Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.

3.17 for the silica polymorphs). Other experimentalists
have used very finely pulverized mineral grains as
starting materials for experiments, but the surface and
strain energy imparted to the minute grains can over-
whelm the small Gs intrinsic to the phases. The nature
of sillimanite grains sometimes used as starting mater-
ial poses yet another difficulty. Other components, or
“impurities,” such as upwards of 1 wt.% of Fe
2
O
3
that
substitutes for Al
2
O
3
, influence the very small free
energy differences between sillimanite and the other
polymorphs. Consequently, reaction conditions and
equilibrium boundaries can be shifted by as much as a
few tens of degrees and stability field boundaries
become narrow divariant bands in P–T space rather
than univariant lines. Minute, practically inseparable
intergrowths of quartz are common in some sillimanite,
further confusing stability relations.
In many rocks, sillimanite occurs not as isolated
single grains but rather as very fine fibrous bundles,
called fibrolite, that are typically intimately intergrown
in muscovite or biotite (Figure 16.2). According to
Penn et al. (1999), the excess grain-boundary energy
of fibrolite resulting from the small size of the fibers,
together with the slight nonparallelism of neighboring
fibers and possible Al–Si disordering in their lattice,
shifts the andalusite–sillimanite reaction by as much as
an estimated 140°C and the kyanite–sillimanite curve
by 30°C. Difficulty in nucleating an isolated grain
of sillimanite is overcome by nucleation on boundaries
of nonparallel mica flakes during prograde metamor-
phism and the resulting fibrolite mimics the misori-
ented flakes.
Al
2
SiO
5
Stability Diagram. Because of these kinetic
factors, widely discrepant univariant boundary lines
delineating polymorph stability fields have been deter-
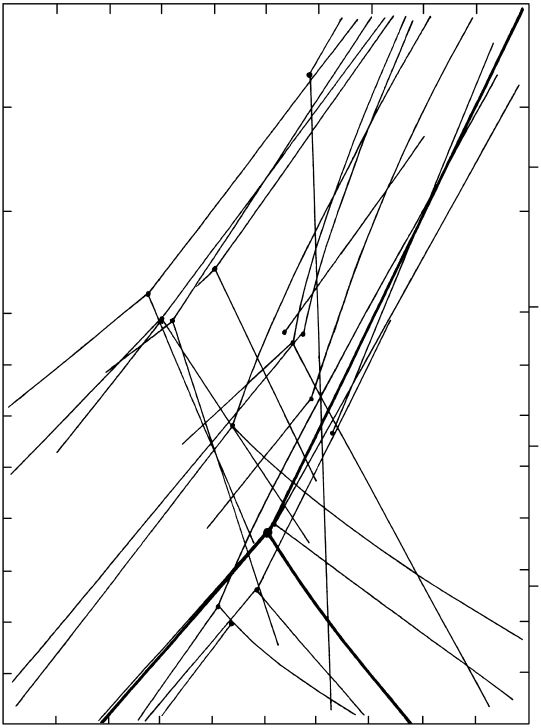
mined by numerous experimentalists (Figure 16.3).
The set of univariant lines determined by Holdaway
and Mukhopadhyay (1993) is preferred by many
petrologists and is adopted here.
It is a useful exercise to recreate the Al
2
SiO
5
poly-
morph stability diagram from the thermodynamic data
provided by Holdaway and Mukhopadhyay (1993) and
presented here in Table 16.1. Qualitatively, kyanite,
having the smallest molar volume, ought to be stable
at highest P, whereas sillimanite ought to be stable
at highest T because its molar entropy is greatest.
An exact P–T point on the boundary lines between
polymorph stability fields can be calculated by com-
bining equations 3.1, 3.4, and 3.7 at constant P and T,
giving G H TS. Here, the differentials dG
and dS in these equations have been replaced by the
change in free energy, G, in enthalpy, H, and in en-
tropy, S, for the polymorphic transition. At the equi-
librium T
eq
between two coexisting polymorphs on
their corresponding boundary line at P 1 atm, G
0 (Section 3.3); hence, H T
eq
S. Using values from
Table 16.1 we can solve for T
eq
in the reaction kyanite
→ andalusite
The Clapeyron equation 3.13 in Section 3.3.1 can be
used to determine the slopes of the boundary lines.
Again, for the kyanite → andalusite transition
11.84 bar/degree
This position and slope of the kyanite–andalusite equi-
librium line may be compared to those in Figure 16.3.
It is left as Problem 16.1 to determine the other two
boundary lines.
Application to Rocks. How reliable are polymorphs of
Al
2
SiO
5
as mineral thermobarometers, indicating the
P–T conditions under which a particular polymorph
was stabilized? Even if experimental difficulties in the
determination of the stability diagram can be overcome,
and it can never be fully certain they have been, there
dP
dT
S
V
(91.60 82.86)J/K
(51.46 44.08)cm
3
462 K 189°C
T
eq
H
S
(2589.66 2593.70)kJ
(91.60 82.86)J/K
Metamorphic Mineral Reactions and Equilibria
477
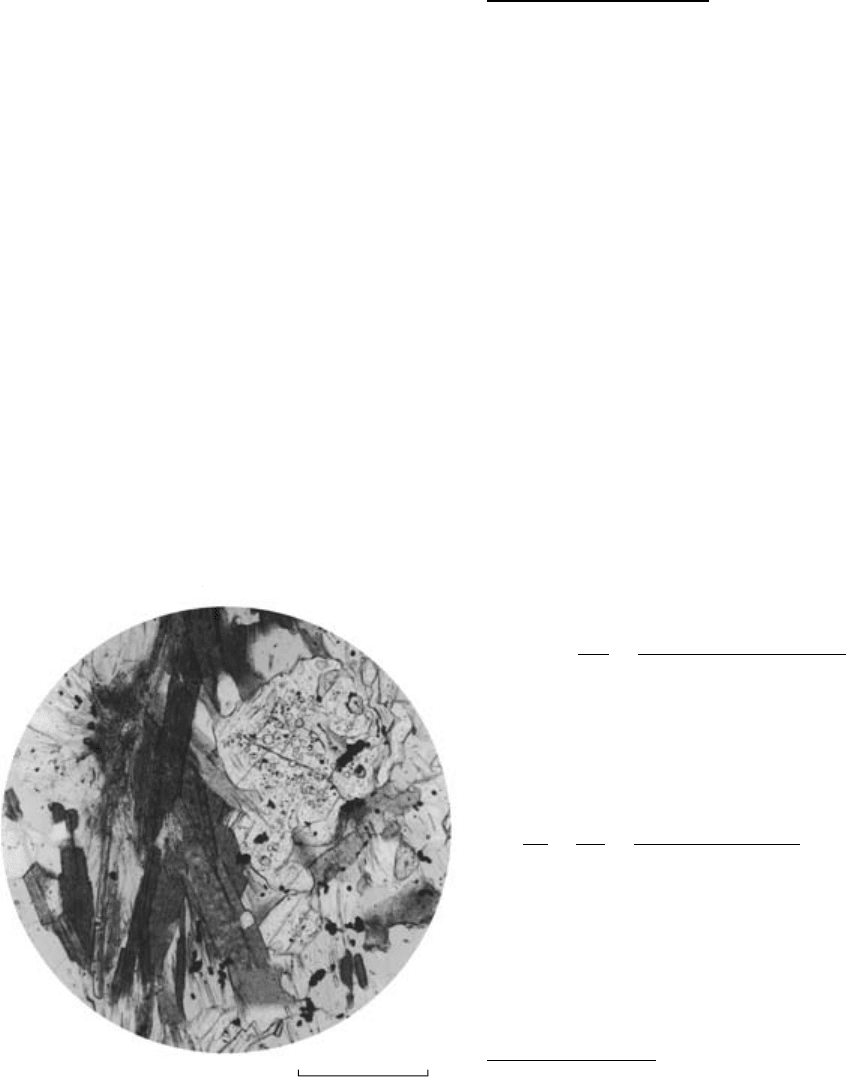
