Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.


474 Lam and Gupta
Specialty Stents
Open-ended ureteral catheters have no coils and thus are not suitable for long-term
urinary drainage. However, they can be secured externally to a urethral catheter to
provide temporary drainage. Open-ended stents are useful in helping to direct and
advance the guidewire into the ureteral orifice. They can assist in the collection of upper
urinary tract urine samples and permit retrograde pyelography. Furthermore, open-
ended ureteral stents are commonly placed intraoperatively to identify the ureters in
order to avoid inadvertent injury during abdominal or pelvic surgery.
Grooved stents have grooves spiraling down the exterior of the stent to improve
extraluminal flow and are aimed at postlithotripsy or holmium laser cases, where pas-
sage of stone fragments is needed. The Towers peripheral stent is a grooved stent manu-
factured by Cook Urological. The outer diameter ranges from 6 to 8 Fr, and the length
from 22 to 32 cm. The LithoStent by ACMI features 3 grooves spiraling down the
exterior of the stent-like gutters designed to prevent ureteral strictures and urinomas; the
lumen accommodates standard guidewires.
Tail stents are designed with a thinner, softer distal segment to increase patient com-
fort. They have a standard pigtail and a 6 or 7 Fr shaft at the proximal end, but the distal
end tapers into an elongated 3-Fr closed-tip tail, which rests in the bladder. Drainage is
achieved around the distal portion of tail stents, and consequently these stents are con-
traindicated when trauma to the distal ureter is suspected. Tail stents resist reflux because
the distal third of the tail is occluded, and approx 3–5 cm of the tail’s occluded portion
lies within the intramural tunnel and the distal ureter. Upper tract symptoms in patients
with tail stents could be attributed to either renal pelvic irritation or intermittent obstruc-
tion. Boston Scientific/Microvasive makes a tail stent called the Percuflex Tail Plus.
Injection stents are single-pigtail catheters that convert into an indwelling double-
pigtail ureteral stent, obviating a second cystoscopy. It is ideal for patients undergoing
SWL that need a double-pigtail stent postoperatively, but simultaneously require con-
trast instillation during the procedure for stone localization. Once the procedure is com-
pleted, the injection apparatus is removed leaving a double-pigtail stent.
Urinary diversion stents, made of various materials, have a single coil for the renal
pelvis and a long straight end that is brought out for external drainage. Drainage holes
are in the proximal end of the stent. They are commonly used to provide internal support
to ureteral anastomoses created during various types of urinary diversion. This allows
for accurate monitoring of urine output from each renal unit and facilitates removal of
the stent after an adequate period of healing.
Endoureterotomy/endopyelotomy stents are used for temporary drainage from the
ureteropelvic junction to the bladder following incision of a stricture. These internal
stents have a tapering diameter with no sideports designed to prevent narrowing of the
ureteral lumen while preventing ingrowth of the ureteral wall to the stent. The larger-
caliber portion of the stent acts as a mold for the healing of the incised ureter. Applied
Medical has a 7/10 Fr endopyelotomy stent for use with the Acucise endopyelotomy
balloon catheter device. Cook Urological makes a 6/10 Fr and 7/14 Fr stent and Boston
Scientific/Microvasive makes the RetroMax Plus in 7/14 Fr.
Subcutaneous urinary diversion (nephrovesical) stents are used as an alternative to
percutaneous nephrostomy for urinary diversion in uremic cancer patients and in those
with benign idiopathic ureteral obstruction, in which placement of ureteral stents have
failed. The proximal end of the stent is inserted into the renal pelvis via a percutaneous
nephrostomy puncture. A subcutaneous tunnel is created from the flank to the bladder,

Chapter 25 / Ureteral Stents 475
where the distal end of the stent is passed into the bladder via a suprapubic bladder
puncture.
Fistula stent sets specifically assist in the internal management of ureteral fistulas.
The stent has drainage ports solely in the pigtails, in order to reduce fluid pressure within
the ureter. It is manufactured in several different diameters and lengths.
