Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.

metamorphic paths causing the metamorphism. (The
homonym “terrain” refers to a geographic feature, such
as a mountainous terrain.) Figure 14.23 is an attempt to
relate the types of metamorphism from the previous
paragraph to geologic settings.
Ocean-ridge Metamorphism. The products of ocean-
ridge metamorphism are concealed on the seafloor
except for scraps that have been locally accreted onto
island arcs and continental margins in subduction
zones as ophiolite (Section 13.6) in accretionary prisms
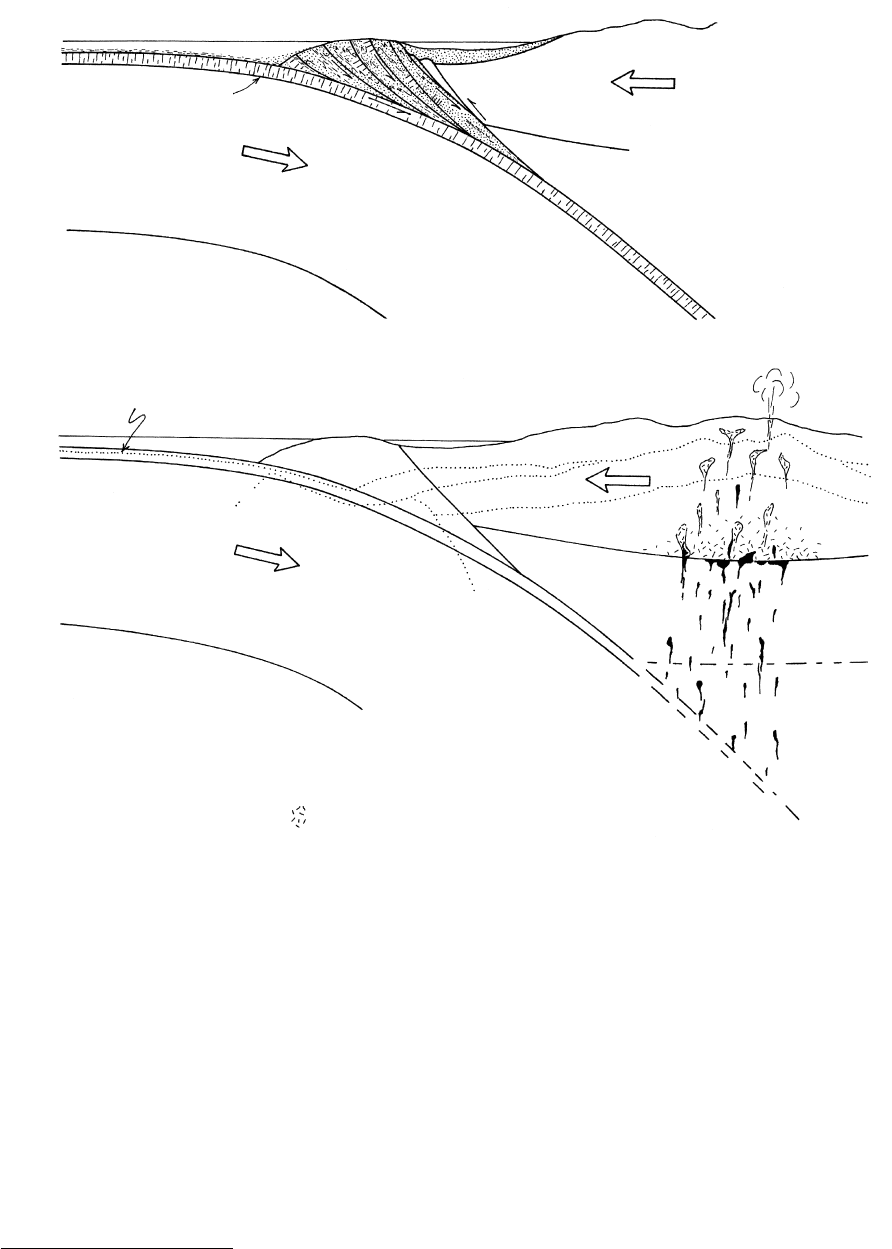
(Figure 14.24).
Hot, highly fractured magmatic rock exposed to
cold seawater at oceanic spreading ridges provides an
exceptional opportunity for movement of heat and
mass to create equilibrating metamorphic changes. The
global ridge system has been referred to as the “radia-
tor atop the oceanic magma engine” (Special Interest
Metamorphic Rocks and Metamorphism: An Overview
427
Trench
Fore-arc
basin
High P/T metamorphic rocks
in accretionary prism
(a)
Magmatic
arc
Oceanic crust
Lithosphere
Asthenosphere
1 and 4 closely
telescoped
(b)
1
2
3
3
4
?
1
4
5
4
2
Lithosphere
Asthenosphere
1. Zeolite
2. Prehnite–pumpellite
3. Blueschist
4. Greenschist
5. Amphibolite
migmatite and other partial
melts in crust
M-discontinuity
14.24 Schematic cross-sections through a continental margin subduction zone showing typical settings for regional and contact metamorphism.
Redrawn from Ernst (1976). (a) Underthrust imbrication of slices of arc-trench sediments and scraped-off oceanic lithosphere builds an
accretionary prism, or accretionary wedge (compare Figures 11.16 and 13.17). (b) Distribution of metamorphic facies (Figure 14.33) in
an orogenic belt, or orogen. Note that boundaries between facies in the magmatic arc in the core of the orogen are deflected nearer the
surface where the geothermal gradient is elevated. This is the setting of regional Barrovian-style metamorphism of the greenschist–
amphibolite facies series along an intermediate P metamorphic field gradient that culminates with migmatites in the deeper crust. Con-
tact thermal metamorphism develops around magmatic intrusions, especially in the shallow crust, perturbing the facies boundaries as
drawn. The high P/T facies series (zeolite, prehnite–pumpellyite, and blueschist) develops in the accretionary prism adjacent to the trench
where the cool subducting oceanic slab acts as a heat sink, depressing geotherms.

Box 11.3). Tensile cracking during cooling and con-
traction of submarine lavas and feeder dikes, coupled
with active extensional rifting, promotes deep propa-
gation of fractures in the oceanic crust (Figure 13.1a).