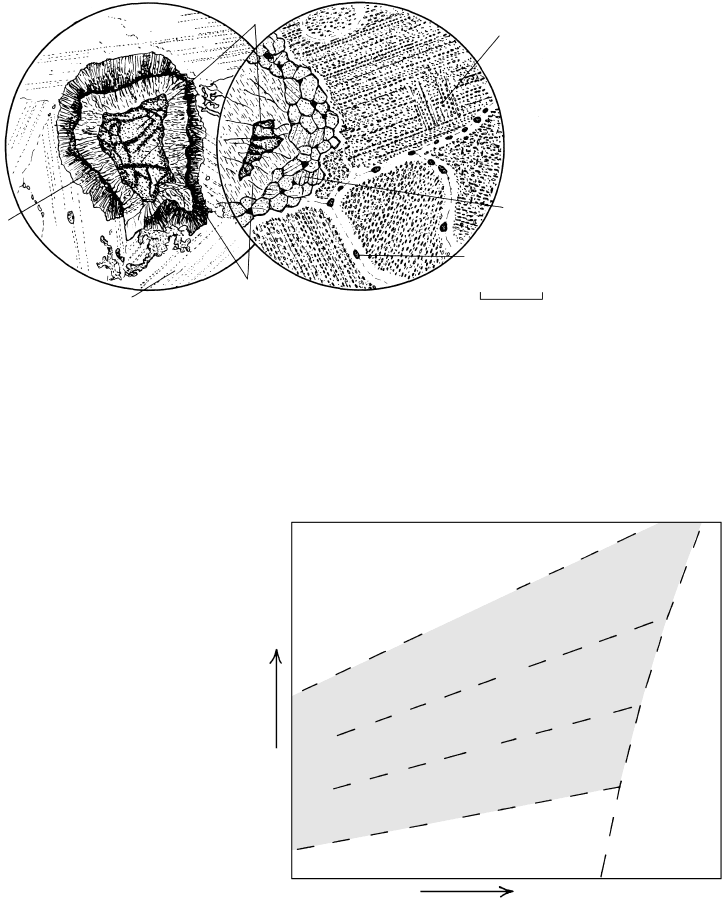
0 0.2mm
Fibrolite
Biotite
Andalusite
16.2 Photomicrograph under plane-polarized light of fibrolite in a
pelite hornfels near Lake Isabella, Sierra Nevada roof pendant,
California. Note that the fibrolite is intimately intergrown with
biotite and does not replace nor lie in contact with andalusite.
remains the nagging question whether a particular
polymorph in a rock truly represents the most stable
phase at the time of metamorphism. Nature apparently
experiences the same kinetic difficulties as experiment-
alists do. Although many rocks contain just one poly-
morph, the presence of two polymorphs in one thin
section (such as andalusite and sillimanite, e.g. Fig-
ures 14.25d and 16.2) is not uncommon, and there are
reports of all three coexisting together.
In zoned terranes, univariant equilibrium prevails at
isograds; for example, at the sillimanite isograd where
kyanite reacts to form sillimanite. In ideal circum-
stances, the mineral equilibria between the polymorphs
can be frozen in the rock in a reaction texture. But the
widespread coexistence of two polymorphs throughout
part of a zoned terrane, where metamorphic conditions
just happened to fall on a univariant reaction boundary,
would seem to be improbable; the probability is indeed
remote that peak metamorphic conditions would fall
on the invariant triple point, preserving all three. It is
more possible that a polymorph nucleated metastably
or, more likely, persisted into the stability range of
another. Or a dissolved impurity component might
have created a divariant field boundary wherein a
slight range in P–T conditions would stabilize two
polymorphs. Clearly, inferring P–T conditions from
Al
2
SiO
5
polymorphs requires caution.
For polymorphs in other chemical systems, kinetics
appear to be less of a problem and they may be more
reliable mineral thermobarometers.
Although the univariant boundary lines and ther-
modynamic data of Holdaway and Mukhopadhyay
(1993) for the Al
2
SiO
5
polymorphs are preferred by
many petrologists, comparison of their stability field of
478 Igneous and Metamorphic Petrology
P (kbar)
Depth (km)
14
12
10
8
6
4
2
0
40
30
20
10
0 200 400 600 800
Kyanite
Sillimanite
Andalusite
1000
T (°C)
16.3 Al
2
SiO
5
stability diagram. Light lines are widely discrepant univariant boundary lines between stability fields of the polymorphs deter-
mined experimentally prior to 1969 by many investigators. Triple point where three boundary lines meet is emphasized by small filled
circle. Redrawn from Althaus (1969). The preferred phase diagram from Holdaway and Mukhopadhyay (1993) is indicated by heavy
lines. The triple point lies at 504 20°C and 3.75 0.25 kbar. Unlike the other two straight boundary lines, the andalusite–sillimanite
line is slightly curved, and three coordinates fix its curvature: 500°C at 3.82 kbar, 600°C at 2.30 kbar, and 700°C at 0.95 kbar.
andalusite in Figure 16.3 with Figure 14.31 reveals an
apparent paradox. The solidus for a water-saturated,
minimum-T granite composition (Figure 5.24) lies at
a T well above the stability field of andalusite. Yet
andalusite-bearing, peraluminous granites are well
known (Section 11.6.2), as are local migmatites and
pegmatites that contain this polymorph. The presence
of substantial concentrations of F and B might lower
the solidus T enough to stabilize andalusite in these
magmas; however, this is unlikely as the necessary
concentrations found by experiments are excessive for
natural systems. Alternatively, sillimanite might have
precipitated in the magma and then transformed into
more stable, lower-T andalusite during slow subsolidus
cooling. In yet other possibilities, andalusite might have
grown metastably above the solidus in the stability field
of sillimanite, or some sort of subsolidus metasomatism
might have been involved, converting an alkali silicate
into andalusite (see Section 16.8.1).