CLINICAL APPLICATIONS AND OUTCOMES
There are multiple indications for the placement of ureteral stents. They are often
placed as part of therapy to relieve ureteral obstruction or promote healing of the ureter,
or as prophylaxis against possible complications by assisting passage of a guidewire into
a ureteral orifice or by passively dilating the ureter before interval ureteroscopy.
Urolithiasis
This is probably the most common indication for ureteral stenting. Contemporary
management of renal calculi relies on endourologic techniques such as SWL, percuta-
neous nephrolithotomy (PCNL), and ureterorenoscopy (URS) (54–57). Stenting can be
performed as a therapeutic or prophylactic procedure. Indications for therapeutic stenting
include: obstructive pyelonephritis secondary to an obstructing stone; renal failure sec-
ondary to bilateral obstructing stones or obstructing stone in a solitary kidney; refractory
renal colic or pain; and relief of high grade and/or long-term obstruction. PCNL can be
performed without need for postoperative ureteral stenting. Specific indications for
postoperative stenting following PCNL include extensive perforation of the collecting
system, need for subsequent SWL for large stone burden, ureteral obstruction secondary
to edema or stone fragments, and persistent urinary leakage following nephrostomy tube
removal.
The indications for ureteral stenting with SWL of renal calculi are less defined.
Prophylactic stenting before SWL has been shown to prevent the development of
‘steinstrasse’ in patients with stones >20 mm (58). However, opponents of stent use
report an increased morbidity associated with stent placement with no differences in
stone-free rates (59,60). Furthermore, patients with ureteral stents placed before SWL
were subjected to higher levels of total power during the procedure with no difference
in stone-free rates among patients treated without a stent (60). Urinary urgency (43 vs
25%), hematuria (40 vs 23%), duration of bladder discomfort (26 vs 13%), and duration
of urinary frequency (31 vs 16%) was also significantly higher in patients with indwell-
ing stents compared to those without (60).
SWL of ureteral stones can be performed either by pushback of stone into the renal
collecting system, bypass of stone with an externalized or internalized stent, or in situ.
Data analyzed by the American Urological Association Ureteral Stones Clinical Guide-
lines panel did not support routine use of ureteral stents to improve efficiency and stone-
free results of SWL, regardless of stone size (61). Ureteral stenting before SWL of middle
ureteral stones, however, may aid in the localization of stones overlying the bony pelvis,
especially in the presence of significant ureteral obstruction, which diminishes the effi-
cacy of intravenous contrast in aiding stone localization (62). Advances in fiberoptics has
allowed for the development of smaller ureteroscopes and advances in intracorporeal
lithotripsy, such as the holmium:yttrium-aluminum-garnet (YAG) laser has allowed for
uncomplicated ureteroscopy to be performed without routine stenting with minimal
discomfort and a low incidence of postoperative complications (63,64). However, ure-

476 Lam and Gupta
teral stenting following ureteroscopy is recommended if complications occur, the stone
is impacted, the ureteral orifice is formally dilated to 18 Fr, fragmentation is incomplete,
or associated with a solitary kidney. In addition, stent placement after unsuccessful
ureteroscopic stone extraction may facilitate spontaneous stone passage and dilates the
ureter, making subsequent ureteroscopic procedures more successful (65).
Pregnancy
Urolithiasis during pregnancy is relatively infrequent with the reported overall inci-
dence of approx 0.07%, with 0.02% being symptomatic (66). Although hypercalciuria,
hyperuricosuria, and pregnancy-induced urinary stasis predispose to stone formation,
the incidence of symptomatic urinary stones in this population is no greater than that for
nonpregnant women of childbearing age (66,67). These findings are likely a result of an
increase in urinary lithogenic inhibitors (urinary magnesium, citrate, and glycoproteins)
and urine volume during pregnancy (67). Pain from renal colic is the most common
nonobstetric reason for admission during pregnancy (68). Approximately two-thirds of
symptomatic stones presenting during pregnancy will pass spontaneously; therefore, a
trial of conservative management with intravenous hydration, analgesics, antiemetics,
and prophylactic antibiotics should be advocated initially (69,70).