Cracks allow seawater to circulate through the hot
rock, promoting hydrating mineral reactions that par-
tially to completely replace primary anhydrous mag-
matic phases. Heated seawater expands and buoys
upward out of the cracks, carrying ions leached from
the rock and dissolved in the hot brine. Sulfides and
other solids precipitate in the cold seawater as this
solution exits from seafloor hot springs, creating
particle-laden “black smokers” at vents along the ridge
(Figure 11.17). Basaltic rocks are metasomatized (or
hydrothermally altered, depending on one’s personal
viewpoint) to varying degrees by addition of Na and
water. Less mobile Ti, Al, and Mg may increase in
concentration in the protolith as a consequence of loss
of substantial Ca. Relict basaltic fabrics are preserved
in these spilites, whose metasomatic mineral com-
position is dominantly sodic plagioclase chlorite
quartz.
Relentless spreading of the seafloor distributes the
products of ocean-ridge metamorphism across two-
thirds of the globe. As the hydrated crust is subducted
and heated at converging plate junctures, mineral
reactions liberate water that fluxes the overlying man-
tle wedge and leads to the generation of arc magmas
(Section 11.4).
Regional Metamorphism. Within the continental crust
the most widespread of any metamorphism is regional
metamorphism. It is developed in the vast roots of oro-
genic belts (orogens) and, hence, is often called oro-
genic metamorphism. It involves broadly concurrent
deformation resulting from contractional stresses dur-
ing convergence of lithospheric plates in the subduc-
tion zone and recrystallization resulting from increases
in P and T in the thickened crustal welt (Figure 14.24).
Classic terranes include the Alps of southern Europe
and the Appalachian Mountains of eastern North
America, continuing, in a predrift connection, into the
Caledonides of the British Isles and Scandinavia. Rem-
nants of deeply eroded roots of Precambrian orogens
are represented in regional terranes in the Canadian
Shield and cratons in Australia, Africa, and other con-
tinents. Tectonites produced by regional metamorphism
are the familiar slates, schists, and gneisses.
Increased temperatures in the orogen are created as
geotherms adjust to the crust that is thickened by con-
tractional overthrusts and folds, magmatic underplates
(Figure 11.2), and stacks of volcanic deposits. Temper-
atures are sufficiently high in the lower crust to cause
partial melting and generation of calc–alkaline magmas
that ascend into the shallower crust as granitoid plu-
tons. Isostatic uplift and erosion during and following
orogeny exposes the crustal welt of metamorphic and
plutonic rock.
Orogens typically evolve over hundreds of millions
of years and experience more or less discrete pulses
of deformation or tectonic events related to changes
in character of converging oceanic plates and their
rates of convergence. Heating of the crustal welt may
accompany these pulses or occur sometime else, also
commonly in distinct episodes. Consequently, regional
terranes in orogens typically evolve through multiple
episodes of deformation and recrystallization, each
several million years in duration. Superposed meta-
morphic imprints may be of insufficient intensity or
duration to completely erase earlier metamorphic
fabrics and mineralogical compositions, making it
possible to unravel at least a part of the history of
polymetamorphism.
Burial metamorphism. In very thick depositional
basins the more deeply buried sedimentary and vol-
canic material can be subjected to temperatures of
200–300°C sufficient to cause recrystallization, or bur-
ial metamorphism. The classic terrane described by
Coombs (1961) is a thick stack (10 km) of Mesozoic
volcaniclastic rocks in southernmost New Zealand
(see Figure 18.30). Although of regional extent, burial
metamorphism has little or no associated penetrative
ductile deformation so that relict depositional fabrics
are usually well preserved (Figure 14.2) in rocks now
composed of low-T minerals such as zeolites. Asso-
ciated granitoids are also absent. Heated advecting
aqueous fluids in the deep basins are likely involved,
so it is a type of hydrothermal metamorphism. A
currently active hydrothermal metamorphic system is
in the thick pile of sediment in the Colorado River
delta in the Salton Sea geothermal field of southern
California and northern Baja Mexico (see Special
Interest Box 18.1).
Thick piles of sedimentary and volcanic rock accu-
mulated along passive continental margins, such as
along the eastern and Gulf of Mexico coasts of the
United States, that experience burial metamorphism
may subsequently suffer orogenesis as changes in plate
motion occur. In this polymetamorphism, the earlier
simple burial effects may be completely obscured.
Contact Metamorphism. Country rocks surrounding a
magmatic intrusion are subject to contact metamor-
phism. Intrusions liberate heat that conducts (Section
8.4.1) and advects via percolating fluids into the coun-
try rock (Figure 4.12), producing a thermal gradient in
which T diminishes away from the magmatic heat
source. Oxygen isotope studies reveal that hydrother-
mal fluids can advect many kilometers from an intru-
sion (Section 4.3.3). Many factors influence the extent
and configuration of the thermal gradient in the
428 Igneous and Metamorphic Petrology
contact metamorphic aureole and how elevated the
temperatures become. Important factors include, for
the country rock, its composition, permeability to ad-
vecting fluids (Section 8.5), pre-existing T, and depth
beneath the surface. Thermal metamorphism is most
conspicuous in shallow crustal country rock because
of the large thermal contrast with intrusive magmas.