16.4 NET TRANSFER SOLID–SOLID
REACTIONS
In discontinuous net transfer reactions, heterogeneous
mineral equilibria among multiple participating phases
can be represented by a written stoichiometric reaction
and by a P–T diagram that shows stability fields of
mineral assemblages and boundary lines between
them. We begin our discussion of net transfer reactions
using only pure solid phases, including ideal end-
members, where there is no solid solution.
16.4.1 Basic Relations in a System of Pure
End-Member Phases
Consider the equilibrium
16.1 Ca
3
Al
2
Si
3
O
12
SiO
2
CaAl
2
Si
2
O
8
grossular quartz anorthite
2CaSiO
3
wollastonite
in which the higher T and lower P assemblage is on the
right (Figure 16.4). LeChatelier’s principle (Section
3.3.1) tells us that this higher grade assemblage should
be the one of greater entropy and molar volume. Ther-
modynamic data in Table 16.2 confirm that it is anor-
thite 2 wollastonite
S
Grs
S
Qtz
S
An
2S
Wo
260.1 41.5 199.3 163.4
V
Grs
V
Qtz
V
An
2V
Wo
12.528 2.269 10.079 7.98
These same entropy and volume data allow us to
quantify the slope (dP/dT) of the reaction boundary
line in P–T space. According to the Clapeyron
equation 3.13
Where this positive sloping line is positioned in P–T
space can be found in the same manner as the field
boundary lines were determined above for the Al
2
SiO
5
polymorphs but using data in Table 16.2. Thus, the
equilibrium T
eq
for reaction 16.1 at 1 atm is
61 J/K
3.262 J/bar
18.7 bar/degree
(199.30 163.4) (260.1 41.5)
(10.079 7.98) (12.578 2.269)
dP
dT
S
V
(S
An
2S
Wo
) (S
Grs
S
Qtz
)
(V
An
2V
Wo
) (V
Grs
V
Qtz
)
Metamorphic Mineral Reactions and Equilibria
479
P (kbar)
3 Anorthite
Depth (km)
25
20
15
10
5
0
80
60
40
20
0
200 400 600 800 1000
Anorthite 2 wollastonite
Grossular quartz
Albite nepheline
2 Jadeite
Jadeite quartz
Albite
Grossular
quartz
2 kyanite
1200
T (°C)
T
eq
498°C
P
eq
8858.6 bar at 25°C
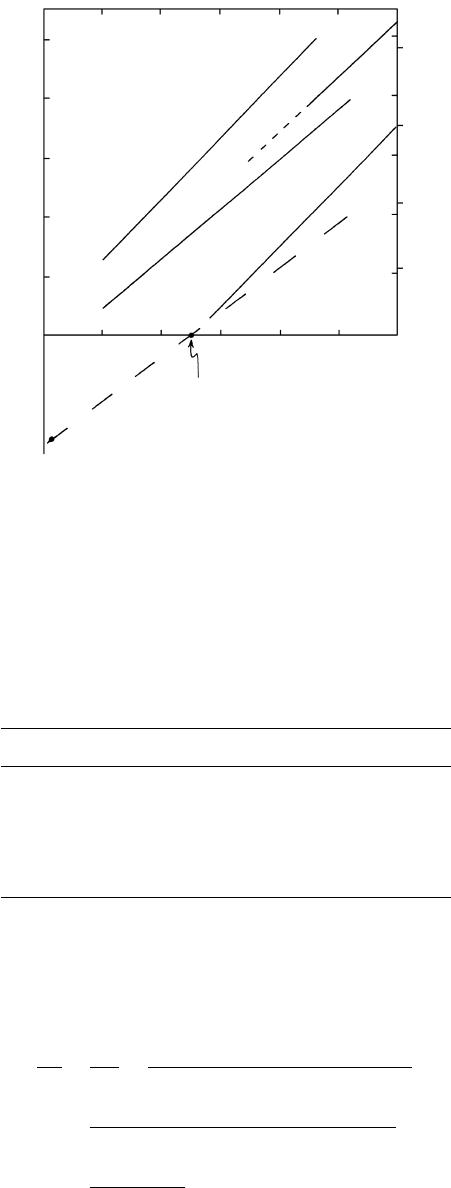
16.4 Univariant discontinuous reaction boundary lines involving
albite and anorthite for pure phases based on laboratory
synthesis experiments. Redrawn from Hays (1967), Newton
and Fyfe (1976), and Koziol and Newton (1988). The dashed
line through P
eq
and T
eq
is based on calculations in text from
data in Robie and Hemingway (1995).
Table 16.2. Thermodynamic Data per Mole for
Some Pure Solid Phases at 25°C (298.15K) and 1 Bar
from Robie and Hemingway (1995)
V (J/
BAR
)* S ( J/K) H (
K
J) G (
K
J)
Anorthite 10.079 199.3 4234.0 4007.9
Grossular 12.528 260.1 6640.0 6278.5
Wollastonite 3.990 81.7 1634.8 1549.0
Quartz 2.269 41.5 910.7 856.3
Coesite 2.064 38.5 907.8 852.5
*1 J/bar 10 cm
3
.

A second method of determining a point on the equi-
librium boundary line begins with an expression for
the free energy, , of Reaction 16.1 at the equi-
librium T
eq
and P
eq
relative to some reference state,
G° , such as 25°C (298.15K) and 1 bar. We first
integrate Equation 3.11, dG
P
SdT, at constant P,
and Equation 3.12, dG
T
VdP, at constant T, between
the reference state and the equilibrium T
eq
and P
eq
and
then combine to give
16.2
S(T
eq
T
ref
)
This expression assumes that V is independent of P
and S of T. Here we note that, at the equilibrium T
eq
and P
eq
, 0; furthermore, the calculations
are simplified if we evaluate P
eq
where T
eq
25°C
T
ref
. Equation 16.2 becomes
16.3
Using data in Table 16.2, V 3.262 J/bar and
G 28 900 J. Remembering that P
ref
1 bar,
we can solve for P
eq
, obtaining 8858.6 bar at 25°C.
Although this is a geologically unrealistic P, extrapola-
tion to higher P–T values along a properly sloping line
(18.7 bar/degree) is quite reasonable.
