heat generated to
fuse silica into branching configurations. These stones are the fulgurites.
Faraday cage An enclosure formed by conducting material or by a mesh of such material;
blocks out external static electrical fields.
Flashover
phenomenon
Given the short duration of a lightning bolt, current is often conducted over the
skin without penetration. When this happens, it significantly lowers the mortal-
ity of a direct strike victim from 85% with signs of penetration to 40% without.
TABLE 55-1. LIGHTNING GLOSSARY
Chapter 55 LIGHTNING AND ELECTRICAL INJURIES 385
at that instant. However, because it is such short duration, it is only enough energy to light a
single light bulb for about a month. The energy is dissipated as light, heat, sound, and radio
waves.
8. Does lightning ever hit airplanes? What happens?
Yes, and usually not much. There are at least 160 lightning strikes on aircraft annually.
Typically, these strikes happen at 10,000 ft to 15,000 ft, in rain and light turbulence, within a
cloud and near the freezing level. Because the aircraft skin is metal, the lightning almost
always flashes over, leaving only minor damage. However, the blast effect of the thunderclap
can interrupt jet engines; the bright flash can temporarily blind pilots, and the induced
electromagnetic field can disrupt avionics and communication equipment—just what you
don’t want at 10,000 ft to 15,000 ft, in rain and light turbulence, within a cloud and near the
freezing level. There have been a few aircraft lost to fuel vapor explosions within the fuel tanks
induced by lightning.
9. How common is lightning?
There are up to 8 million lightning flashes worldwide each day. Across the globe, there are
2,000 active thunderstorms right now. Those storms produce 100 cloud-to-ground strikes per
second. In the United States, there are 50 to 300 lightning fatalities every year. This number is
hard to pin down exactly due to the majority of data being gathered from newspaper and news
accounts. “Storm Data” reports an average of 58 fatalities per year for the past 30 years, but it
suggests that there are probably closer to 70 deaths per year.
10. In what types of locations do lightning injuries occur?
In 40% the location goes unreported. Of those that are reported, 27% happen in open fields
and recreation areas; 14% happen under trees; 8% are water-related (e.g., boating, fishing, or
swimming); 5% happen on golf courses; 3% are related to the use of heavy equipment and
machinery; 2.4% are telephone related, and 0.7% are related to radio transmitter and
antennae. Central Florida is thunderstorm alley with the greatest number of thunderstorm days
in the United States.
States that are consistently in the top 10 for both total casualties and the highest casualty
rate are Florida, Colorado, and North Carolina. See Table 55-2.
11. Am I more likely to get struck as a woman or a man?
Remarkably, 84% of victims are male, likely reflecting a higher number of men engaging in
outdoor recreational and occupational activities.
Top Three States for Lightning Incidence Bottom Three States for Lightning Incidence
Florida Alaska
Texas Hawaii
Colorado North Dakota
Top Five States for Lightning Injuries Top Five States for Lightning Deaths
Florida Florida
Michigan Michigan
Pennsylvania Texas
North Carolina New York
New York Tennessee
TABLE 55-2. Top States for Lightning Incidence and Injury

Chapter 55 LIGHTNING AND ELECTRICAL INJURIES386
12. What time of year am I more likely to get struck?
Summer seems to be the most dangerous time: the monthly incidence is June 21%; July 30%;
August 22%.
13. What factors predispose somebody or something to be hit by lightning?
n
Height of the object
n
Isolation
n
Pointiness of the object (this does not apply to humans)
14. I’m treating a farmer who was found unconscious in his field after a
thunderstorm. He has no recollection of what happened. How can I tell if
he was struck by lightning?
The easiest way to tell is to look at his skin and look in his ears. His memory of the
events won’t help; in fact, it is said that 100% of direct-strike victims have amnesia.
Victims of nondirect strikes may be able to recall being hit by lightning, but they often
develop anterograde amnesia. However, arborescent superficial erythema (known as
Lichtenberg’s flowers, ferning, arboration, or fractals) is pathognomonic for lightning.
See Figure 55-1.