Increasingly deeper, and therefore hotter, country
Metamorphic Rocks and Metamorphism: An Overview
429
45°25′N
0
mi 3
0
km 4
Slate
Granodiorite
Hornfels
Spotted slate
and semihornfels
Slate
Onawa
69°15′W69°25′W
(a)
(b)
Quartz
0 mm 0.2
(c)
Biotite
0 mm 0.2
Quartz
Muscovite
Andalusite
Cordierite
(d)
Perthite
Andalusite
0 mm 0.2
Biotite
Quartz
Cordierite
Sillimanite
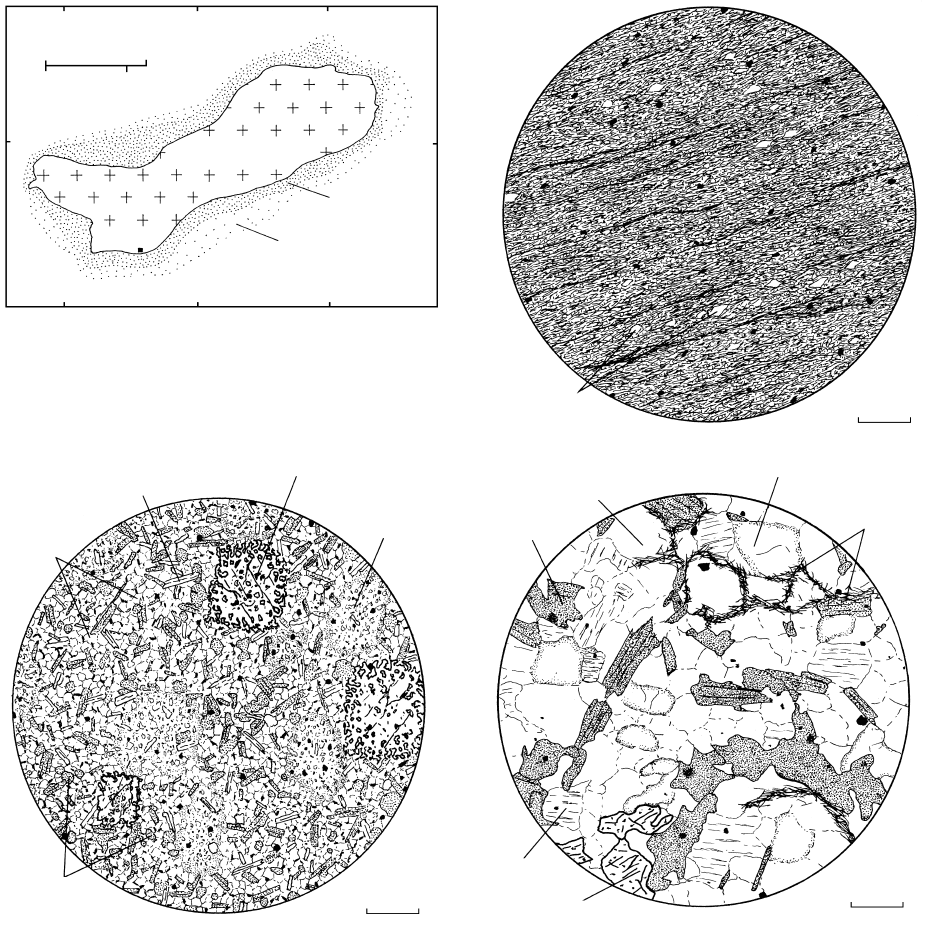
14.25 Contact metamorphic aureole surrounding the Onawa, Maine pluton in the Appalachian Mountains. (a) Generalized geologic map of
the Onawa granodiorite pluton and surrounding zoned aureole. Redrawn from Moore (1960). (b) to (d) Thin sections showing progres-
sive thermal metamorphism of pelitic country rocks in the aureole. (b) Slate. Country rock into which the magma was emplaced had
experienced previous regional metamorphism with development of a low-grade mineral assemblage of quartz chlorite phengitic
white mica Fe–Ti oxides graphite. Preferred orientation of platy grains defines a slaty cleavage. Angular quartz grains are relics of the
original clastic fabric of the parent shale. (c) Spotted semihornfels in outer part of contact aureole. Recrystallization under hydrostatic
conditions has completely obliterated the anisotropic fabric of the slate protolith, producing a somewhat coarser granoblastic
fabric. Most of the rock is composed of quartz biotite muscovite graphite (black opaque grains). Large poikiloblasts of andalusite
(high relief ) and cordierite (low) are crowded with numerous tiny inclusions of matrix phases. On weathered rock surfaces, andalusites
stand out in relief, whereas cordierites etch out because of replacement by fine-grained sericite and other alteration products; both form
spots. (d) Hornfels. Coarser granoblastic rock from the inner part of the zoned contact aureole. Anhedral andalusite was apparently
unstable and decomposing when the metamorphic reactions stopped. Samples provided courtesy of James M. Moore, Jr.

rock can exhibit wider aureoles but the thermal effects
may be progressively less evident because of ongoing
metamorphic processes and equilibration. Significant
factors for the intrusion include its volume, shape,
T, composition, volatile content and composition,
whether the magma cools conductively (Section 8.4) or
convectively (Section 8.6), and whether magma replen-
ishment occurs. Contact aureoles range from barely
perceptible ones on the order of a centimeter wide next
to thin dikes that cool in less than a year to aureoles
measured in kilometers around large plutons that may
take tens of thousands of years to cool.
Aureole fabric around passively emplaced intru-
sions usually in the shallower crust (Section 9.4.3) is
commonly isotropic and granoblastic (Figure 14.25b,
c) because the country rocks are simply “cooked”
under hydrostatic conditions. Hence, metamorphism is
essentially thermal and deformation is so limited that
little or no preferred orientation of mineral grains re-
sults. The rock is typically a hornfels. However, around
forcefully intruded magmas usually in the deeper crust,
ductile deformation can create anisotropic fabrics (Fig-
ure 9.19b) that are indistinguishable in outcrop from
tectonites created by regional metamorphism.
In orogens, many granitoid plutons are post-tectonic
(Section 9.4.3) and postdate regional metamorphism
because magma ascent from deep crustal sources
may require a long interval of time after the thermal
“maximum” associated with peak tectonic activity and
magma generation. In such cases contact metamor-
phism is overprinted on earlier regionally metamor-
phosed country rocks, such as shown in Figure 14.25;
this is yet another expression of polymorphism.
In essentially closed-system aureoles little change in
chemical composition of the rocks can be discerned,
other than loss of water during progressive mineral
reactions. The metamorphism is virtually isochemical
as well as thermal.
However, in more open-system, metasomatic aure-
oles, major changes in country-rock composition take
place. Depending on the nature of the advecting fluids
and country rock, a compositional gradient will be
established in addition to the thermal gradient. Com-
positional gradients are most evident where there are
sharp contrasts between compositions of the advecting
fluids and country rock. The most striking common
examples occur where calcareous or impure carbon-
ate rocks (limestones and dolostones with variable
amounts of admixed quartz and clays) are increasingly
silicated nearer granitoid intrusions from which silica-
rich fluids are expelled. The resulting metasomatic
calc-silicate skarns are made of actinolite, diopside,
andradite–grossular garnet, vesuvianite (idocrase), and
other Ca–Al–Mg–Fe silicates.
The source of the metasomatizing hydrothermal
solutions can be:
1. Exsolved water liberated from the crystallizing
magma intrusion.
2. Heated fluids (“meteoric water”) in openings in the
country rocks.
