Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.


Metamorphic Rocks and Metamorphism: An Overview
407
at the solidus is dependent on rock composition,
regimes of metamorphism and partial melting in het-
erogeneous rock bodies overlap hundreds of degrees.
Such overlapping conditions are thought by many
petrologists to be represented in migmatites that con-
tain leucocratic granite intimately mingled with more
refractory mafic metamorphic rock (Figure 11.24). At
the lower limit, the distinction is equally blurred be-
tween incipient metamorphism and diagenetic changes
in sedimentary and volcaniclastic deposits. In this tran-
sition, the typically very fine grained rocks are generally
not amenable to study using thin sections but require
other laboratory techniques, such as X-ray diffractom-
etry, transmission electron microscopy, and reflected
light microscopy (Frey and Robinson, 1999). The trans-
itional continuum between diagenesis and metamor-
phism can be seen in thick sedimentary and volcanic
sequences in arc-related depositional basins where P
and T increase with depth of burial. The next two
paragraphs describe the nature of this continuum in
volcaniclastic and clay deposits.
Volcanic glass is metastable from the moment of
its formation but can persist for very long periods of
time, especially in dry climates. However, vitroclasts
deposited in saline lakes can be diagenetically altered
to zeolites in a few thousand years. In addition, during
burial at progressively increasing P and T, glass in
vitric tuffs can be replaced by zeolites and analcite
(Figure 14.2). Stabilization of analcite with quartz, or
of the zeolites heulandite and clinoptilolite with quartz,
is generally considered to be indicative of the onset of
metamorphism resulting from burial. Large variations
in the depth of burial necessary for incipient meta-
morphic equilibration have been found by drilling into
active geothermal areas and from stratigraphic rela-
tions in exhumed basin deposits. In geothermal areas,
such as the Salton Sea in southern California and
the Wairakei-Broadlands in northern New Zealand,
Table 14.1. (Continued).
Orthopyroxene Opx (Mg,Fe)SiO
3
Paragonite* Pg Na
2
Al
4
(Si
6
Al
2
O
20
)(OH)
4
Periclase Per MgO
Phengite Phe K
2
(Al,Mg,Fe)
4
Si
6–7
Al
2–1
O
20
(OH,F)
4
Phlogopite* Phl K
2
Mg
6
Si
6
Al
2
O
20
(OH)
4
Plagioclase Pl (Ca,Na)Al
2–1
Si
2–3
O
8
Prehnite Prh Ca
2
Al(AlSi
3
O
10
)(OH)
2
Pumpellyite Pmp Ca
4
(Mg,Fe
2
,Mn)(Al,Fe
3
,Ti)
5
O(OH)
3
(Si
2
O
7
)
2
(SiO
4
)
2
·2H
2
O
Pyrite Py FeS
2
Pyrope* Prp Mg
3
Al
2
Si
3
O
12
Pyrophyllite Prl Al
4
Si
8
O
20
(OH)
4
Pyrrhotite Po Fe
1–x
S where x 0–0.125
Quartz Qtz SiO
2
Rutile Rt TiO
2
Sapphirine Spr (Mg,Fe
2
,Fe
3
,Al)
8
O
22
(Al,Si)
6
O
18
Scapolite Scp (K,Na,Ca)
4
Al
4
(Al,Si)
3
Si
6
O
24
(Cl,CO
3
,SO
4
)
Serpentine Srp Mg
3
Si
2
O
5
(OH)
4
Siderite Sd FeCO
3
Sillimanite Sil Al
2
SiO
5
Spessartine* Sps Mn
3
Al
2
Si
3
O
12
Spinel* Spl MgAl
2
O
4
Staurolite St (Fe
2
,Mn,Zn)
2
(Al,Fe
3
,Ti)
9
O
6
[(Si,Al)O
4
]
4
(O,OH)
2
Stilpnomelane Stp (K,Na,Ca)
0.6
(Mg,Fe
2
,Fe
3
)
6
Si
8
Al(O,OH)
27
·2–4H
2
O
Talc Tlc Mg
6
Si
8
O
20
(OH)
4
Titanite (sphene) Ttn CaTiSiO
5
Tourmaline Tur complex boron silicate
Tremolite* Tr Ca
2
Mg
5
Si
8
O
22
(OH,F)
2
Vesuvianite (idocrase) Ves Ca
19
(Al,Fe)
10
(Mg,Fe)
3
(Si
2
O
7
)
4
(SiO
4
)
10
(O,OH,F)
10
Wairakite Wa CaAlSi
2
O
6
·H
2
O
Wollastonite Wo CaSiO
3
Zoisite* Zo Ca
2
Al
2
O·AlOH(Si
2
O
7
)(SiO
4
)
Chemical compositions from Deer et al. (1997). Select chemical analyses listed in Appendix A. Abbreviations from Bucher and Frey (1994).
Some minerals(*) are end-members of solid solutions that can also occur in nearly pure form. Clay minerals are in italics.

increases during burial to more than 100°C, smectites
are gradually replaced by more stable, less water-rich
illites, another complex clay mineral group. Interlay-
ered clay particles (0.0039 mm in diameter) of illite
and smectite are common. The expulsion of water—
both physically entrapped between clay grains during
deposition and chemically bound in the clay minerals
—and conversion to higher T minerals is one manifes-
tation of diagenesis, making mud into shale. (Some
geologists prefer to use the term mudrock for any rock
that is made mostly of clay minerals and to restrict the
name shale for laminated or platy mud rocks. The inter-
action between clay minerals and organic material is
explored in Special Interest Box 14.1.) Still higher
temperatures, to perhaps as much as 300°C, convert
mixed-layer clays into chlorite, while illites are recon-
stituted into sericite. This is a fine-grained white mica
which may include muscovite, paragonite, and pyro-
phyllite but is usually phengite, a high-silica musco-
vite in which there is coupled substitution of Si and
Fe
2
or Mg for 2Al so that the atomic ratio Si/Al 3.0
(Table 14.1; see also Dempster, 1992). These chlorites
and white micas that are produced at the onset of
metamorphism are harder, less hydrous, and generally
slightly coarser grained than the clay minerals in shale
and are typical of the aphanitic platy metamorphic
rock called slate. Water liberated during diagenesis
and incipient metamorphism carries dissolved ions
leached from the unstable recrystallizing clays and
other phyllosilicates. Thus, these changes at the onset
of metamorphism are not strictly isochemical.
In magmatic rocks where the water fugacity is very
low, primary high-T minerals, such as feldspars and
pyroxenes, can persist indefinitely over a fairly wide
range of metamorphic pressures and temperatures.
However, where the water fugacity is sufficiently high
under subsolidus conditions, hydrous metamorphic
minerals are stabilized and can partially to completely
replace primary minerals, depending on the availability
of water. Water not only stabilizes new phases but also
catalyzes mineral reactions by enhancing kinetic rates
of atomic diffusion and crystal growth.