From the alternate reaction points at T
eq
498°C
and 1 atm and P
eq
8858.6 bar at 25°C on the field
boundary line determined by the two methods, a slop-
ing line can be plotted in Figure 16.4 by taking some
arbitrary “rise and run” of, say, (400 18.7) bar/400°C
7480 bar/400°C. This line based on thermodynamic
data deviates increasingly at higher P from the bound-
ary line based independently on laboratory synthesis
experiments. One reason for the discrepancy is a P de-
pendence on the V of the reaction, which we ignored.
In many cases, different approaches yield significantly
different results, forcing the petrologist to critically
examine the data, rejecting one or more or all sets, and
possibly performing additional experimental work to
try to resolve the discrepancies.
It may be noted that the boundary lines in Figure
16.4 are straight lines; that is, they are linear functions
of P and T. However, some solid–solid equilibria define
slightly curved lines where molar volumes and en-
tropies vary with respect to P and T; the andalusite–
sillimanite reaction line in Figure 16.3 is an example.
Using computer spreadsheets, it is easy to take into
account heat capacities, compressibilities, and so on to
T
ref
,P
ref
G
0
T
ref
,P
ref
V(P
eq
P
ref
)
G
T
eq
,P
eq
G
T
eq
,P
eq
G°
T
ref
,P
ref
V(P
eq
P
ref
)
T
ref
,P
ref
G
T
eq
,P
eq
47 100 J
61.1 J/K
771K 498°C
(4234 000 2{1634 800}) (6 640 000 910 700)
(199.30 163.4) (260.1 41.5)
T
eq
H
S
create more accurate reaction lines from thermody-
namic data (Nordstrom and Munoz, 1994, p.101).
It is important to note in Figure 16.4 that the stabil-
ity field of a phase, or phase assemblage, shrinks in the
presence of an additional reacting phase. Thus, the P–T
stability field of anorthite wollastonite lies within,
and at lower P and T than, the field of anorthite alone.
Where kyanite is present, grossular quartz is stable
only at pressures higher than in systems lacking kyan-
ite. Albite encompasses the more restricted stability
field of the assemblage albite nepheline, and the
stability field of jadeite quartz is a part of the larger
field of stable jadeite. This same concept explains why
olivine in basaltic systems is stable to only deep levels
of the continental crust (Figure 5.11), whereas in peri-
dotite it is stable to depths of a magnitude greater in
the mantle (Figure 1.3). In basaltic systems, olivine is
consumed in reactions with plagioclase at modest P,
whereas in peridotite olivine does not react with any of
the other coexisting phases. (Nonetheless, beginning
near 410 km depth in the mantle, olivine undergoes a
phase transformation into a denser spinel-like mineral.)
In the three component system SiO
2
–CaO–Al
2
O
3
,
the phase rule indicates that the boundary line repre-
sents a state of univariant equilibrium where all four
solid phases (grossular quartz anorthite wollas-
tonite) coexist in equilibrium (F 2 C 2
3 4 1). For P–T values off the univariant line, no
more than three phases can coexist stably in states of
divariant equilibrium where T and P can freely and
independently vary without affecting the phases in
equilibrium. Examination of reaction 16.1 indicates
which three phases can coexist. In the low-T divariant
field, grossular and quartz occur stably together but
wollastonite or anorthite may also be present, but not
both. In the high-T divariant field, wollastonite and
anorthite are stably coexistent but grossular or quartz
may also be present, but not both.
In most stability diagrams only the critical deter-
mining phases are indicated, such as the lower T
assemblage grossular quartz and the higher T
assemblage wollastonite anorthite in Figure 16.4. In
most instances, the molar proportions of grossular and
quartz would not be exactly the same in a rock under-
going prograde metamorphism at, say, 10 kbars and
900°C. So what happens is that the lesser of one of
these reactant phases, let’s say it is quartz, is entirely
consumed while the more abundant and excess grossu-
lar persists stably above 900°C, together with the prod-
uct phases wollastonite and anorthite. The reaction
at 10 kbar progresses at 900°C until quartz is entirely
consumed and only then can T increase in the heating
system, bringing it into the higher T divariant field.
In the very unusual instance where the number of
moles of grossular is exactly equivalent to that of
quartz in a rock, an increase in T above 900°C at
480 Igneous and Metamorphic Petrology
10 kbars will result in the total consumption of both of
these two reactants into anorthite and wollastonite.
This product phase assemblage is divariant because it
consists of two components, CaSiO
3
and Al
2
SiO
5
.
Phase relations for the model reaction grossular
quartz anorthite 2 wollastonite are conveniently
depicted in a triangular composition, or compatibility,
diagram for the three components SiO
2
, CaO, and
Al
2
O
3
that is drawn for a restricted range of P–T
conditions (Figure 16.5). Chemographic devices such
as this were described in Section 15.3. Stably coexisting
phases are connected by tie lines. At low T, the divari-
ant assemblage grossular quartz is stable. A rock
whose proportions of components place it at point A
consists of only grossular and quartz in proportions
given by the lever rule. However, two three-phase
assemblages are also stable possibilities. In rock B,
an excess of Al
2
O
3
stabilizes anorthite in addition to
grossular quartz whereas a deficiency in rock C sta-
bilizes wollastonite in addition to grossular quartz.
At, say, 10 kbars an increase in T above 900°C (Figure
16.4) will result in a tie-line switching reaction in
which grossular quartz react to form more stable
anorthite wollastonite. As a result of this discontinu-
ous reaction, rock A now consists of equal proportions
of only anorthite wollastonite, while in rocks B and
C the modal excess of quartz cannot be consumed in
the reaction, resulting in the three-phase assemblage
anorthite wollastonite quartz.
16.4.2 Model Reactions in the Basalt–Granulite–
Eclogite Transition
Metabasites are among the most common rocks in
regional metamorphic terranes in orogens. Signific-
ant changes in mineral assemblages in their mafic
protoliths reflect the effects of increasing pressures in
deepening parts of the mountain belt. In this section, to
simplify the nature of reactions during increasing P we
utilize model reactions between pure idealized end-
member phases, ignoring the widespread solid solution
that prevails in the rock-forming minerals.
Changing mineral equilibria in metabasites are
commonly recorded in corona and symplectite reaction
textures. Because of the commonly dry composition of
basaltic protoliths and possibly also because of limited
time, the sphere of effective diffusion is restricted
and recrystallization is commonly incomplete. Con-
sequently, a jacketing corona of more stable product
phases surrounds a destabilized reactant grain. In
Figure 16.6, more stable orthopyroxene (hypersthene)
crystallized at the contact between incompatible
olivine and plagioclase. Excess Mg and Fe from this
replacement diffused into the plagioclase where it
reacted to form spinel, and Ca from decomposing
plagioclase diffused in the opposite direction toward
the olivine, where diopside formed.