3. Water liberated from nearby decomposing hydrous
minerals undergoing prograde dehydration reac-
tions (see below).
In addition to the normal rock-forming elements
(e.g. Si, Al, Fe) introduced into country rock by the
metasomatizing solutions, other elements (e.g. S, Zn,
Pb, Cu, Ag, Au) can also locally be introduced to form
ore deposits.
Dynamic Metamorphism in Ductile Shear Zones.
Compressional stresses in orogens create shallow
crustal brittle faults and deeper crustal ductile shear
zones. Major continental-scale faults and shear zones
include the San Andreas in California (see Figure 18.5),
the Brevard in the Appalachian Mountains (see Figure
18.7), the Moine Thrust in Scotland (see Figure 14.30),
the Alpine fault in New Zealand (see Figure 18.30),
and many others. Along any zone of displacement, high
rates of strain create cataclastic rocks by brittle defor-
mation, mylonite by ductile deformation, and very
locally pseudotachylite by frictional melting (Special
Interest Box 11.1).
Impact Metamorphism. Unrelated to tectonic settings
are bodies of rock impacted by meteorites that cause
impact metamorphism, also called shock metamor-
phism. Extremely high rates of strain resulting from the
transient high-speed shock wave create cataclastic fab-
ric, high-P phases, such as the silica polymorphs coesite
and stishovite, intracrystalline plastic slip (see Section
17.2.2), and local melting, producing pseudotachylite
(Grieve et al., 1995).
14.2.4 Metamorphic Grade
In the Onawa contact aureole (Figure 14.25), pelitic
hornfelses closer to the intrusion that contain higher T
minerals are said to be of higher metamorphic grade
than the pelites farther away made of lower T minerals.
Grade corresponds to equilibration T without restric-
tions as to P when comparing rocks of similar bulk
chemical composition. Differences in fabric are pos-
sible and, in fact, common in different grades; in the
Onawa aureole, the lower grade rocks are spotted
slates in contrast to the higher grade coarser hornfelses
near the contact. However, reliance on fabric, espe-
cially grain size, to infer metamorphic grade can be
very misleading because of the complex factors on
which fabric depends. Mineralogical differences are
more critical in establishing grade, especially as they
result from differences in the T of equilibration during
metamorphism.
430 Igneous and Metamorphic Petrology
How does a petrologist distinguish between high-T
minerals characterizing a high grade of metamorphism
from low-T and low-grade?
In Figure 14.25c and d, the major difference in the
mineral assemblage in the Onawa contact hornfelses is
the presence of muscovite and quartz in the outer,
lower T part of the aureole and the absence of this
mineral pair in the higher grade hornfels closer to the
intrusion that instead contain alkali feldspar and an
Al
2
SiO
5
polymorph. This change in mineral assem-
blage can be modeled by the mineralogical reaction
14.1 thermal energy (K,Na)Al
2
AlSi
3
O
10
(OH)
2
muscovite
SiO
2
(K,Na)AlSi
3
O
8
Al
2
SiO
5
H
2
O
quartz alkali feldspar
Al
2
SiO
5
can be any of the polymorphs andalusite,
sillimanite, or kyanite, depending on P–T conditions.
Breakdown of muscovite quartz is an endothermic
dehydration reaction, consuming heat and liberating
water. (Because of excess quartz in the Onawa aureole
rock relative to muscovite, it was not entirely con-
sumed in the reaction and higher grade hornfelses still
contain quartz.)
Thus, a general rule is that lower-grade metamorphic
mineral assemblages contain more volatile-rich phases
whereas higher-grade assemblages equilibrated at higher
T are made of less volatile-rich or volatile-free minerals.
Remember that in considerations of metamorphic
grade we compare rocks of similar bulk chemical com-
position: pelites with pelites, metabasites with meta-
basites, and so on; we don’t attempt to compare toads
with horses!
Devolatilizing prograde reactions liberate volatiles
with increasing T. Dehydrating prograde reactions,
such as the breakdown of muscovite, give off water as
T increases; the hydrous mineral assemblage, in effect,
“dries out” at higher T as a result of the endothermic
reaction. Prograde decarbonation reactions liberate
CO
2
from calcite, dolomite, and other CO
2
-bearing
minerals. A metamorphic path that progresses toward
a peak or maximum T is referred to as prograde meta-
morphism. Thus the aureole rocks toward the intru-
sion at Onawa experienced prograde metamorphism,
as did the sequence of mafic rocks in Figure 14.6a to d.
After a peak T is reached during prograde meta-
morphism, there must logically be a reversal going
down T. This is clearly the case for rocks now exposed
at the surface. Reversal may happen soon (in a geolo-
gical sense), or many, possibly tens or hundreds of, mil-
lions of years after attaining the peak T. But high-grade
metamorphic mineral assemblages (and also magmatic
rocks made of high-T minerals) exposed at the surface
all over the world indicate that retrograde reactions
during the decreasing T of retrograde metamorphism
do not generally occur. Prograde mineral reactions are
not automatically reversed during cooling and uplift to
the surface for two apparent reasons. The first follows
from the nature of reaction kinetics (Section 3.6.2)
whereby phases formed at high T typically persist
metastably at falling T because of slowing kinetic rates
of equilibrating reactions. The second is that prograde
reactions progressively liberate volatile fluids, which
migrate out of the metamorphosing body. As T falls
after the peak metamorphic event, no volatiles are avail-
able within the system and little can enter into typically
impermeable bodies. Without available water, hydrous
phases more stable at lower T cannot replace high-T
anhydrous minerals. Primary magmatic minerals in
granitic rocks are typically only partially replaced (e.g.
Plate IV), if at all, during the excursion of the rock
system from the solidus to lower temperatures. In
metamorphic rocks, high-T anhydrous silicates, such
as garnet, are commonly incompletely replaced by a
hydrous Mg–Fe alumino-silicate, such as chlorite, if at
all. Retrogressive metamorphism (or alteration, as it
might be called by some geologists) is commonly local-
ized along fractures or shear zones in aggregates of
high-T minerals. Shearing not only produces a higher
energy state in the strained grains, making them less
stable and more amenable to replacement, but also
provides an avenue for advective influx of volatile fluid
into the otherwise impermeable rock body. Planar
veins of hydrous Ca–Al silicates, especially epidote, are
common in granitic rocks (Figure 14.26) and form by
reaction between plagioclase and water that invaded a
fracture, possibly during cooling below the solidus. In
Figure 14.27, an anhydrous eclogite assemblage has
been retrograded to a lower T hydrous assemblage in a
vein.