The water required for this metamorphism (or altera-
tion, as some geologists prefer to call it) can have
different sources. In closed magma systems, juvenile
water exsolved from the crystallizing melt reacts at
subsolidus temperatures with the primary magmatic
minerals so that the rock effectively “stews in its own
juices” during autometamorphism or deuteric alteration.
This is particularly common in granitic intrusions
where primary minerals are partially re-equilibrated to
lower-T hydrous minerals (Plate IV). In open magma
systems, water may be drawn into a cooling dike from
water-bearing wallrock or water may enter a cooling
lava flow emplaced in a lake or the ocean, as at the
global spreading ridge system. Tensile fractures created
408 Igneous and Metamorphic Petrology
reconstituted tuffs and sedimentary rocks occur at
depths as little as 1–2 km, based on drilling. In basins
with lower geothermal gradients, reconstitution occurs
at depths 8 km. Hence, T appears to be a more signi-
ficant factor in reconstitution than P. The presence of a
separate aqueous fluid phase is also necessary because
zeolites and analcite contain more water (10 and
about 8 wt.% H
2
O
, respectively; Appendix A) than is
typically found in silicic glass. Also, the concentration
of CO
2
in the fluid phase cannot be very large or car-
bonate minerals are stabilized instead of zeolites and
analcite. Figure 14.2 shows delicate glass shards that
have been perfectly preserved through the conversion
into analcite. Growth of the fine-grained metamorphic
mineral grains has not erased the original vitroclastic
texture of the tuff that is preserved as a relict fabric.
Kaolinites and more widespread smectites, of which
montmorillonite is a principal variety, are complex clay
minerals formed by weathering of feldspars and other
alumino-silicates and are a major constituent of soils
and of mud deposited in sedimentary basins. Chemic-
ally, clay minerals are typified by their relatively high
contents of H
2
O and Al (Tables 14.1 and A.2 in
Appendix A). Rocks made largely of clays, or their
metamorphic equivalents, are called pelites. As T
Feldspar
phenocrysts
10mm
Relict
glass shards
replaced by analcime
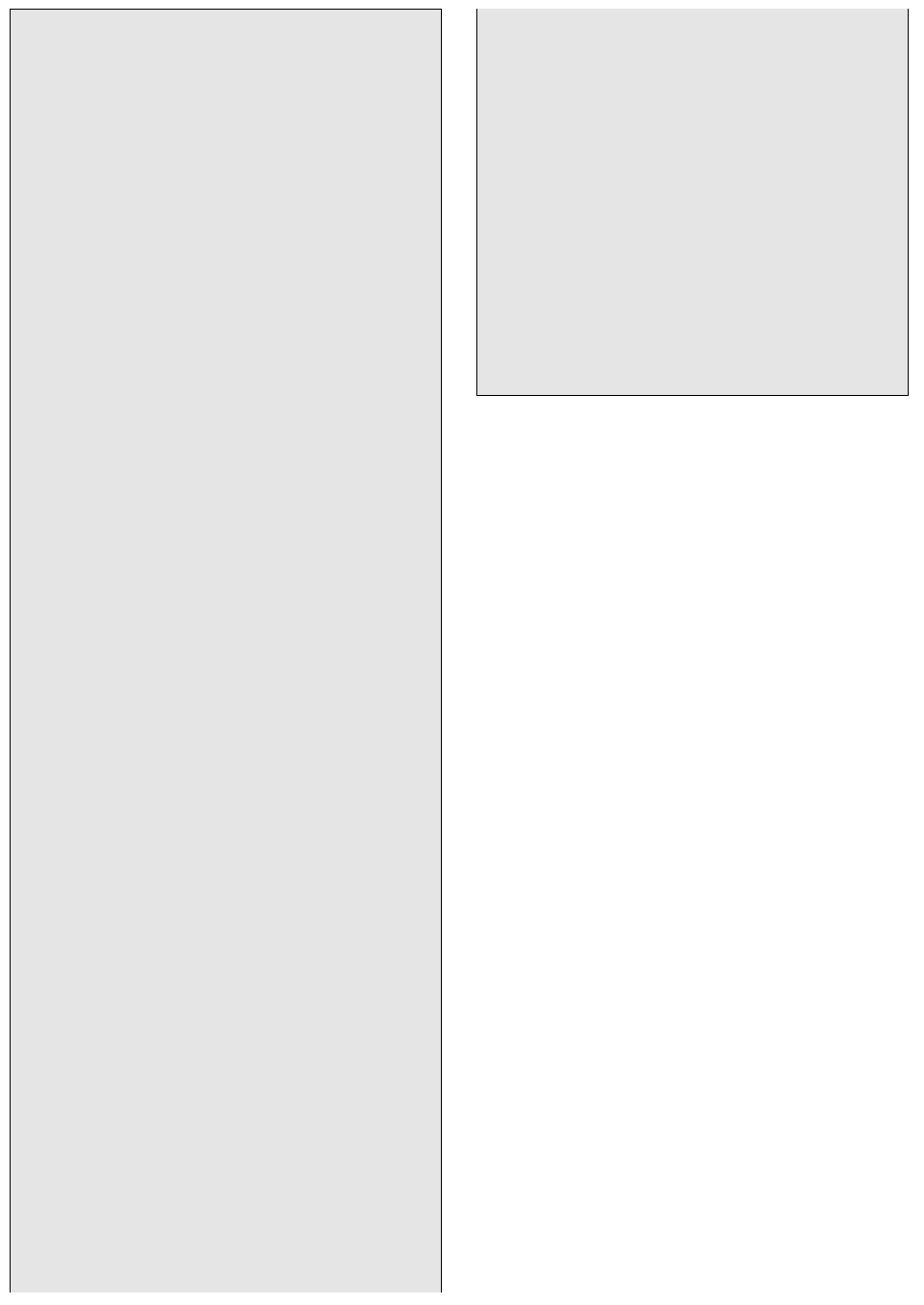
14.2 Relict vitroclastic texture in a vitric–crystal tuff subjected to
incipient burial metamorphism. Compare this photomicro-
graph under plane polarized light of a rock from New South
Wales, Australia (Wilkinson and Whetten, 1964) with Figure
7.32b. Shards of glass have been entirely replaced by analcime
(analcite). Fine-grained quartz, alkali feldspar, heulandite
zeolite, chlorite, and montmorillonite occur in the matrix
around the relict shards and phenocrysts. Black circles are air
bubbles in the thin section cement. Thin section provided
courtesy of John Whetten.