To a first approximation, corona development in the
metagabbros in Figure 16.6 can be modeled by simple
reactions between pure end-member phases, ignoring
minor components. For the replacement of olivine by
orthopyroxene
16.4 2Mg
2
SiO
4
2MgSiO
3
2MgO
forsterite enstatite
and for the formation of the rim of diopside spinel
surrounding the orthopyroxene
16.5 CaAl
2
Si
2
O
8
2MgO CaMgSi
2
O
6
MgAl
2
O
4
anorthite diopside spinel
Metamorphic Mineral Reactions and Equilibria
481
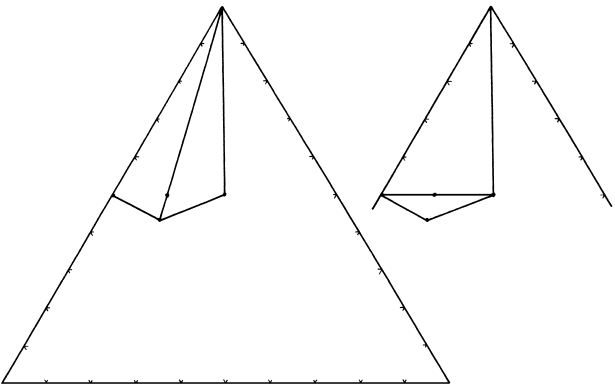
Wollastonite
Low T High TMole
proportions
Quartz
Anorthite
Grossular
CaO Al
2
O
3
SiO
2
A
BC
A
BC
16.5 Phase relations for the equilibrium 16.1 in terms of the components CaO–SiO
2
–Al
2
O
3
.
These two reactions can be added together to give
16.6 2Mg
2
SiO
4
CaAl
2
Si
2
O
8
forsterite anorthite
2MgSiO
3
CaMgSi
2
O
6
MgAl
2
O
4
enstatite diopside spinel
Molar entropies and volumes for these assemblages at
25°C and 1 atm are listed in Robie and Hemingway
(1995) as follows:
S
(2FoAn)
387.5 J/K
S
(2EnDiSpl)
364.0 J/K
V
(2FoAn)
188.09 cm
3
V
(2EnDiSpl)
168.42 cm
3
If the relative values of these quantities do not change
at high P and T, they indicate that the assemblage
2Fo An ought to be stable at higher T because of
its greater entropy compared to 2En Di Spl.
Similarly, it is stable at lower P because of its greater
volume. Therefore, because S/V is positive, the
Clapeyron Equation (3.3.1) indicates the univariant
boundary line for the reaction 2Fo An 2En Di
Sp has a positive slope on a P–T diagram (Figure 16.7).
Because anorthite has a relatively large molar volume,
with increasing P it reacts with MgO (from breakdown
of olivine) forming a smaller volume (denser) assem-
blage of diopside spinel according to reaction 16.6.
Al can be sequestered in high-P pyroxenes as the Mg-
and Ca-tschermak end-members (Special Interest Box
16.1). Tschermak end-members do not exist as distinct
pure minerals. In a reaction between plagioclase and
olivine, previously modeled by reaction 16.6, alternate
products would be aluminous pyroxene solid solutions
containing the tschermak end-members, as follows
16.7 3Mg
2
SiO
4
4CaAl
2
Si
2
O
8
forsterite anorthite
2MgSiO
3
·MgAl(AlSi)O
6
Al-enstatite
2CaMgSi
2
O
6
·CaAl(AlSi)O
6
SiO
2
Al-clinopyroxene quartz
482 Igneous and Metamorphic Petrology
P
T
BASALT (Pl
Cpx Opx Ol)
Crystals
melt
GRANULITE
Solidus
ECLOGITE
Al-Cpx
Grt Ky Opx Qtz (or Cs)
Low-P ~3–5 kbar
Pl
Cpx
Opx
Ol
Spl
Medium-P
Pl Al
-
Cpx Al
-Opx Qtz
High-P ~10 kbar
Al
-
Cpx Grt Pl Qtz
16.8 – 16.10
16.9
16.7
16.6
16.7 Schematic stability fields of basaltic, granulitic, and eclogitic
mineral assemblages separated by model univariant reactions
16.6–16.10. Boundary line slopes are schematic and assumed
to be linear. Eclogite and granulite facies assemblages are
stable in either the crust or upper mantle depending upon T.
Minerals listed in approximate order of modal abundance.
Plagioclase
Diopside
+ spinel
Hypersthene
Spinel
Diopside
Plagioclase with
minute spinel
inclusions
Relict
olivine
(a) (b)
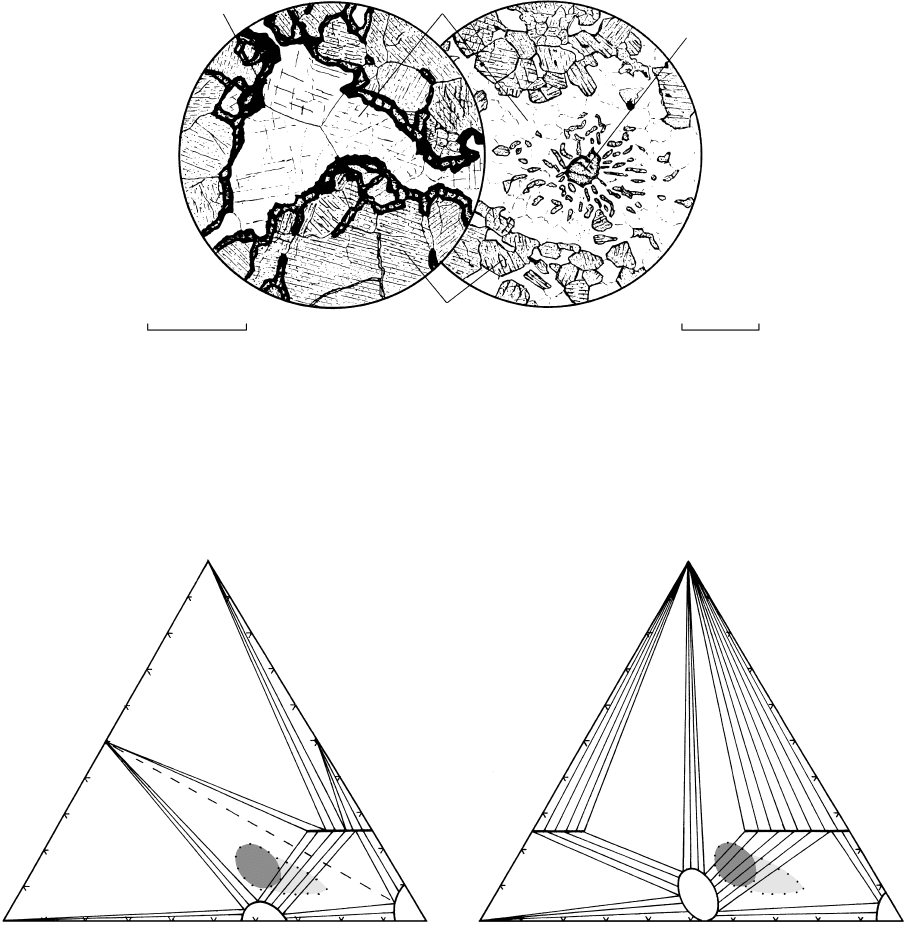
0 0.3mm
16.6 Corona reaction textures in metagabbros developed at original olivine–plagioclase grain contact. (a) Sample from Risor, southern
Norway, showing an inner corona of fibrous orthopyroxene (hypersthene) that has replaced original olivine and surrounded by an
outer corona of a fine intergrowth of diopside spinel that has replaced plagioclase. (b) Sample from the Barton Garnet Mine, Gore
Mountain, New York. Corona of hypersthene and diopside that is texturally more stable than (a) because of coarser granoblastic grains.