Azotemia, fever with obstruction, and urosepsis in the setting of urinary stone disease
requires urgent percutaneous nephostomy (PCN) or ureteral stent drainage, and the
gravid state should not alter this approach. Indwelling ureteral stent or PCN drainage is
recommended for interim treatment with definitive management delayed until postpar-
tum for symptomatic urinary stones that do not pass or if obstruction persists for more
than 3 or 4 wk (71,72). Both can be placed with ultrasound guidance to avoid radiation
exposure to the fetus. Encrustation is a concern during pregnancy because of gestational
hyperuricosuria and hypercalciuria (67). The authors recommend that ureteral stents be
changed in gravid patients at 4–6-wk intervals. PCN offers a good alternative to stent
drainage, especially early in pregnancy. The need for repeated stent changes is elimi-
nated, and it can be converted to an indwelling stent for comfort as the patient nears term.
Definitive stone management during pregnancy has been performed with few com-
plications. Both flexible and rigid ureteroscopy with holmium:YAG laser lithotripsy
have allowed for successful and safe endoscopic removal of symptomatic stones during
all stages of pregnancy, although this is not considered standard (73–75). Other forms
of intracorporeal lithotripsy that can be considered for treating stones during pregnancy
are the pulsed-dye laser and pneumatic lithotriptor because these modalities deliver
localized energy. In contrast, electrohydraulic lithotripsy (EHL) and ultrasonic litho-
tripsy may present theoretical risks to the fetus because electrical energy discharged
from EHL could precipitate premature labor and the vibratory energy of ultrasonic
lithotripsy may pose a risk to the developing ears of the fetus (75). PCNL is rarely
indicated during pregnancy; however, it has been safely performed in cases of repeated
infection or obstruction despite percutaneous drainage (72). Other options have included
open stone surgery and early parturition. SWL is contraindicated in pregnancy because
of a theoretical risk to the fetus and/or ovaries (71).
Renal Transplantation
Urinary tract stones after renal transplantation can be a result of unsuspected donor
calculus or caused by persistent hyperparathyroidism, recurrent urinary tract infections,

Chapter 25 / Ureteral Stents 477
foreign body such as suture or staple, obstruction, habitual decreased fluid intake, and
type I renal tubular acidosis (76). Because the renal transplant is denervated, the kidney
transplant patient will not experience typical renal colic, and the diagnosis is suspected
when renal function suddenly deteriorates or transplant pyelonephritis occurs. Upper
tract stones are managed by the same techniques as stones in the normal urinary tract;
however, negotiation of the transplanted ureter may be difficult or impossible because
of tortuousity, thus favoring percutaneous techniques. SWL has been used successfully
with the stone positioned in the shock wave path (77).
Ureteral strictures and fistulas are the most frequent urologic complications in renal
transplantation, occurring in 1–12% of patients (78,79). Prospective studies have con-
cluded that ureteral stenting is beneficial in reducing vesicoureteric leakage rates and
obstruction in renal transplantation (80). Furthermore, presence of a ureteral stent does
not result in an increased incidence of urinary tract infection (81).
Obstructive Urosepsis
Management of an obstructed urinary tract can be performed with ureteral stent or
PCN drainage. The advantage of ureteral stenting is complete internalization of the stent
without the need for an external drainage device; however, the advantage of PCN is a
more definitive and reliable urinary drainage. Disadvantages of an indwelling stent
include pain, encrustation, infection, and bladder irritability. Disadvantages of PCN
include risk of renal and other organ injury during placement, their external nature, and
the potential for dislodgment, erosion, bleeding, or infection. Both ureteral stents and
PCN can be placed under intravenous sedation, although stenting usually occurs in the
operating room with an anesthesiologist present. Both ureteral stenting and PCN have
been demonstrated to effectively relieve obstruction and infection caused by ureteral
stones and neither modality is superior in promoting more rapid recovery following
drainage (82). The decision on which mode of drainage to use should be based on
surgeon preference and skill, presence of skilled interventional radiologists or urologists
trained to perform PCN, operating room availability, and stone characteristics.