14.2.5 Metamorphic Zones
Terranes of metamorphosed rock commonly display
geographically limited, or mappable, metamorphic
zones. Each zone is defined by some distinctive fabric
attribute or mineral assemblage in rocks derived from
compositionally similar protoliths. The sequence of
zones reflects the existence of gradients, or spatial vari-
ations, in conditions of metamorphism. Zones range in
dimension from that of an outcrop, or small portions of
it, to terranes measured in hundreds of square kilome-
ters. Zones are of considerable significance in the elu-
cidation of transfers of heat that caused changes in the
T of metamorphism, of changes in P that were caused
by variations in depth of burial, and of changes in the
nature of advecting fluids from different potential
sources. Where successive zones in a regional terrane
or contact aureole correspond essentially to a thermal
gradient they will be of different grade. Zones based
on some fabric attribute can be used to infer something
of states of stress and deformation. Zones and the
Metamorphic Rocks and Metamorphism: An Overview
431

gradients they reflect in regional terranes are of partic-
ular utility in understanding the evolution of orogens
and continents. The following three examples of meta-
morphic zones illustrate their connection to gradients
in equilibrating conditions.
Thermal Metamorphism in the Onawa Contact Aureole.
This aureole (Figure 14.25) was developed in previ-
ously regionally metamorphosed pelitic country rocks
and exhibits conspicuous textural and mineral zones
432 Igneous and Metamorphic Petrology
14.26 Vein of epidote (black) in granodiorite. Light-colored borders on the epidote consist of chloritized biotites and feldspars whose chalky
appearance is the result of replacement by very fine grained sericite and other alteration products. Pocket knife 8 cm long rests on fresh,
unaltered granodiorite.
that resulted from a longer duration of conductive and
possibly advective heating and more elevated temperat-
ures closer to the intrusion. The first visible indication
in hand sample of the thermally driven recrystallization
in the country rock slates in the outermost part of the
aureole is ill-defined spots made of variably altered (to
fine-grained phyllosilicates) poikiloblasts of cordierite
and/or andalusite. These poikiloblasts are slightly
lighter colored than the surrounding darker graphitic
matrix of the slate but tend to weather out on expos-
Metamorphic Rocks and Metamorphism: An Overview
433
ures, creating pits. With progressive recrystallization
toward the intrusion, slaty cleavage is obliterated and
the rock takes on a more massive appearance, becom-
ing first a “semi” hornfels and finally nearer the intru-
sion a relatively coarse granoblastic hornfels (Figure
14.25d). Mineralogically, the transition from semihorn-
fels to hornfels was marked by the decomposition of
muscovite reacting with quartz to form alkali feldspar
plus sillimanite. Thus, one can recognize at least two
mineralogical zones, an outer lower grade zone of mus-
covite quartz ( other phases) succeeded inward by
a higher grade zone of alkali feldspar sillimanite (
other phases). The lower grade outer zone contains
more hydrous minerals—muscovite biotite—than
does the inner zone—biotite only.
Metasomatic Skarn Zones at Crestmore, California.
This is an unusually well developed and now classic
example of mineral zones developed in calcareous
country rocks around a small granitic porphyry intru-
sion (Figure 14.28). The thermal gradient attained
exceptionally high temperatures next to the intrusion
but the successive zones are chiefly the result of a
strong chemical gradient imposed by hydrothermal
solutions expelled from the freezing magma.
The sequence of zones and ten subzones and their
constituent mineral assemblages as determined by
Burnham (1959) are, beginning with the lowest grade
outermost zone
Forsterite 1. calcite brucite clinohumite
zone spinel
2. calcite clinohumite forsterite
spinel
3. calcite forsterite spinel
clintonite
Monticellite 4. calcite forsterite monticellite
zone clintonite
5. calcite monticellite melilite
clintonite
6. calcite monticellite
spurrite (or tilleyite) melilite
7. monticellite spurrite merwinite
melilite
Vesuvianite 8. vesuvianite monticellite
zone spurrite merwinite melilite
9. vesuvianite monticellite
diopside wollastonite
Garnet zone 10. grossular diopside wollastonite
Note that once a particular “index” mineral, such as
monticellite, appeared in subzone 4 it persisted, pre-
sumably stably, not only through its corresponding
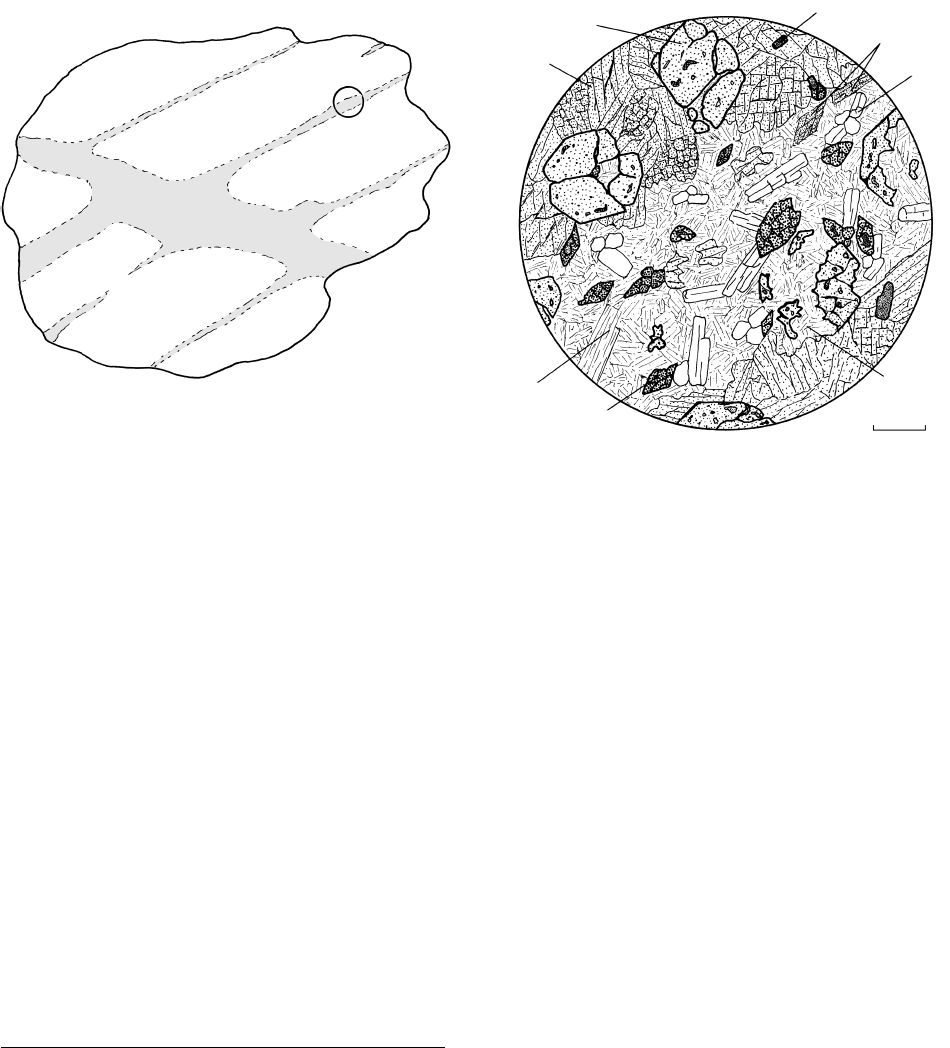
Retrograded eclogite
Fresh eclogite
(b)(a)
White mica
Sphene
0.5 mm
Jadeitic
pyroxene
Garnet
Rutile
Glaucophane
Epidote
Chlorite
14.27 Retrograde metamorphism of eclogite. (a) Hypothetical, schematic field relations showing retrograde effects (shaded) controlled by two
sets of fractures along which hydrous fluids gained access into the rock. Circle shows where thin section in (b) would be located.