Although a few small spinels (black) occur in the diopside aggregate, most of them cloud plagioclase grains and have grown preferen-
tially along twin planes and grain boundaries. Both reproduced with permission from Nockolds SR, Knox RWO’B, Chinner GA. 1978.
Petrology for students. New York, Cambridge University Press. Copyright © 1978. For additional corona textures in high-grade mafic rocks
see Yardley et al. (1990, Figures 102–104).

The albite end-member in plagioclase has a smaller
molar volume than anorthite and, therefore, albitic
plagioclase is stable to higher P. But ultimately, it too
becomes unstable. Albite decomposes to form the
aluminous pyroxene, jadeite, NaAlSi
2
O
6
, according to
16.8 NaAlSi
3
O
8
NaAlSi
2
O
6
SiO
2
albite jadeite quartz
Jadeite is a naturally occurring phase, locally in vir-
tually pure form, in high P/T mineral assemblages
(Figure 16.4) of the eclogite and blueschist facies.
Jadeite is also a prominent end-member, along with
diopside (CaMgSi
2
O
6
) and other lesser components, in
omphacite (Appendix A). Ca–Mg–Fe–Al garnet and
omphacite are major constituents of eclogite together
with smaller amounts of other possible accessory min-
erals including kyanite, quartz (or coesite), orthopy-
roxene, rutile, phengite, paragonite, and epidote, but
never plagioclase. High-P omphacites can equilibrate
during decompression to a symplectitic intergrowth of
plagioclase in diopside (Figure 15.11).
Garnet is yet another dense repository for Al, Mg,
and Ca at high P. Anorthite can react with enstatite to
form garnet according to the reaction
16.9 CaAl
2
Si
2
O
8
2(Mg,Fe)SiO
3
anorthite orthopyroxene
Ca(Mg,Fe)
2
Al
2
Si
3
O
12
SiO
2
garnet quartz
Reaction textures resulting from the forward and
backward progress of this reaction are shown in Fig-
ure 16.8.
Another reaction consuming anorthite is
16.10 3CaAl
2
Si
2
O
8
Ca
3
Al
2
Si
3
O
12
anorthite grossular
2Al
2
SiO
5
SiO
2
kyanite quartz
where grossular is dissolved in garnet solid solutions.
The univariant line for this reaction is shown in Figure
16.4.
The reactions listed above serve as models for uni-
variant boundary lines in P–T space between fields
where basaltic, granulitic, and eclogitic mineral assem-
blages are stable (Figure 16.7). This figure is highly
schematic and no exact values are placed on the coor-
dinate axes because of the chemical variability of nat-
ural basaltic systems. Furthermore, reactions involving
solid solutions in rocks are at least divariant and are
thus “smeared” over P–T bands, in contrast to the uni-
variant lines indicated.
Further insights into the continuous mineralogical
transitions depicted in P–T space in Figure 16.7 are re-
vealed in ACF diagrams (described in Section 15.3.3)
that show solid solution of the compatible phases. In
unmetamorphosed basaltic and gabbroic rocks, the sta-
ble mineral assemblage is plagioclase clinopyroxene
Fe–Ti oxides orthopyroxene olivine (Figure
15.26). With increasing P under low volatile fugacities,
olivine ceases to be stable in the presence of calcic pla-
gioclase and is consumed by reaction 16.6. Low- to
mid-P granulite-facies metabasites consist of increas-
ingly more sodic plagioclase aluminous clinopyrox-
ene aluminous orthopyroxene. At higher P, garnet-
forming reactions 16.9 and 16.10 take place, causing a
tie-line switch to Fe–Mg–Ca–Al garnet clinopyrox-
ene compatibility from the lower P plagioclase or-
thopyroxene compatibility, changing the topology of
the the ACF diagram in Figure 16.9a. Recall that tie-
line switching is one diagrammatic manifestation of a
discontinuous reaction, in this case plagioclase ortho-
pyroxene → garnet clinopyroxene. Nonetheless,
plagioclase remains stable in more aluminous metaba-
sites but is absent in more mafic protoliths. At highest
P in the deep, thick continental crust or upper mantle,
further discontinuous reactions occur in metabasites,
creating plagioclase-free mineral assemblages of the
eclogite facies and causing further changes in configura-
tion of the compatibility diagram (Figure 16.9b). In
these highest P reactions, plagioclase is fully consumed
in what are called terminal disappearance reactions
because this phase is not stable in any rock composi-
tion. Dense garnets of a wider range of composition are
Metamorphic Mineral Reactions and Equilibria
483
Special Interest Box 16.1 Coordination of
oxygen around Al and Si in high-P minerals
At relatively low pressures, Al and Si cations are
tetrahedrally coordinated with oxygen anions in
aluminosilicate minerals but with increasing pres-
sures a more compact, lower molar volume octa-
hedral configuration develops where six oxygens
surround a cation instead of four. This transition to
a denser packing and higher coordination begins
for Al in pyroxene end members at mid-crustal
pressures. In the Mg- and Ca-tschermak end-
members, MgAl
VI
(Al
IV
Si)O
6
andCaAl
VI
(Al
IV
Si)O
6
,
one Al has a tetrahedral coordination (IV) while the
other is octahedral (VI). In the jadeite end-member,
NaAl
VI
Si
2
O
6
, all of the Al is in six-fold coordination.
The only silica polymorph possessing octahedral
coordination is the densest one, stishovite (atmos-
pheric density 4.28 g/cm
3
compared to 2.65 g/cm
3
for -quartz). Stishovite is only stable at pressures
(Figure 5.1) well beyond those to which continental
sialic rocks are normally subjected but it has been
found in some meteorite impact rocks that were
subjected to extreme transient pressures in the
shock wave.
stabilized, together with the densest Al
2
SiO
5
poly-
morph, kyanite, in relatively aluminous protoliths.