Extrinsic Ureteral Obstruction
Ureteral stenting has been widely used in the management of ureteral obstruction
caused by pelvic malignancies or retroperitoneal fibrosis. Nevertheless, obstruction can
still persist even after placement of large-caliber indwelling stents (83). The combina-
tion of extrinsic compression against the stent and aperistaltic ureteral segments may
impair urine drainage around well-placed stents. Stenting with single indwelling stents
has been reported to fail in 50% of prostate cancer patients and 89% of patients with
cervical cancer (84,85). An option for patients failing previous stenting with a single
6-, 7-, or 8-Fr stent is to place two parallel ipsilateral ureteral stents, usually 8 Fr, which
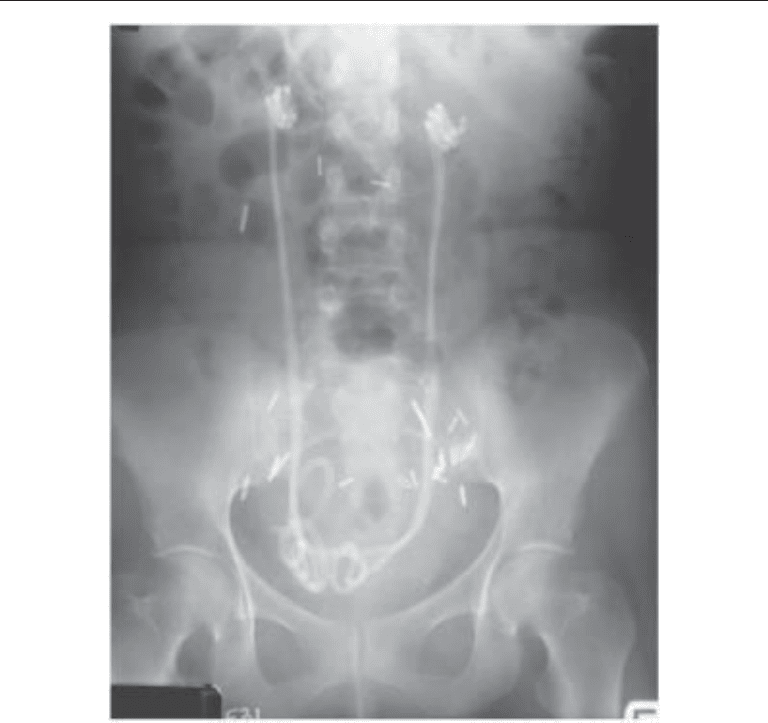
have been successfully used in the relief of obstruction (Fig. 1) (86). Another option is
the use of metal ureteral stents, which are intended for permanent implantation and have
a radial force higher than the pressure exerted by the surrounding tissues. Varying
success rates using these devices have been reported, ranging from 14 to 100% in
patients with strictures of various etiologies and locations (87,88). Multiple overlapping
metal stents can be placed either alone or in combination with conventional indwelling
ureteral stents in patients with long strictures. Not all patients with malignant strictures
are candidates for metal stent implantation, especially those patients with distal ureteral
478 Lam and Gupta
strictures because the stents may project into the bladder, prevent epithelization and
result in early encrustation blockage and patient discomfort. Subcutaneous urinary
diversion with nephrovesical ureteral stenting has also been reported to be successful
in patients with ureteral obstruction secondary to malignancy or medical conditions,
excluding them from more invasive procedures (89,90).
Endoscopic and Open Surgery
Ureteral stenting has become complementary to ureteroscopic procedures to prevent
either post-ureteroscopic ureteral edema or scarring of small mucosal lesions. Success
rates for ureteral stenting following endoscopic balloon dilatation of ureteral strictures
has been reported to be about 60% (91). Primary insertion of ureteral stents has been
shown to prevent or reduce the incidence of anastomotic stenosis and leakage following
open ureteral repair (92). Ureteral stents promote healing by providing a scaffold for
epithelialization and by avoiding early flow urine through the defect. However, other
studies have shown that ureteral healing is the same whether a stent is present or not (93).