Metamorphic Rocks and Metamorphism: An Overview
409
Special Interest Box 14.1 Low-grade meta-
morphism of mudrock and the origin and
migration of oil
Oil is created from incompletely oxidized organic
debris, chiefly plant material, that is deposited with
clay and other fine-grained sediments in sediment-
ary basins. With time, the mud and organic debris
become buried to greater depths within the basin as
they are covered by younger sediments. As burial
depth increases, T also increases. As T rises to about
50°C, the organic debris converts to a dark, solid,
complex hydrocarbon called kerogen. With further
burial, as T rises from about 50 to 100°C, the solid
kerogen transforms into the liquid hydrocarbon
called crude oil.
At this point, crude oil is a sticky, viscous liquid
that is finely dispersed among and stuck to the
grains of clay and other sedimentary particles in the
basin. An oil well drilled into this material would
recover no oil because the crude oil is too widely
dispersed and the molecules adhere to the sediment-
ary grains. Therefore, the oil cannot flow through
the rock to the well. For the oil to become recover-
able, it must first migrate out of this source rock and
concentrate in reservoir rock. What causes the oil to
move from the clay-rich source rock and eventually
concentrate elsewhere?
A clay mineral called smectite is the most abund-
ant clay formed by weathering and thus the most
abundant clay deposited in sedimentary basins.
Smectite converts metamorphically into another clay
called illite as the T rises from about 50 to 100°C
during burial in sedimentary basins. Therefore, illite
is the most abundant constituent of shales.
Smectite contains about 40% water by volume;
illite contains only a few percent. Thus, as smectite
converts to illite, large quantities of water are for-
cibly expelled from the rock. This expulsion of water
during low-grade metamorphism of clay occurs in
the same T range in which kerogen transforms to
liquid oil. The forcibly expelled water then flushes
the oil from the clay-rich source rock and causes
it to migrate into reservoirs. [Hydraulic fractures
likely play an important role in this migration.]
Calculations of the amount of energy required to
convert kerogen to oil in sedimentary basins show
that, in most basins, there is not enough thermal
energy available to form liquid oil in the T range
50–100°C. The conversion should not occur until
much higher temperatures are attained. Yet 50–
100°C is the T range of observed oil formation in
most sedimentary basins.
Recent experiments and theoretical work show
that the reaction in which smectite converts to illite
during cooling and contraction facilitate advective
penetration of water into dikes and lava flows.
In hydrating changes, exact stoichiometrically bal-
anced reactions are difficult to write because of their
complexity. The following serve only to indicate in
a general manner the fate of the high-T, primary
magmatic mineral(s) on the left that convert into a
lower-T, secondary, more hydrated subsolidus mineral
or mineral assemblage on the right. Note the liberated
elements that can be removed as soluble ions in an
aqueous fluid.
Primary magmatic → Secondary subsolidus
mineral(s) water mineral(s)
biotite water → chlorite rutile
(or titanite) K Si
hornblende water → chlorite rutile
(or titanite) Si Ca
calcic clinopyroxene → actinolite or epidote
water
olivine/orthopyroxene → serpentine Fe-oxides
water
plagioclase Ca Fe → epidote
water
feldspars water → sericite Si K
(higher T )
feldspars water → clay minerals Si
(lower T ) Ca Na
Quartz remains stable under a wide range of geologic
conditions.
acts as a catalyst that causes transformation of kero-
gen to oil at much lower temperatures than would
be necessary otherwise. A catalyst is a substance
or process that causes or accelerates a chemical
reaction without being permanently changed by the
reaction.
This research on clay mineral reactions in sedi-
mentary basins suggests that, in many of the world’s
great oil-producing basins, low-grade burial meta-
morphism of clay minerals is important to both the
formation of oil and the migration of oil from
source rocks to reservoirs.
Excerpt from Modern Physical Geology by
Graham R. Thompson and Jonathan Turk, copyright
© 1991 by Saunders College Publishing, reprinted
by permission of the publisher.

410 Igneous and Metamorphic Petrology
In a thin section of a metamorphosed andesite (Fig-
ure 14.3), the formation of new, more stable hydrous
minerals has not obliterated the original magmatic
aphanitic–porphyritic texture. Because of this pre-
served relict magmatic texture, perhaps fortified by
field relations, there is little doubt of the ancestry of the
rock. Hence, the metamorphic rock may accurately be
called a meta-andesite.
Note the important contrast in the nature of the
equilibrating changes in the foregoing examples. In
the shale, reconstitution was driven by increasing T
and the ensuing endothermic mineral reactions liber-
ated water while producing less hydrous minerals. On
the other hand, in initially “dry” magmatic protoliths,
water was added to stabilize hydrous minerals at
subsolidus temperatures (Figure 5.31).
14.1.2 Recrystallization under Hydrostatic
Conditions: Newly Imposed Granoblastic Fabric
In most metamorphic equilibration processes, new
fabrics are progressively overprinted over protolith
fabrics, obliterating them. Only in finer-grained, less
metamorphosed rocks are relict features preserved.
The newly imposed solid-state growth fabrics are,
for the most part, distinctly different from magmatic
fabrics and from the texture of clastic sedimentary
rocks. We first examine metamorphic fabrics created
under hydrostatic states of stress. The influence of non-
hydrostatic states is considered in Section 14.1.3.
Recrystallization as used in this textbook refers to
solid-state production of new mineral grains from
pre-existing ones. Two distinct processes can be
recognized:
1. Recrystallization sensu stricto. Boundaries of exist-
ing grains are texturally modified in some way. No
new phases are created. Static heating can coarsen
grain size whereas recrystallization of strained
grains can yield smaller grains, the stored “strain
energy” derived from the work of deformation
being an important driving force for grain bound-
ary modification.
2. Solid-state crystallization. Nucleation and growth
of crystalline grains of a new phase or phases are
stabilized by changing metamorphic conditions, such
as formation of white mica and chlorite from illite
and smectite in the shale to slate transition cited
above. Once nuclei of the new phase or phases are
viable, requisite ions for grain growth diffuse from
nearby decomposing unstable, or reacting, mineral
grains. Some sort of fluid is commonly involved in
the mineral reaction.
Increase in Grain Size without Changes in Constituent
Phases. Increasing T, or an elevated T maintained for
a significant period of time, can modify grain size and
shape as grain boundaries adjust to a minimal surface
energy (Section 6.6.2). Aggregates experience Ostwald
ripening whereby smaller, higher energy grains are
(a)
Plagioclase
Pyroxene
(b)
Relict
plagioclase
phenocryst
Fe–Ti
oxide
mm01 mm01
Relict
pyroxene
phenocryst
14.3 Thin section views of andesite and meta-andesite. (a) Andesite made of phenocrysts of clinopyroxene and plagioclase in a felty matrix of
glass, feldspar microlites, Fe–Ti oxides (black), and pyroxene. (b) Low-grade meta-andesite that has a relict aphanitic–porphyritic
texture. Note similarity in shapes of relict pyroxene and plagioclase phenocrysts to fresh phenocrysts in (a) even though they are replaced
(pseudomorphically) by secondary minerals, including epidote after clinopyroxene and epidote, albite, and sericite after plagioclase.