Unfortunately, it is seen in only seen in 20% of confirmed cases and fades away over
hours. It is not a true burn but the effect of a strong electromagnetic field on wet skin. It has
been postulated to be secondary to red blood cell extravasation into the superficial layers of
the skin from capillaries from the electrical injury. In addition, there may be partial-thickness
linear or punctate burns in moist areas. Tympanic membrane rupture (one or both) occurs in
50% of victims. Tinnitus is also common and usually resolves in hours to days. Seven
percent to 12% of victims experience temporary hearing loss, and a few have permanent
hearing loss.
15. The farmer is also tachycardic and hypertensive, with cool, pale skin, and
diminished peripheral pulses. Although awake, he seems unable to move his
extremities. Why?
He is likely suffering from lightning paraplegia (keraunoparalysis or Charcot’s paralysis). It
is probably primarily due to intense adrenergic stimulation, vasospasm, and hypoperfusion,
not direct nerve injury. If that is the case, it typically resolves over the next several hours.
However, you should be wary of occult injuries and diligent in your work-up for traumatic
injuries.
Figure 55-1. Ferning developed after the victim received a side flash lightning
strike. (From Auerbach PS: Wilderness medicine, ed 5, Philadelphia, 2007,
Mosby. Courtesy Sheryl Olson, RN.)

Chapter 55 LIGHTNING AND ELECTRICAL INJURIES 387
16. My practice at multiple casualty incidents (MCIs) is to allocate resources to
victims who are not breathing and not moving only after I have taken care of
those with signs of life. Is that rule applicable in a lightning strike MCI?
No. Lightning strike is the one exception to the usual MCI triage rule. The first priority should
go to those who are not breathing and not moving. The reasons are that only those who
present in cardiac arrest are at high risk of dying. Bystander cardiopulmonary resuscitation
(CPR) doubles survival from about 24% without CPR to 50% with CPR. Those who have not
arrested have little chance of dying, and so in this case the first priority goes to “the dead.” Of
note, victims’ cardiac automaticity often returns before spontaneous respirations; as such,
they may need prolonged rescue breathing during CPR.
17. I have heard that prolonged CPR is beneficial in lightning injuries. Is this
true?
No. There is no evidence that prolonged CPR improves recovery. It is reasonable to stop CPR
after 20 to 30 minutes as long as other reversible causes of cardiopulmonary arrest have been
addressed.
18. I am caring for a patient who has been struck by lightning and he has fixed
and dilated pupils. Can I stop CPR based on this finding?
No. One should not use dilated/fixed pupils as an indicator of brain death or to gauge the
prognosis in lightning victims until all other anatomical and functional etiologies have been
evaluated.
19. True or false: Lightning strike victims typically suffer extensive burns?
False. The crispy critter phenomenon is largely untrue. Victims do not burst into flame and
become reduced to a pile of ashes. Of the lightning strike victims who have burns, only 5%
have deep or significant burns. Lightning most often flashes over a victim with few, if any,
burns. Victims who experience burns typically demonstrate linear burns, punctuate burns,
feather burns (Lichtenberg’s Flowers), or thermal burns.
20. Which organ systems can be damaged by lightning?
See Table 55-3.
21. True or false: A lightning strike is always fatal?
False. Lightning is a surprisingly inefficient killer. The mortality may be as low as 10% to 30%.
However, lightning is the third most frequent cause of death caused by natural phenomena
(after floods and extremes of temperature).
22. True or false: If a victim of lightning is not killed outright, he or she will likely
be fine?
False. The majority of victims suffer some sequelae. These complications can include
neurologic, psychiatric, cardiac, pulmonary, otic, ophthalmologic, and musculoskeletal
disorders (see Question 20).
23. What are the best ways to reduce lightning risk?
n
Avoiding the following: thunderstorms, being the tallest target, holding a lightning rod,
touching conductors.
n
Seeking shelter indoors or in a car.
n
Staying away from groups (especially people who know CPR; they are your potential
rescuers).
n
Holding feet together and crouching down to reduce your stride potential.
24. What’s the 30-30 rule?
It is a lightning safety recommendation. If you see lightning and cannot count to 30 before
hearing thunder, you are in danger and should seek shelter. This is based on the flash-to-
bang method of determining your distance from a lightning strike: the time between the