Fig. 1. KUB radiograph of bilateral parallel double-pigtail stents for malignant ureteral obstruc-
tion.

Chapter 25 / Ureteral Stents 479
Endopyelotomy and endoureterotomy have become treatments of choice for uretero-
pelvic junction obstruction and ureteral stricture, respectively, owing to a short conva-
lescence period and absence of incision-related morbidity related to these procedures.
Stenting is an integral part of the ureteral endoincision that prevents leakage of urine into
the retroperitoneum and provides a mold for the ureteral epithelium to grow over. There
remains debate over the size of the stent and optimal duration of postoperative stenting.
A 6-wk duration of postoperative stenting is considered the gold standard based on
experimental work by Davis, who showed that after a full-thickness incision through the
narrowed segment of ureter, regeneration of the muscular layer was 90% complete by
6 wk (94). However, others have demonstrated shorter duration of postoperative stenting
in the successful outcome of endopyelotomy (95). The size of ureteral stent to use
following ureteral stricture incision remains just as controversial. A specialized
endopyelotomy stent, one that measures 14 Fr at the ureteropelvic junction and tapers
to 7 Fr, can be used (96). These can be placed antegrade or retrograde, and can drain
externally or internally. Placement of smaller 7 or 8 Fr internal ureteral stents have been
reported to produce comparable results with that achieved by the standard 14/7 Fr
endopyelotomy stent (97). Smaller caliber stents, ranging in size from 4 to 10 Fr, have
yielded satisfactory outcomes in 78–95% of patients (97).
Preoperative ureteral stenting has been used to prevent ureteral injuries caused by
gynecologic procedures, as well as general surgical procedures. However, some inves-
tigators have reported that preoperative catheterization is not effective in preventing
trauma and could even provoke it (98,99). Transilluminating ureteral stents have been
used for preventing ureteral injuries during gynecologic procedures (100).
Trauma
Ureteral injuries can be secondary to external trauma, either blunt or penetrating, or
more commonly are iatrogenic. In most cases, injury to the ureter diagnosed during the
initial evaluation of the trauma is generally treated by open surgical repair. External
ureteral trauma is almost exclusively associated with other injuries (101). Principles of
open surgical repair of ureteral injury include debridement and spatulation of the ureter,
followed by a tension-free closure with absorbable suture over an indwelling ureteral
stent. Delayed diagnosis of a missed or iatrogenic injury may be treated by percutaneous
drainage of urinoma and retrograde or antegrade placement of a ureteral stent if the ureter
is partially intact (102). Following tangential injury to a portion of the ureteral wall by
penetrating trauma or clamp or suture injury during surgery, passage of an indwelling
ureteral stent may allow for complete healing with reasonable long-term results (103).
Ureteral stents may also be used safely and effectively to treat persistent or recurrent
urinary extravasation resulting from major blunt renal trauma in appropriately selected
patients (104).
Fistulas
Ureteral fistulas can be classified based on location: ureterovaginal, ureterocutaneous,
ureteroenteric, lymphaticoureteral, and ureteroretroperitoneal (urinoma) (105). One of
the earliest uses of ureteral stents was for treatment of ureterovaginal fistula in which
polyethylene tubing was employed to affect closure (5). The use of percutaneous ureteral
stents to successfully treat urinary fistulas was first reported by Goldin (106). Andriole
and colleagues reported a 50% closure rate with use of an indwelling double-J stent in

480 Lam and Gupta
the management of upper urinary tract fistulas (84) and Chang and co-workers reported
successful resolution in 10 of 12 ureteral fistulas treated with percutaneous antegrade
ureteral stenting (107).
STENT REMOVAL
There are no controlled studies or consensus on the ideal length of time to leave
indwelling ureteral stents in place. In uncomplicated cases, stents can be removed in 2–
3 d after ureteroscopy. In cases of ureteral perforation or concern regarding ureteral
obstruction after instrumentation, an indwelling ureteral stent should remain in place for
at least 1–2 wk. The majority of indwelling ureteral stents can be removed in the office
with topical anesthesia using a flexible cystoscope and grasper. If the patient is unable
to tolerate an office procedure, stent removal can be performed under anesthesia in the
cystoscopy suite. In patients that are hospitalized, externalized ureteral stents can be
used, and before stent removal, a ureterogram can be performed to document ureteral
anatomy, passage or removal of stones, and lack of extravasation or obstruction.