(b) Thin section showing retrograde vein cutting diagonally from lower left to upper right through anhydrous omphacite garnet
rutile eclogite. Vein is made mostly of chlorite epidote glaucophane white mica, which are all hydrous minerals. Note two garnet
grains on right partially pseudomorphed by chlorite, leaving embayed remnants; this is an example of a reaction texture in which the
reactant phase (garnet) and the product phase (chlorite) are both preserved. Sphene (titanite) has partially to completely replaced
rutile.
434 Igneous and Metamorphic Petrology
monticellite zone but also through the next higher
grade vesuvianite zone. It should also be noted that
calcite is stable in the six lower grade subzones but
is absent at higher grades, where only calc-silicate
minerals occur. Noting that the country rocks of
brucite marble (formed during an earlier metamorphic
event) far from the intrusion contain no quartz or
silicate minerals, it appears that the succession of zones
corresponds to increasing concentrations of silica and
elimination of CO
2
. Significant amounts of Al were
introduced into the monticellite, vesuvianite, and gar-
net zones, in addition to Si, thereby stabilizing melilite,
vesuvianite, and grossular.
Many reactions could be written to account for the
progress of mineralogical changes through the zones
that consumed a lower grade mineral or mineral assem-
blage as a higher grade one was stabilized. A possible
silicating, decarbonation reaction producing some
vesuvianite in subzone 8 from the mineral assemblage
in 7 is
CaMgSiO
4
2(2Ca
2
SiO
4
CaCO
3
) 3Ca
3
MgSi
2
O
8
monticellite spurrite merwinite
4Ca
10
Mg
2
Al
6
Si
7
O
35
15SiO
2
12H
2
O
melilite in solution
→ 6Ca
10
Mg
2
Al
4
Si
9
O
34
(OH)
4
2CO
2
vesuvianite
Another way to consider the progressive meta-
somatism is to graphically display (Figure 14.29) the
aureole chemical system in terms of the compositions
of the major minerals; these are made up essentially of
CaO, MgO, and SiO
2
and so can be represented in a
triangular diagram. (These composition diagrams are
described in Section 15.3.) This graphical analysis
ignores substantial Al in some minerals, as well as
minor Fe and the different temperatures that prevailed
in the successive zones. Despite the approximations,
the diagram clearly demonstrates that successively
higher grade zones have increasing proportions of
silica but decreasing CaO; MgO remains fairly constant.
These compositional variations are consistent with out-
ward migration into the country rock of silica-bearing
solutions derived from the intrusion and with outward
diminishing silication, or fixation of SiO
2
, by reaction
with CaCO
3
and Mg(OH)
2
in the brucite marble
protolith.
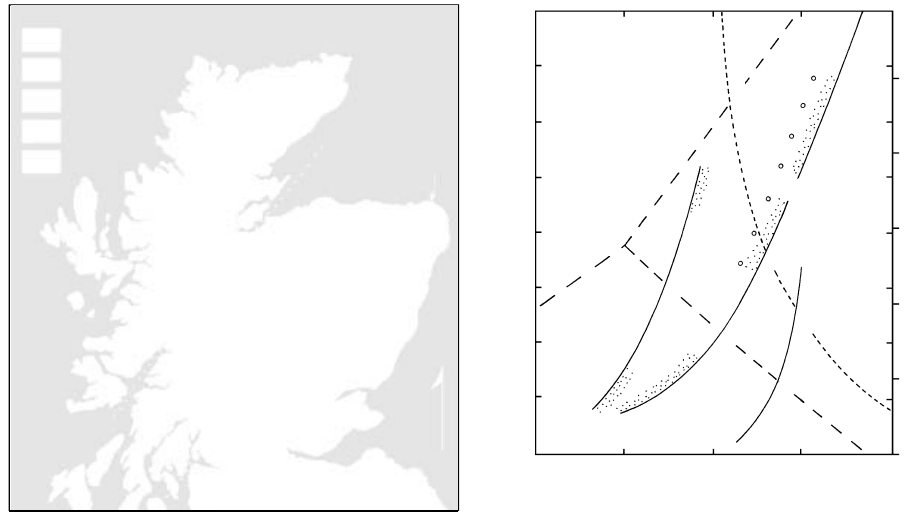
Barrovian Zones in Pelites in Scotland. Historically,
the first systematic study of mineralogical variations in
progressively metamorphosed rocks was made near the
turn of the last century by George Barrow (1912) in the
regional terrane just north of the Highland Boundary
fault in Scotland southwest of Aberdeen (Figure 14.30).