Metamorphic Rocks and Metamorphism: An Overview
411
consumed at the expense of larger, more stable ones.
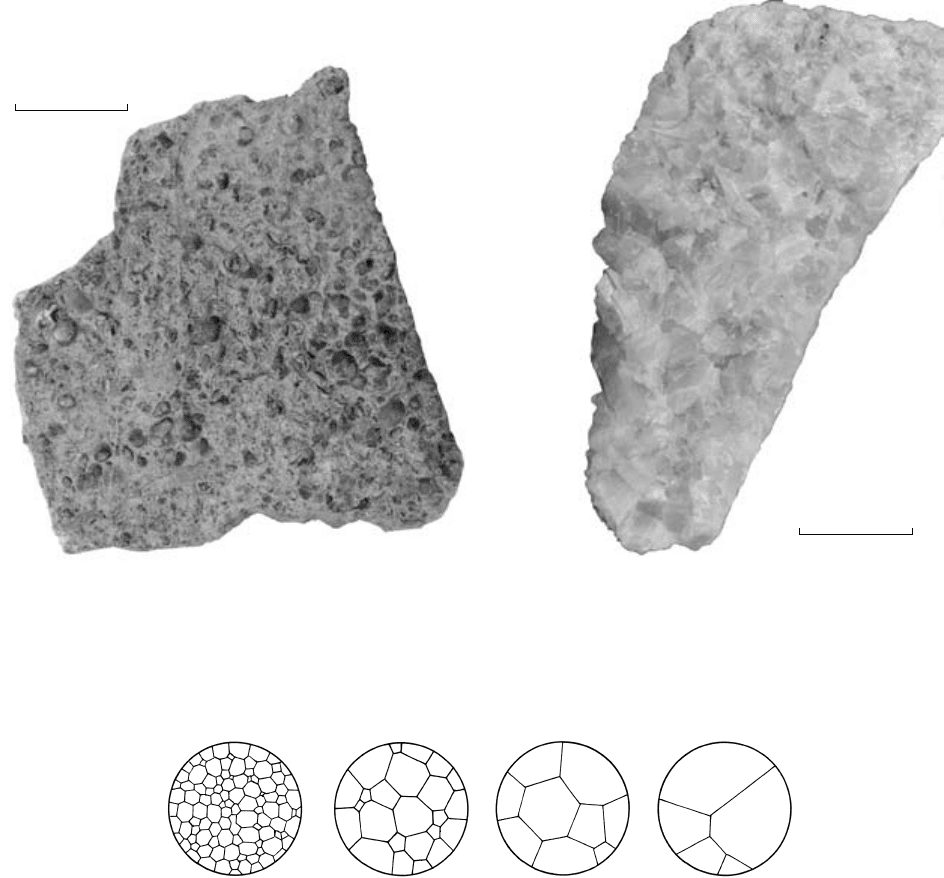
An example of grain coarsening is seen in the con-
version of limestone to marble (Figure 14.4), where
protolith features such as fossils defined by fine-
grained arrays are completely erased. A familiar analog
of grain coarsening is found in aggregates of soap bub-
bles in which, over time, bubbles increase in size while
decreasing in number (Figure 14.5). Grain boundary
adjustments can also change grain shapes. For aggreg-
ates of common felsic and carbonate minerals, the
equilibrium shape, as viewed in two-dimensional sec-
tions, is five-, six-, or seven-sided polygons with triple
grain boundary junctions forming angles near 120°
(Figures 6.23 and 6.24).
Marble and the olivine rock in Figure 6.24 illustrate
granoblastic texture that results from grain boundary
equilibration in the solid state. This texture, also
seen in polyphase rocks (Figure 14.6c, d), consists of
an isotropic aggregate of polygonal grains of more
or less similar size, perhaps within an order of magni-
tude. Inequant grains, such as micas, are randomly
oriented.
The blastic suffix on some names for metamorphic
fabrics is derived from the Greek blast, meaning lump.
11 minutes 49.5 156 225
(a) (b)
4 cm
3 cm
14.4 Contrasting textures at about the same scale of (a) fine-grained, gray fossiliferous limestone and (b) coarse-grained granoblastic white
marble. Textural features in gray limestones are defined by aggregates of minute black carbonaceous particles that are eliminated by
reaction with water during metamorphism, releasing CO
2
and CH
4
from the rock (see Special Interest Box 16.2). Thorough recrystal-
lization by grain boundary adjustments of calcites and growth of larger grains at the expense of smaller ones has obliterated the texture
of the original limestone. The end product is a coarse white marble.
14.5 Increase in size of soap bubbles in a flat cell as a function of time. Numbers are minutes after creation of bubbles by agitation. Note the
120° triple junctions that mimic the grain boundary configuration in granoblastic texture (compare Figure 6.24). The evolution of the
bubbles serves as a model for Ostwald ripening, or grain coarsening, during recrystallization in rocks that can perhaps require millions
of years instead of minutes. Redrawn from Smith (1954).