Nonendourological techniques have been described for stent removal. Some stents
are manufactured with a nylon suture attached to the distal end, which can be left at the
urethral meatus allowing for removal after short-term drainage without the need for
repeat cystoscopy. However, patients must be cautioned not to place tension on the string
because this could potentially lead to unintentional dislodgment of the stent. The dan-
gling suture may also be associated with a slight degree of incontinence. A wire intro-
ducer with a snail head coil at its distal end has also been described with some success
for blindly grasping a stent within the bladder of women only (108). Stent retrieval has
been reported with indwelling stents incorporating a distal magnetic tip that can be
retrieved by using a magnet (109), and in stents with a stainless steel bead attached to its
distal end, which can be removed by a urethral catheter that has a rare earth magnet
attached to its proximal end (110). The development of biodegradable stents will elimi-
nate the need for invasive removal procedures (42–44). All patients with indwelling
ureteral stents should be told of the importance of appropriate follow-up and eventual
stent removal. Extracting forgotten ureteral stents can be technically challenging and
may require multiple cystoscopic and/or percutaneous procedures owing to potential
stent friability and breakage.
COMPLICATIONS
Despite recent innovations and improvements in stent materials and designs, prob-
lems relating to indwelling ureteral stent use still occur, such as migration, occlusion,
encrustation, breakage, and stone formation (111).
Symptoms and Quality of Life
Patient discomfort associated with irritative voiding symptoms, flank and abdominal
discomfort, and hematuria in the absence of urinary tract infection is the most common
complication involving patients with indwelling ureteral stents (112–114). It is pre-
sumed that the etiology for lower urinary tract symptoms is the distal portion of the stent
traversing the intramural ureter and impinging on the bladder floor (115,116). The cause
of upper tract symptoms, which may occur in as many as 50% of patients, is thought to
be secondary to vesicoureteral reflux. Many earlier studies comparing ureteral stents
failed to report any significant differences between stents of different compositions and

Chapter 25 / Ureteral Stents 481
diameter (113,114). However, more durable clinical studies have shown that “softer”
stents composed of biomaterials with smaller durometer, likely result in a lower inci-
dence of symptoms (114). In another randomized, blinded study, smaller diameter stents
were associated with less pain and improved patient tolerance (117).
Migration
Migration of ureteral stents is a well-known complication (118). Proximal or distal
migration may occur despite the J shape of the stent and can be problematic. Stents with
a full coil rarely migrate compared to those with the J configuration. Materials with good
memory, such as polyurethane, have the least tendency for migration, whereas stents
composed of softer materials, such as silicone, have the highest incidence of migration.
Stent migration may occur if the stent is too short, resulting in proximal migration, or
placed in massively dilated systems. One trick that can help prevent migration of the
proximal coil into the ureter in a patient with a widely patent UPJ is to place the coil into
a calyx with a relatively narrow infundibulum. Direct ureteroscopic removal and occa-
sional use of percutaneous techniques may be needed to extract proximally migrated
stents (119). These stents can be tricky to retrieve ureteroscopically because they can be
slippery and difficult to grasp, the distal coil displaces and distorts ureteral anatomy, and
the tip to the stent can dig into the ureteral wall. One trick the authors have found useful
in retrieving stents that have migrated into the distal ureter is the use of a stone basket
to “catch” the distal tip instead of using a grasper. Another trick is to use rat tooth or
3-prong graspers and to place one of the prongs into a side-hole of the stent to prevent
slippage when withdrawing.