The regionally metamorphosed rocks of the Scottish
Highlands comprise a 13-km thick sequence of shale,
sandstone, limestone, and mafic lava that was folded
and thrust into nappes during the early Paleozoic Cale-
donide Orogeny. Following more or less concurrent
metamorphism during the deformation, numerous
post-orogenic granitoid plutons were intruded.
Barrow found significant variations in the miner-
alogical composition and fabric of the pelites across the
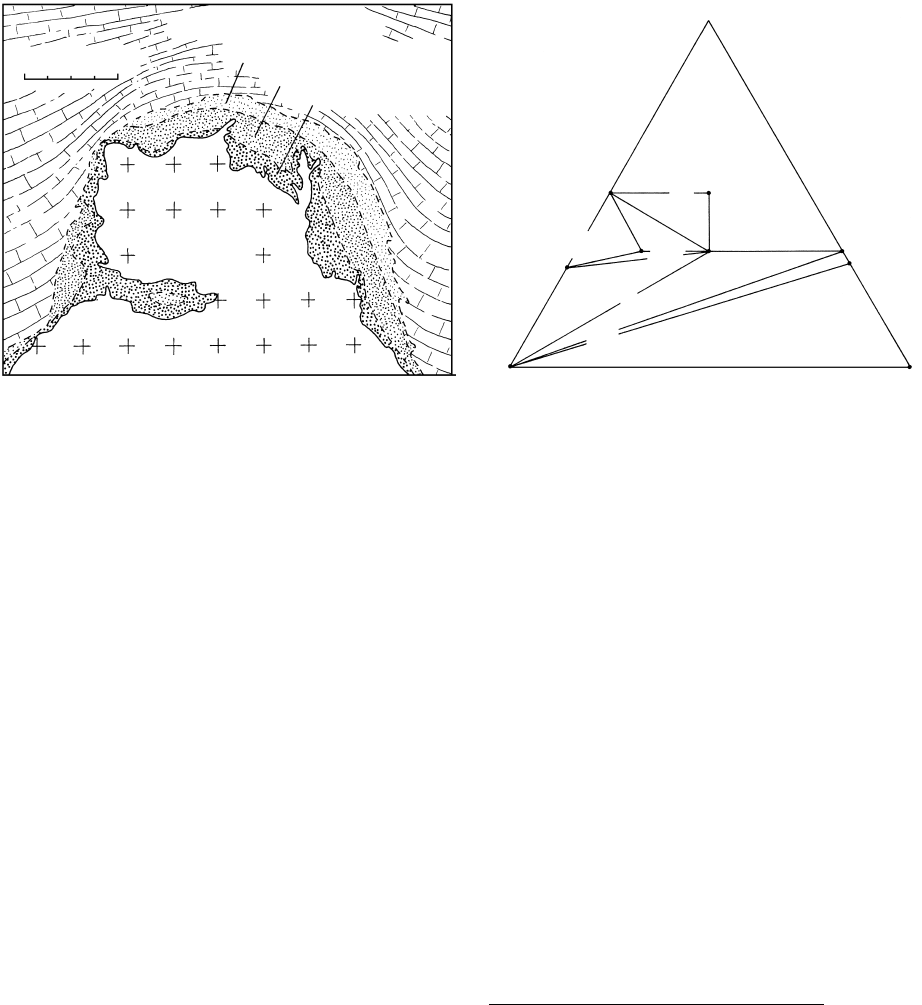
Brucite marble
Monticellite zone
Idocrase zone
Garnet zone
Quartz monzonite
porphyry
0 m 400
14.28 Schematic cross-section through the granitic porphyry and
surrounding metasomatic zones at Crestmore. California. See
also Figure 14.29. Outermost forsterite zone lying between the
brucite marble country rocks and the monticellite zone is
omitted. Redrawn from Burnham (1959).
Calcite Periclase
CaO
MgO
Spurrite
and
tilleyite
Merwinite
Wollastonite Diopside
Monticellite
Forsterite
Clinohumite
10
9
7,8
6
5
4
3
2
1
SiO
2
CO
2
14.29 Compositional relations in the system CaO–MgO–SiO
2
with
excess CO
2
at more or less fixed P but variable T. The path of
progressive silication of the carbonate country rocks at Crest-
more is shown by the sequence of numbers 1–10 representing
the skarn mineral assemblages that make up the metasomatic
zones. See also Figure 14.28. Redrawn from Burnham (1959).
during progressive metamorphism requires careful
study of mineral phase equilibria and reactions (Chap-
ter 16). In the thermal metamorphism in the Onawa
contact aureole, the first appearance of the index
mineral pair alkali feldspar sillimanite, produced by
breakdown of muscovite quartz (reaction 14.1; Fig-
ure 14.31), likely depended on a critical change in T;
if so, the isograd represents the line of intersection of a
paleo-isothermal surface with the ground surface. In
the Crestmore metasomatic zones, successive isograds
were dictated chiefly by changes in silica activity.
In Figure 14.30, northwest of Aberdeen, there are
regional Buchan zones defined on the basis of andalu-
site and cordierite in pelitic rocks. If equilibrium pre-
vailed, pressures cannot have exceeded about 4 kbar
(Figure 14.31), otherwise the other two Al
2
SiO
5
poly-
morphs would have been stabilized, as in the zonal suc-
cession from kyanite to sillimanite in typical Barrovian
zones in the remainder of the Scottish Highlands. The
lower pressures of Buchan metamorphism are also
consistent with the presence of Mg–Fe aluminosili-
cate cordierite rather than denser almandine garnet
(another Mg–Fe aluminosilicate) that is widespread in
Barrovian zones.
Geographic relations of Buchan and Barrovian
zones and variations in the grade of the latter provide
Metamorphic Rocks and Metamorphism: An Overview
435
terrane, whereas other protoliths showed less variation
in their constituent mineral assemblages because of
less sensitivity to changing metamorphic conditions.
Northward from the boundary fault, he mapped a
sequence of pelitic mineral zones beginning with the
lowest grade slates and slightly coarser phyllites of the
chlorite zone made basically of chlorite white mica
quartz. Succeeding zones were identified by the first
appearance of a particular index mineral that com-
monly persisted into succeeeding higher grade zones
(see Figure 18.13). The onset of the next higher grade
biotite zone was marked by the first appearance of
biotite in phyllites and coarser schists that contain addi-
tional chlorite white mica quartz. The beginning
of the garnet zone was delineated by the appearance of
an almandiferous garnet and of succeeding zones by
the first appearance of staurolite, kyanite, and finally
sillimanite in the highest grade schists and gneisses.