(a)
Chlorite
Calcite
Fe–Ti oxide with
rim of sphene
(b)
Chlorite
Plagioclase
Actinolite
Sphene
Relict
plagioclase
0.5 mm 0.5 mm
Actinolite
Epidote
Calcite
(c)
Plagioclase
Hornblende
(d)
Plagioclase
Fe–Ti
oxide
Fe–Ti
oxide
0.5 mm 0.5 mm
Pyroxene
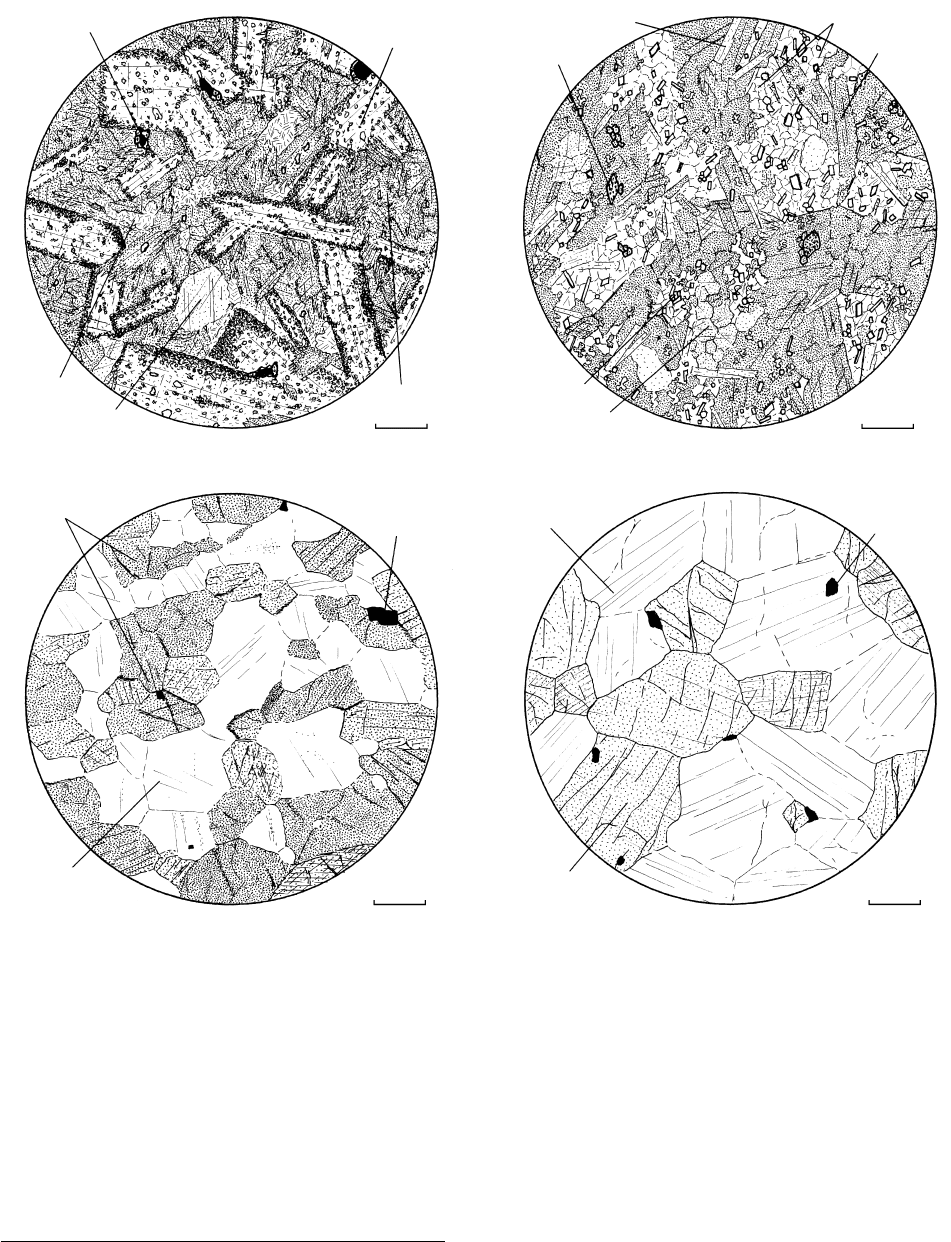
14.6 Prograde thermal metamorphism of diabase under essentially hydrostatic stress conditions. (a) Weakly metamorphosed diabase (meta-
diabase) or greenstone. Relict ophitic fabric (Figures 7.15 and 7.22) is well preserved because recrystallization has not produced large
enough grains to obliterate original grain boundaries. Magmatic pyroxenes have been replaced by aggregates of randomly oriented,
chemically zoned actinolites. Embedded within the actinolite aggregate are patches of fine-grained chlorite and tiny epidotes. Ti-bearing
oxides have partially reacted with mobile Ca and Si to produce rims of sphene (titanite). Original lath-shaped calcic plagioclases
(labradorite) are partly replaced by aggregates of minute epidote (high relief ) and white mica grains; remaining plagioclase is more
albitic. Local large calcite grains represent relict amygdules. (b) Greenstone or fine-grained amphibolite. An isotropic aggregate of the
same minerals comprising (a), except here grains formed by solid-state growth are larger with simpler, cleaner outlines. The original
magmatic fabric is virtually obliterated; only vaguely defined rectangular areas of untwinned albite and epidote aggregates suggest the
former existence of magmatic plagioclases. (c) Amphibolite. Coarser, well developed granoblastic fabric. All vestiges of the original
magmatic fabric have been erased. (d) Granoblastic plagioclase–pyroxene granofels. Mineral phases in this high-grade rock have
compositions of a near-solidus gabbro but the texture is clearly metamorphic (compare Figure 7.16a).
412 Igneous and Metamorphic Petrology
Crystallization Yielding New Minerals and New Fabrics.
Subsolidus equilibration generally yields new mineral
phases as well as new imposed fabrics, both stable
under new conditions. This is well illustrated by the
progressive metamorphism at increasing T under high
water fugacities of an ophitic diabase in Figure 14.6.
In the initial stages of subsolidus crystallization at
low temperatures, primary magmatic labradorites are
mutual competition for space brings into play the
differing stabilities of grain boundaries (Section 6.6.2),
rather than growth velocities in different crystallo-
graphic directions as occurs in an isolated crystal freely
suspended in a melt.
There is, therefore, a significant contrast between
the metamorphic fabric of the granoblastic amphibo-
lite and plagioclase–pyroxene granofels (Figure 14.6c
and d, respectively) and the mineralogically similar
magmatic rocks shown in Figures 7.15 and 7.16. This
contrast centers on plagioclase—the dominant mineral
in magmatic rocks as well as many metamorphic rocks.
In magmatic rocks, plagioclases are typically euhedral
to subhedral tablets—their characteristic habit result-
ing from uninhibited growth in a melt. Polysynthetic
twinning and compositional zoning (Figures 5.14d and
6.17) developed during growth are present in virtually
all magmatic plagioclases. In metamorphic rocks, on
the other hand, equilibrium plagioclase shapes are dic-
tated by the demands of minimal surface energy dur-
ing solid-state recrystallization, typically resulting in
anhedral polygonal outlines. Twinning tends to be less
conspicuous in plagioclases grown wholly in the solid
state. Zoning, if present, is generally more subtle and
oscillatory zoning is absent.
In some recrystallizing rocks, limited nucleation pro-
duces grains of a phase that are substantially larger than
grains of other more readily nucleating phases in the
rock. Although this fabric resembles the phenocryst-
matrix relation in porphyritic magmatic rocks, its sub-
solidus origin is significantly different and warrants a
different name—porphyroblastic. The large, generally
euhedral to subhedral crystals called porphyroblasts
are typically some type of metamorphic alumino-
silicate such as garnet, staurolite, andalusite, or kyanite
(Figures 14.8–14.10). Garnet and andalusite are rare
in magmatic rocks and kyanite and staurolite non-
existent. Whereas quartz is a common phenocryst in
magmatic rocks, it is nonexistent as a porphyroblast
in metamorphic rocks. Feldspars, micas, amphiboles,
pyroxenes, and olivine are common phenocrysts but
only locally are they porphyroblasts.