Infection and Encrustation
Stent-related infection and encrustation are common complications, causing signifi-
cant morbidity, and are the major limiting factors in long-term use of biomaterials within
the urinary tract. The mechanism of encrustation in infected urine is identical to the
formation of infected urinary stones, involving alkalization of urine owing to hydrolysis
of urea by urease-producing organisms (120). Magnesium and calcium precipitate in the
alkaline environment produced by urea hydrolysis, forming magnesium ammonium
phosphate (NH
4
MgPO
4
2H
2
O) and calcium hydroxyapatite (Ca
10
[PO
4
]6H
2
O). It begins
with the development of an organic biofilm, often consisting of albumin, Tamm-Horsfall
protein, and alpha
1
-microglobulin, covering the plastic surface, and can enhance crystal
precipitation and aggregation events on the surface (121). This biofilm also allows
bacteria to be trapped and potentially be protected from antibiotics (122). Stent encrus-
tation in sterile urine is not completely understood, but it appears to be dependent on both
the urinary constituents and properties of the biomaterial. Sterile encrustations are often
composed of calcium oxalate (123). Metabolic disorders, such as hypercalciuria, certain
physiological states such as pregnancy, and even the intestinal microbial flora may
contribute to accelerated encrustation of stents within a sterile urinary environment
(123–125). Presently, there are no biomaterials used in the urinary tract that are able to
completely withstand the effects of the urinary environment.
Once established, stent-related encrustations and infections are often severe compli-
cations, necessitating stent removal if clinical cure is to be achieved. Stent colonization
is frequent with rates ranging from 28 to 90% (126). Use of urinary cultures to predict
stent colonization has been reported to have a sensitivity of 31% and incidence of stent

482 Lam and Gupta
colonization does not correlate with indwelling time (127). In addition, administration of
prophylactic antibiotic treatment does not prevent bacterial adherence to ureteral stents
(127). Sterile pyuria is not infrequent and reflects foreign body reaction to the stent. In
the absence of infection proven by culture, pyuria is generally inconsequential (30).
Encrustations may develop on ureteral stents intraluminally and extraluminally, reduc-
ing ureteral flow and causing obstruction of the renal unit, leading to impaired renal
function. Encrustations may be multifactorial and risk factors include poor compliance,
long indwelling times, sepsis, pyelonephritis, chronic renal failure, recurrent or residual
stones, lithogenic history, metabolic abnormalities, congenital renal anomalies, and
malignant ureteral obstruction (128). Interactions involved in the deposition of encrus-
tation on stents also appear to be influenced by the chemical composition of the polymer,
physical properties of its surface, presence of graft polymer coating conferring a hydro-
philic/hydrophobic nature on the biomaterial, contact time with urine, solute content of
urine, and intestinal microbial composition (123–125). The exact interval for changing
or removing an indwelling ureteral stent to avoid significant encrustation is difficult to
determine owing to multiple and unclear etiologies of stent encrustation. However, stent
encrustation rates increase with the duration that the stent remains indwelling. At less
than 6 wk, a 9.2% encrustation rate has been reported, which increases to 47.5% at 6–
12 wk and 76.3% at more than 12 wk (128). The optimal indwelling period based on
different series is 2–4 mo (111,128), however, it should be shorter in those patients with
risk factors that predispose them for developing encrustations. Some recent develop-
ments in stent design, including the incorporation of antibiotic coatings covalently bound
to the outer stent surface, are being used to decrease biofilm production and stent colo-
nization, but are not yet proven to be clinically effective.