Similar Barrovian metamorphic zones, or segments of
them, in pelitic rocks have since been documented in
many other orogenic belts around the world.
One can envisage a three-dimensional surface of
constant grade during the metamorphism whose inter-
section with the present ground surface is a mappable
line, called an isograd, that is marked by the appear-
ance of a particular index mineral. Determining what
changing intensive variable or variables caused the first
appearance of a particular index mineral at an isograd
Barrovian zones
Sillimanite
Kyanite
Garnet
Biotite
Chlorite
Ocean
0
0
miles
50
N
Buchan
andalusite
zone
Fault
a
a
a
Aberdeen
Edinburgh
Glasgow
Highland Boundary Fault
Atlantic
Great Glen ]
Moine
Thrust
50
km
14.30 Simplified map of Barrovian mineralogical zones in regional
metamorphic rocks of the Caledonide orogen in Scottish
Highlands. Thin staurolite zone between garnet and kyanite
zones is omitted. Areally restricted Buchan zones lie north of
Aberdeen. Redrawn after Gillen (1982).
P (kbar)
Depth (km)
8
7
6
5
4
3
2
1
0
25
20
15
10
5
0
KYANITE
SILLIMANITE
ANDALUSITE
granite
solidus
Pa Qtz
Ab Al
2
SiO
5
H
2
O
Ms Qtz
Ms
Kfs Al
2
SiO
5
H
2
O
K
f
s
C
r
n
H
2
O
400 500 600
T (°C)
700 800
Water-Saturated
14.31 Breakdown conditions for paragonite and muscovite at P
P
fluid
P compared to stability fields of Al
2
SiO
5
poly-
morphs (long dashed boundary lines). Decomposition of nat-
ural paragonite–muscovite solid solutions in the presence of
quartz occurs between the stippled bands. In systems where
P P, muscovite quartz breaks down at lower T (line
of open circles). See Table 14.1 for key to mineral abbrevia-
tions. Redrawn from Spear (1995).
H
2
O
H
2
O
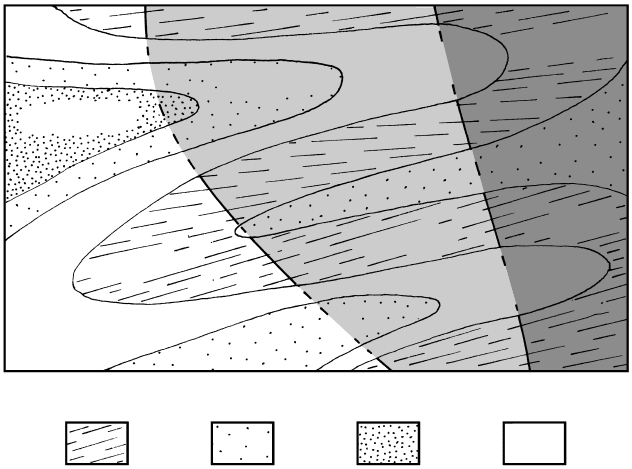
436 Igneous and Metamorphic Petrology
as it equilibrated under peak conditions. For example,
it can be inferred that the kyanite-bearing schist in
Figure 14.9 equilibrated at a higher P than did the
andalusite-bearing slate in Figure 14.10 because kyanite
is denser than andalusite. (Kyanite is actually the densest
Al
2
SiO
5
polymorph.) However, we must hasten to add
that this may only be true if the kyanite schist equili-
brated at an equal or higher T than the andalusite slate,
as may be appreciated from the stability diagram for
the Al
2
SiO
5
polymorphs in Figure 14.31. Because of
the positively sloping boundary line between the sta-
bility fields of kyanite and andalusite, it may be pos-
sible for kyanite to have equilibrated at a lower P and
lower T than the andalusite in the slate. Other minerals
in the two rocks may provide additional information
indicating their relative temperatures of equilibration,
resolving ambiguities such as this.
Hence, two or more minerals comprising the meta-
morphic mineral assemblage in a rock provide the cru-
cial information regarding the equilibrating intensive
variables. The minerals occurring in a metamorphic
rock—its mineralogical composition—may differ from
its mineral assemblage equilibrated under some particu-
lar set of intensive variables at the peak of prograde
conditions. Because of sluggish kinetics, relict phases
surviving metastably from the protolith or formed
along the prograde path, as well as possible phases
crystallized along the retrograde path, may occur in the
Chlorite
B
B
G
zone
zone
zone
G
Biotite
Garnet
B Biotite isograd G Garnet isograd
Pelitic
rocks
Quartzo-feldspathic
rocks
Mafic
rocks
Calcareous
rocks
14.32 Hypothetical, schematic Barrovian mineralogical zones and bounding isograds (thicker lines). Mineralogical variations in interlayered
mafic, quartzo-feldspathic, and calcareous rocks are independent of those in pelitic rocks on which the zones and isograds are based;
consequently, position of defining isograds can only be interpolated through these compositions. Isograds are independent of contacts
between chemical rock groups and rock structure, premetamorphic folds in this example.
insights into gradients in P and T that prevailed in the
Scottish segment of the Caledonide Orogen.
Metamorphic zones and their bounding isograds do
not necessarily correspond to stratigraphic and struc-
tural contacts on geologic maps (Figure 14.32). Condi-
tions of metamorphism are commonly independent of
patterns of pre-metamorphic protolith deposition and
deformation. On the other hand, isograd patterns can
be useful in unraveling the effects of tectonism and
magmatism. For example, isograd “surfaces” in a con-
tact terrane might dome over an underlying intrusion
and sharply offset isograds might be the result of post-
metamorphic faulting, such as across the Great Glen in
Figure 14.30.
14.2.6 Intensive Variables and Stable Mineral
Assemblages
Intensive thermodynamic variables prevailing in a
metamorphic system govern phase equilibria. The final
equilibrium state of the system is generally assumed
to be that attained at the peak T in the course of a
progressive metamorphic path where reaction kinetics
are the most favorable. Unless proven otherwise, equi-
librating mineral reactions during the subsequent retro-
grade path are less prominent or even nonexistent.
A single mineral can provide limited insight into
what intensive variables—P, T, and concentrations of
chemical components—prevailed in the rock system