Growth of isolated porphyroblasts that differ in
composition from the pre-existing protolith volume
which they now occupy can be considered as a local
grain-scale metamorphic differentiation. Thus, the
volume of rock occupied by the large kyanites in the
metamorphosed shale (Figure 14.9) lacks the K, Na,
Fe, Ca, and H
2
O occurring in the surrounding matrix,
which has less Al than the kyanite. There was substan-
tial movement of different ions into and away from the
growing kyanite porphyroblasts.
Porphyroblasts commonly contain inclusions of
other minerals (Figure 14.10; see also Figure 14.25c), in
which case they are called poikiloblasts. They can
furnish significant insight into the metamorphic history
Metamorphic Rocks and Metamorphism: An Overview
413
replaced by a sodic plagioclase, commonly albite. This
albitization liberates Ca and lesser Al which allow
formation of phases such as calcite (if CO
2
fugacity is
also appropriately high) as well as hydrous Ca–Al sili-
cates including prehnite, pumpellyite, epidote, and
zoisite (the Al analog of Fe-bearing epidote). Fe–Ti
oxides can be converted into titanite. Primary clino-
pyroxene is replaced by actinolite (Figure 14.7) and
possibly chlorite. At higher temperatures, the hydrous
Ca–Al silicates, albite, and aluminous chlorite react
together, creating calcic plagioclase and aluminous
amphibole (hornblende). At still higher temperatures,
water-liberating reactions and recrystallization yield an
anhydrous assemblage of pyroxene and plagioclase that
is stable as the system approaches the basalt solidus.
Once all of the relict magmatic grains have been
consumed to form the granoblastic aggregate of new
phases the fabric is distinctly metamorphic.
Although metamorphic fabrics produced by sub-
solidus recrystallization may superficially resemble
magmatic fabrics produced by crystallization of a melt,
especially in rocks of similar modal and mineralogical
composition, they are really quite distinct. In the
former, growing minerals compete with one another
for common space in the solid aggregate, whereas only
in the closing stages of crystallization of magmas where
melt fractions are small does this competition occur. In
wholly solid-state recrystallization, the ever-prevailing
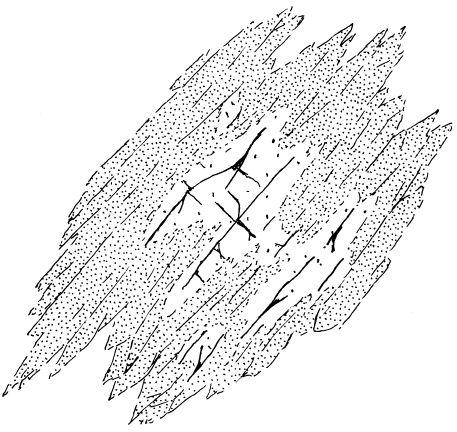
14.7 Overgrowth and replacement of primary magmatic clinopy-
roxene by a bundle of subparallel amphibole needles. Devel-
opment of this specific texture is called uralitization but is a
type of epitaxy or epitaxial growth in which a secondary phase
nucleates and grows on a crystalline substrate that has a sim-
ilar atomic structure and influences the orientation of the
overgrowth. In this case, amphibole and clinopyroxene are
both chain silicates.

414 Igneous and Metamorphic Petrology
of a rock. For example, some included minerals may
not be the same as those in the surrounding matrix and
might be armoured relics of former reactant phases
incorporated into the growing poikiloblast that were
eliminated in the matrix through progressive mineral
reactions. Other poikiloblasts have encompassed fabric
features, such as microfold hinges (see Figure 17.34),
developed during an earlier episode of deformation
that were erased elsewhere in the rock during progres-
sive recrystallization. Thus, poikiloblasts can preserve
records of past mineral reactions and deformation, in
the same way that photographs provide records of
bygone events.
14.1.3 Recrystallization under Nonhydrostatic States
of Stress: Tectonite Fabric
The rheologic response of rocks to nonhydrostatic
stress (Sections 8.1 and 8.2) is such that rocks under
near-surface conditions of low P and T behave in a brit-
tle manner at high strain rates. In shallow crustal faults
(Figure 8.9; see also Twiss and Moores, 1992, Chapter
4), brittle rocks are broken, crushed, and pulverized
by cataclasis to form dilatant, unconsolidated fault
breccia or fine-grained clay-rich gouge. Subsequent
percolation of mineral-laden groundwater along the
fault may cement the loose particles into a coherent
cataclasite rock (Figure 8.7a). The clasts in usually
Andalusite
10mm
14.10 Poikiloblasts of andalusite in slate, Sierra Nevada Foothills,
California. Photomicrograph in plane-polarized light. Minute
graphite inclusions in the form of a cross in the tetragonal
prisms of andalusite define the variety called chiastolite. These
poikiloblasts lie in a very fine-grained matrix of biotite
chlorite white mica quartz but are absent in the lighter-
colored, relict sedimentary bed made mostly of quartz that had
insufficient Al to stabilize the Al
2
SiO
5
polymorph.
14.9 Porphyroblastic kyanite–mica (pelitic) schist north of Kaladar,
Ontario. Porphyroblasts of kyanite lie in a fine phaneritic
matrix of white mica, biotite, and quartz.
14.8 Porphyroblastic garnet amphibolite, Barton Mine, Gore
Mountain, near North Creek, New York. Porphyroblasts of
almandine-rich garnet, some the size of soccerballs (30 cm dia-
mter), are surrounded by a rim of virtually pure hornblende
(black hornblendite) several cm thick. Amphibolite matrix is
made of white plagioclase and hornblende. Camera lens cap
for scale.
Metamorphic Rocks and Metamorphism: An Overview
415
randomized (isotropic) cataclastic fabric have sharp,
angular shapes and many are polygranular. Through-
going fractures cut across many grains. Very locally,
brittle comminution (pulverization) in concert with
frictional melting at high strain rates may create
pseudotachylite (Special Interest Box 11.1).
At greater depths in the crust at elevated P and T,
metamorphic rocks respond to nonhydrostatic stress,
especially at low strain rates, by a more continuous
and homogeneously distributed solid-state ductile
deformation or, as it is frequently called, ductile flow,
because of its overall resemblance to viscous flow. Duc-
tility is accomplished by straining (changing the shape
of ) individual grains in the rock through movement of
individual ions or packets of ions in a systematic way,
including grain boundary migration, recrystallization,
and plastic slip. During ductile flow the rock retains its
cohesion—it does not break apart as in brittle deform-
ation. Downslope movement of glaciers is an example
of solid-state ductile flow (Special Interest Box 14.2
and Figure 14.11).