Ca and Al sequestered in plagioclase at lower P are
lodged in garnet, while Na and Al, together with Ca,
Mg, and Fe, occur in solid solution in omphacite
clinopyroxene.
16.5 CONTINUOUS REACTIONS
BETWEEN CRYSTALLINE SOLID
SOLUTIONS
Because of extensive solid solution in almost all
metamorphic rock-forming minerals, most reactions,
484 Igneous and Metamorphic Petrology
01mm 0.50mm
Pyroxene
Plagioclase
Garnet
Garnet
(a) (b)
16.8 Reaction textures in granulite-facies rocks modeled by reaction 16.9 anorthite orthopyroxene garnet quartz. (a) Sample of a
deep crustal inclusion in a kimberlitic pipe, Delegate, New South Wales, Australia. Well developed crystalloblastic fabric has been
modified by growth of more stable garnet (black borders) along boundaries between incompatible orthopyroxene and plagioclase grains.
(b) Sample from the Granulitgeberge, Hartmannsdorff, Sachsen, Germany. The same reaction in reverse has produced a symplectite
(vermicular (“wormy”) intergrowth) of orthopyroxene plagioclase from garnet, only a small remnant of which remains. Both repro-
duced with permission from Nockolds SR, Knox RWO’B, Chinner GA. 1978. Petrology for students. New York, Cambridge University Press.
Copyright © 1978.
(a) A
Kyanite
GRANULITE
FACIES
magnetite
ilmenite
Spinel
Garnet
Plagioclase
C
Calcite
F
OrthopyroxeneClinopyroxene
(b) A
Kyanite
ECLOGITE
FACIES
rutile
quartz
(or coesite)
Garnet
Grossular
C
Calcite
F
OrthopyroxeneOmphacite
16.9 ACF diagrams showing the transition between mineral assemblages in metabasites of the granulite facies and eclogite facies. Most basalts
and gabbros plot in the dark shaded area but some more mafic ones, such as picrite, fall in the lighter shaded area. Compare the
assemblage in basaltic protoliths in Figure 15.26. (a) Dashed tie-line represents compatibility (stable coexistence) of plagioclase
aluminous orthopyroxene in mid-P granulites. At higher P, the anorthite molecule in plagioclase reacts with orthopyroxene to form
garnet as in reaction 16.9. Remaining plagioclase in less mafic metabasites is more sodic and coexists with garnet and Al-rich clinopy-
roxene. In the eclogite facies (b), plagioclase is not stable and most metabasites consist of a Ca–Fe–Mg–Al garnet solid solution
plus Ca–Fe–Mg–Na–Al omphacitic clinopyroxene (Appendix A, 13) together with accessory rutile. Kyanite appears in more Al-rich
eclogites, orthopyroxene in more mafic ones, while quartz (coesite at higher pressures, Figure 5.11) may be stabilized in more silica-rich
eclogites.
including those just discussed for metabasites, take
place over a range of changing P and T in which both
reactant and product phases coexist. In such contin-
uous mineral reactions both modal proportions and
chemical compositions of reactant and product solid
solutions change until the reactant, or reactants, is
entirely consumed, terminating the reaction.
16.5.1 Solid Solution in the Continuous Net
Transfer Reaction Plagioclase Jadeitic Clinopyroxene
Quartz
The discontinuous reaction involving pure phases
albite jadeite quartz was shown above in Figure
16.4 and equation 16.8. This equilibrium is applicable
to high P/T rocks of the blueschist facies where, how-
ever, the widespread presence in the rocks of Ca, Mg,
and Fe leads to solid solution of these components in
the plagioclase and clinopyroxene. Accordingly, a little
anorthite is dissolved in the plagioclase and typically
greater concentrations of diopside and aegirine in
clinopyroxene resulting from Ca–Mg b Na–Al and
Fe
3
b Al substitutions, respectively. Because of the
complexity of the solid solutions, exact balanced stoi-
chiometric equations are impossible to write where
Ca–Al substitutes in plagioclase and Ca–Mg–Fe in
clinopyroxene; hence, there are many degrees of com-
positional freedom, guaranteeing continuous reactions
where all three phases coexist stably over a range of P
and T. The compositional variability and dilution of
the jadeite end-member results in a shift of the equilib-
rium in P–T space, extending the stability field of the
less pure clinopyroxene in the presence of quartz
to lower pressures. Hence, this equilibrium has the
potential of serving as a geobarometer.
The extent of the shift can, in principle, be calcu-
lated using thermodynamic tools and data tied wher-
ever possible to experimental data. For reaction 16.8
where all phases are pure the univariant reaction line is
governed by the relation
16.11 G G
Jd
G
Qtz
– G
Ab
0
But where there is solid solution in the plagioclase and
especially the clinopyroxene the condition for equilib-
rium is (Section 3.5)
16.12 G
or
16.13 G RT lnK
eq
where G is the calculated change in free energy
(equation 16.8) for pure phases at the P and T of inter-
est and K
eq
is the equilibrium constant
16.14 K
eq
The activities (thermodynamically effective concen-
trations) of jadeite, NaAlSi
2
O
6
, in clinopyroxene and
a
NaAlSi
2
O
6
Cpx
·a
SiO
2
Qtz
/a
NaAlSi
3
O
8
Pl
NaAlSi
2
O
6
Cpx
Qtz
SiO
2
NaAlSi
3
O
8
Pl
0
albite, NaAlSi
3
O
8
, in plagioclase must then be evaluated.
(The activity of silica in quartz, a 1.) Although
the chemical compositions of the stably coexisting pla-
gioclase and clinopyroxene in the mineral assemblage
of a blueschist are readily and accurately determined
by electron microprobe analyses, determination of activ-
ities from measured compositions requires a suitable
model relationship. Several theoretical models are in
use (Spear, 1993, pp. 176–205) depending on the ex-
tent to which the constituent ions in specific crystallo-
graphic sites interact with one another in the natural
solid solution. Which model to apply depends on the
desired rigor and how well it fits independent infor-
mation obtained from experiments. Simply equating
activity with measured concentration generally yields
inaccurate results.
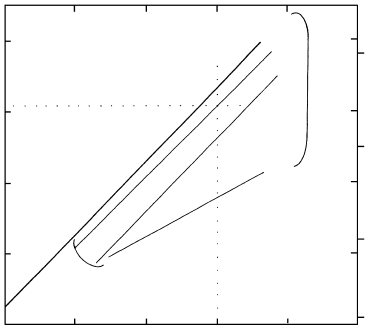
Results of experiments by Holland (1983) on the
equilibria plagioclase jadeitic clinopyroxene quartz
are summarized in Figure 16.10. For clinopyroxenes
with about 0.8 mole fraction of the jadeite end-member
that coexist with quartz the equilibrium P is depressed
about 1 kbar from that for pure jadeite. Decreasing P
significantly reduces the equilibrium concentration of
the jadeite end-member.