Retained/Fractured Stents
Retained stents, especially those encrusted, occur infrequently but can be a difficult
and challenging problem that can lead to severe morbidity and sepsis if not managed
carefully. Successful management of a retained ureteral stent requires careful planning
and may require a combination of endourologic approaches that can be safely performed
to remove the retained stent and any associated stone burden during a single anesthestic
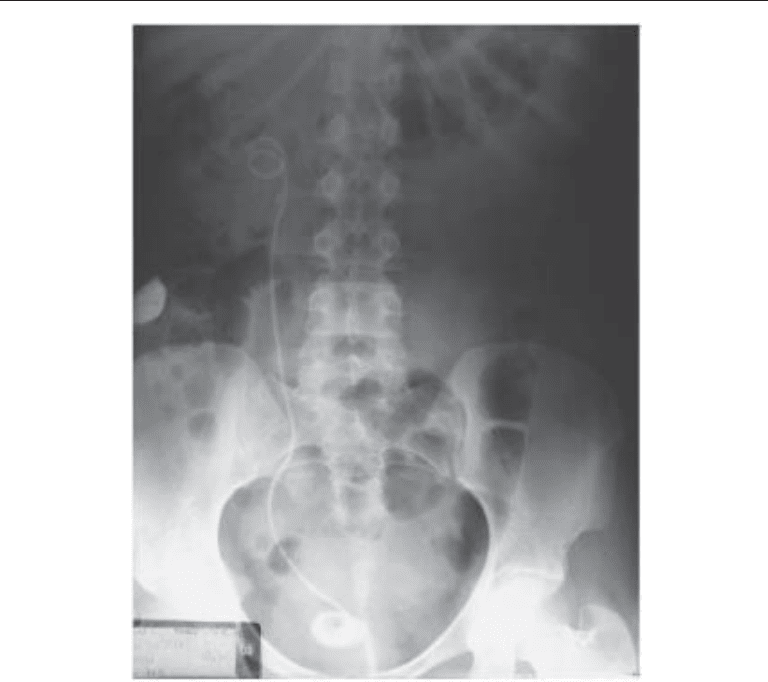
session (111). The authors feel the most important preliminary step is to obtain an
excellent kidney-ureter-bladder (KUB) radiograph with tomographic views paying care-
ful attention to the proximal coil and ureteral portions of the stent in order to determine
whether it appears slightly “thicker” than it should (Fig. 2). These are subtle signs often
missed by radiologists who are not familiar with the clinical situation. If no encrusta-
tions are present, cystoscopy is performed and gentle traction of the retained stent is
attempted. If the patient complains of pain or the stent does not move easily, the authors
do not proceed any further to avoid the risk of damaging the ureter. Occasionally, it is
possible to remove enough of the stent beyond the urethral meatus and attempt to pass
a guidewire through the stent to determine if the lumen of the stent is occluded and to
possibly uncoil the proximal portion of the stent.
If encrustations are visualized on KUB radiograph or fluoroscopy, the authors do not
advise attempted removal of the stent. Instead, ureteral access should be maintained with
a wire placed adjacent to the encrusted stent. In some cases, this may be sufficient for
facilitating removal of the retained stent. The authors recommend treatment of any
bladder component of encrustation first. If bladder component encrustations are minor,
a flexible or rigid alligator forceps or biopsy forceps can be used to separate the coils after
Chapter 25 / Ureteral Stents 483
breaking off pieces of encrustation. A cystoscopic view of a large bladder stone encom-
passing the distal coil of the retained stent is shown (Fig. 3A). Otherwise, EHL can be
used to break apart bladder encrustations and remove any stone-burden within the blad-
der (Fig. 3B). The least invasive and most efficacious way of managing encrustations on
the proximal or ureteral portions of the retained stent is SWL, and subsequently gently
tugging on the stent until it releases. If a lithotriptor is present in the room, this can be
performed immediately and simultaneously. Otherwise, a small (4.7 Fr) ureteral stent
can be placed adjacent to the existing stent. This will provide drainage if the renal unit
is obstructed and passively dilate the ureter. The patient is then brought back for SWL
at a later session. In no case should significant force be used to attempt stent extraction
because this may result in severe ureteral injury, or the stent may break off, especially
if it is brittle, making a terrible situation even worse. If a stent breaks off, ureteroscopy
can be performed to retrieve the broken stent fragments within the ureter.
If the above fails, or if a ureteral component of encrustation is visible, retrograde
ureteroscopy may be attempted after placing a safety wire. A small caliber (6 Fr) semi-
rigid ureteroscope can be passed under direct vision along side the existing stent and
encrustations can be fragmented using a holmium:YAG laser under low power (5 W).
Fig. 2. KUB radiograph showing a retained ureteral stent with a calcified bladder stone encom-
passing the distal coil of the retained ureteral stent and a “thickened” proximal coil.