Ductile deformation resulting from nonhydrostatic
stress is responsible for the development of imposed
anisotropic fabrics in metamorphic rock bodies. (It
may be recalled from Section 7.9 that an anisotropic
fabric has different attributes in different directions in
the rock body.) Such fabrics are often claimed to be the
hallmark of metamorphism, serving as a distinguishing
criterion from the effects of diagenesis and alteration.
The most common anisotropic fabric is a planar foli-
ation, a term derived from the Latin folium, which
means “leaf.” Schist is a typical metamorphic rock
that possesses foliation. In some rocks, a linear fabric
feature, or lineation, lies on foliation surfaces.
Special Interest Box 14.2 Glaciers as
metamorphic bodies
Ice is a mineral, and a solid aggregate of ice crystals
is a rock, large bodies of which are called glaciers.
Although requiring cold climatic conditions to
form, glaciers otherwise satisfy the criteria of a
metamorphic rock. They deform during downslope
movement under their own weight by solid-state
ductile flow, producing folds in dirty layers within
glaciers (Figure 14.11b) that resemble folds in deep
crustal metamorphic bodies (Figure 8.4). To permit
flow, individual ice crystals experience internal
plastic deformation and recrystallization, not unlike
that which occurs in tectonites. Thin sections of
glacier ice show well developed granoblastic fabric
characteristic of metamorphic rocks (Figure 14.11a).
As in other metamorphic rocks, melting occurs in
glacier ice if temperatures become sufficiently high.
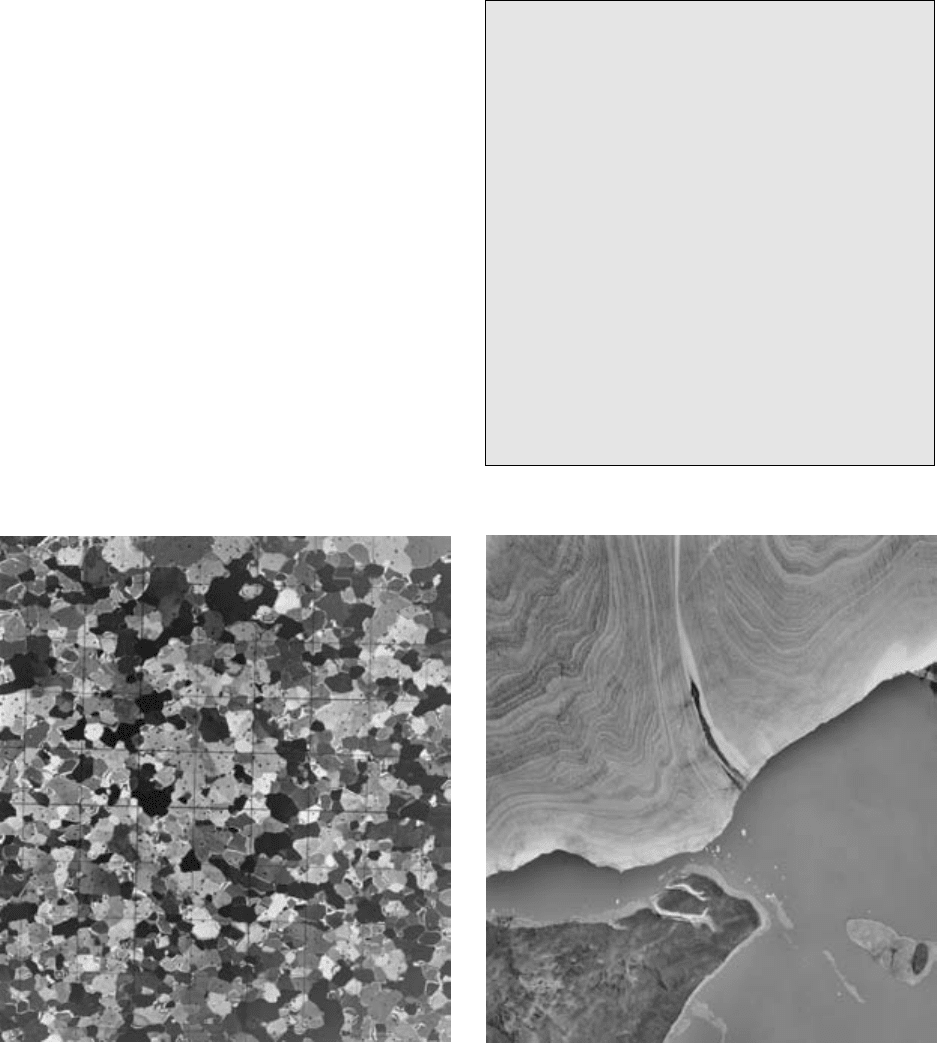
14.11 Glaciers as metamorphic rock bodies. (a) Photomicrograph under cross-polarized light of granoblastic glacier ice. Each grid in this thin
section is 1.0 cm. Small black spots are air bubbles in the ice. Photograph courtesy of A. J. Grow, US Army Cold Regions Research and
Engineering Laboratory. (b) Aerial photograph of the southern margin of the Barnes Ice Cap, Baffin Island, Canada showing ductile-flow
folds in dirty layers. Photograph courtesy of Canadian National Air Photo Library.
(a) (b)
416 Igneous and Metamorphic Petrology
In many metamorphic rocks, ductile deformation
is accompanied by concurrent recrystallization; com-
monly one enhances the other. The elevated temperat-
ures necessary for ductile flow also promote mineral
reactions and grain boundary adjustments. However,
some metamorphic rocks experience deformation after
recrystallization or vice versa; in still other polymeta-
morphic rocks multiple episodes of each process are
recorded. Because deformation and recrystallization
typically occur during contractional tectonism in oro-
genic belts, the Alpine geologist Bruno Sander in the
mid-1900s introduced the term tectonite for rocks
possessing anisotropic tectonite fabric (Turner, 1981).
Because tectonites are exposed over vast areas of
deeply eroded ancient orogens the question immedi-
ately arises whether there is a consistent geometric
relation between the orientation of the prominent
foliation, and lineation if present, and the directions of
the principal axes of nonhydrostatic stress (Section 8.1)
that produced it. Whether such a relation exists has
been one of the enduring controversies in geology, un-
like the simple relation that exists for magmatic dikes
intruded into hydraulic extensional fractures that
formed perpendicular to
3
(Figure 8.2). The question
of the geometric relation between fabric orientations
and stress directions is examined in Section 17.3.6. For
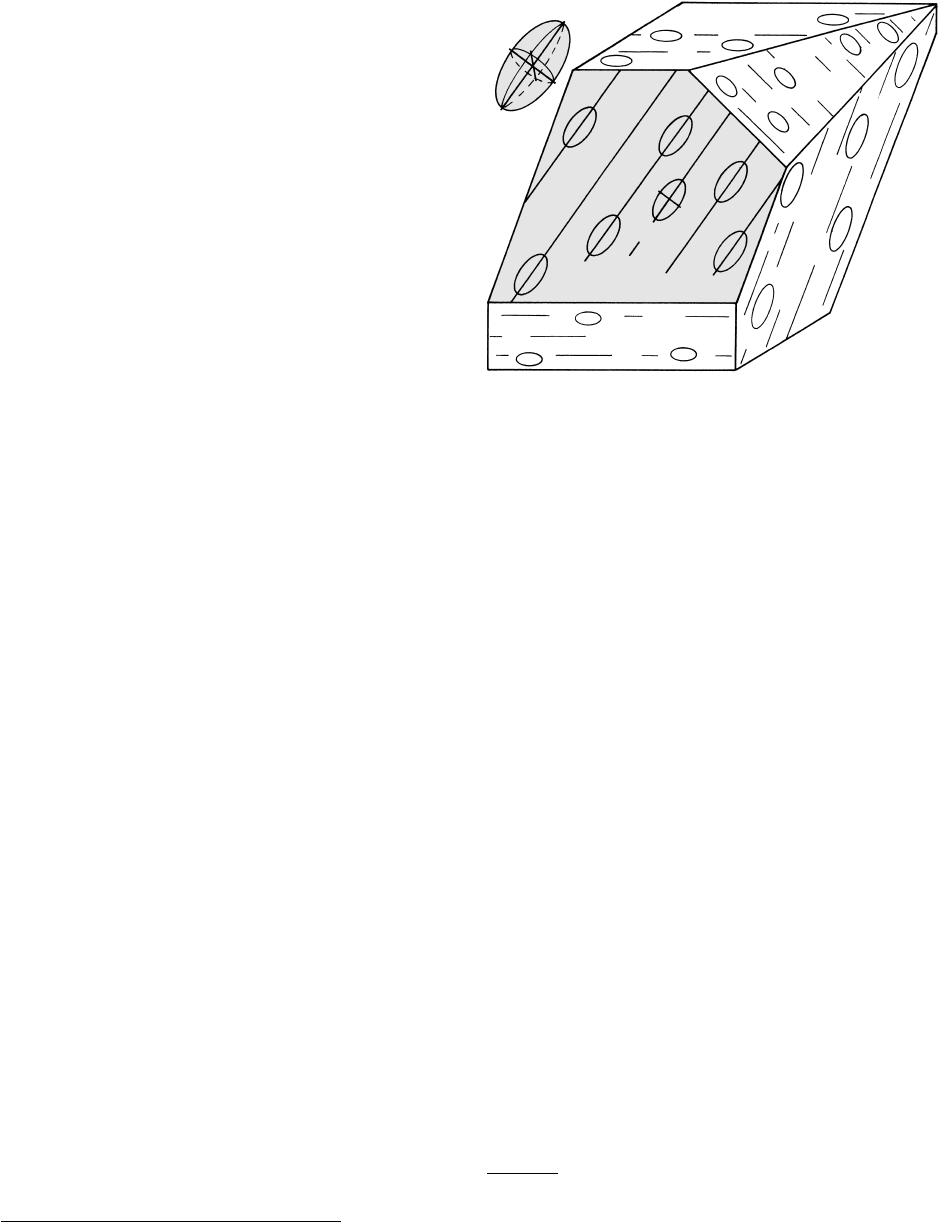
now, we make the provisional working assumption that
foliations are more or less parallel to the plane of flat-
tening (Twiss and Moores, 1992, p. 331), or the plane
that parallels the maximum and intermediate axes of
the triaxial strain ellipsoid (Figure 14.12). Metamor-
phic lineations can have different geometric relations
to the pattern of deformation depending on their par-
ticular nature and origin. One common possibility is
that the lineation parallels the direction of extension,
or the maximum axis of the strain ellipsoid.
It was once believed that nonhydrostatic states of
stress stabilized certain minerals, such as kyanite, that
were created during concurrent solid state crystalliza-
tion. But all such “stress minerals” have been shown to
grow in the laboratory under hydrostatic conditions, so
the concept has been abandoned. Nonetheless, defor-
mation can exert a catalytic effect on recrystallization
because the work of straining grains imparts additional
energy that can overcome activation energy barriers,
hastening the kinetics of mineral reactions and elimin-
ating otherwise metastable phases.
Examples of tectonite development are considered
next.
Foliated and Lineated Metaconglomerate. One of the
most obvious compound effects of nonhydrostatic
stress and recrystallization is found in a metaconglom-
erate that contains ductilely deformed relict cobbles in
a quartz-mica matrix. In the original conglomerate
protolith, quartzite cobbles approximated spheres, or
at least nearly equidimensional ellipsoids whose ratio
of major to minor axial dimension was no more than
two or three. In the metaconglomerate tectonite, they
are intensely flattened and elongated ellipsoids whose
ratio is as much as 30 (Figure 14.13). Examination in
thin section of the quartz grains making up the relict
cobbles reveals a similar flattened and elongate shape
that resulted from recrystallization and ductile strain in
a nonhydrostatic stress field. The integrated effect of
these ellipsoidal grains that make up individual relict
cobbles and the integrated effect of their common
orientation through the metaconglomerate rock body
expresses a scale-independent foliation and lineation.
In other words, the tectonite body possesses the same
foliated and lineated tectonite fabric on all scales, from
the outcrop to the hand sample to the thin section. It is
not unusual to find similar fractal-like scale invariance
extending to entire mountain ranges in terranes of
metamorphic tectonites (Figure 14.14).
Mylonite. Mylonite was first described in the late 1800s
by Charles Lapworth from the Moine thrust of north-
ern Scotland (see Figure 14.30). Since then, the origin
of this type of tectonite has been controversial (e.g.,
Snoke et al., 1998). Relative to its protolith, mylonite
is much finer grained and has enhanced anisotropic
fabric. Mylonite occurs as sheets as thin as a cm or less
to broad zones as much as 10 km thick. The common
b
a
c
Trace of
b
a
TRUE FOLIATION
Trace of
TRUE LINEATION
foliation
foliation
14.12 Block diagram of foliation and lineation in a hypothetical tec-
tonite related to strain ellipsoid. Redrawn from Twiss and
Moores (1992). The strain ellipsoid (upper left) represents the
result of homogeneous deformation of a reference sphere in
the initial undeformed state. Foliation (shaded) is parallel to
plane of flattening in the strain ellipsoid; this plane is defined
by the longest axis a and the intermediate axis b and is per-
pendicular to the shortest axis c. Lineation that lies within
the foliation is parallel to the direction of elongation, or the
longest ellipsoid axis a. On randomly oriented rock surfaces
the obliquely intersecting foliation appears as line traces that
are not a true lineation.