16.5.2 Continuous Exchange Reactions in Fe–Mg
Solid Solutions
We now deal with a more common type of continuous
reaction that involves Fe–Mg substitution in many mafic
rock-forming minerals. Similar continuous reactions
SiO
2
Qtz
Metamorphic Mineral Reactions and Equilibria
485
P (kbar)
Depth (km)
25
20
15
10
5
80
60
40
20
200 12001000800600400
T (°C)
Jadeite quartz
Albite
0.2
0 5
0 8
1 0
Clinopyroxene
quartz plagioclase
16.10 Clinopyroxene plagioclase quartz equilibria. Heavy line
is for the univariant discontinuous reaction involving the pure
end members (compare Figure 16.4). Light lines are for equi-
libria involving jadeite–diopside solid solutions (0.8, 0.5, and
0.2 mol fractions of jadeite) with coexisting quartz and sodic
plagioclase. Decreasing purity of jadeite corresponds to de-
creasing equilibrium P. At 800°C and 20.5 kbar, for example,
in an assemblage of quartz plagioclase clinopyroxene the
equilibrium composition of the latter phase would be
Jd
80
Di
20
. Redrawn from Holland (1983).
occur in micas, amphiboles, and feldspars where
there are substitutions among Na, K, and Ca. Clearly,
continuous reactions are of particular significance in
metamorphic rocks.
In contrast to the univariant polymorphic trans-
itions and discontinuous reactions that result in sharp
isograds in the field, continuous reactions occur over
broad geographic domains in metamorphic terranes
but an isograd line can nonetheless be positioned at the
first appearance of a solid solution, such as Fe–Mg–Ca
garnet. In compatibility diagrams, continuous reactions
cause continuously changing topologies manifest in
shifts and sweeps in tie-line orientations and configura-
tions that accompany expansions and contractions of
stability fields.
Because of differing crystal lattices, different miner-
als have different preferences for Fe and Mg. Con-
sequently, the mole fraction of the Mg end-member, or
the mole ratio Mg/(Mg Fe
2
) X
Mg
, will differ in
stably coexisting mafic minerals in a rock. In pelitic
rocks, five mafic minerals are common, including
garnet, staurolite, biotite, chlorite, and cordierite. The
relative partitioning of Fe and Mg is
16.15 X
Although X
Mg
is different for each stably coexsiting
mineral, the ratio by which Mg and Fe are partitioned
between any two minerals is essentially constant under
the same metamorphic conditions regardless of bulk
rock composition. Hence, garnet and biotite in Fe-rich
pelites will contain more Fe than these same phases in
Mg-rich rocks but the distribution ratio, or distribu-
tion coefficient, K
D
, is constant for a given P and T.
Mg
Grt
6 X
St
Mg
6 X
Bt
Mg
6 X
Chl
Mg
6 X
Mg
Crd
486 Igneous and Metamorphic Petrology
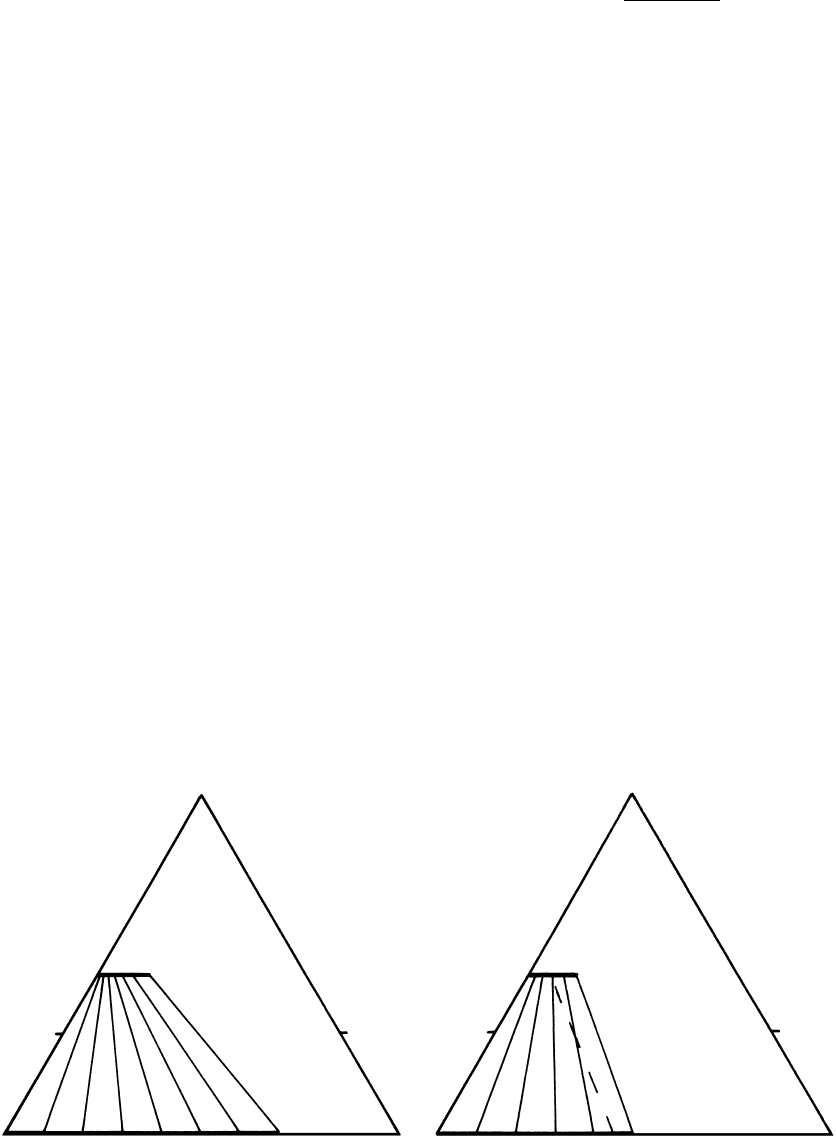
Biotite
FeO MgO
Garnet
K
D
0.14
500°C
Al
2
O
3
Biotite
FeO MgO
Garnet
K
D
0.30
800°C
Al
2
O
3
16.11 Contrast in the distribution coefficient, K
D
, for Fe and Mg between stably coexisting garnet and biotite at two temperatures depicted
in AFM projections (Section 15.3.3). In the 800°C diagram, a representative tie-line from the 500°C plot is shown as a dashed line to em-
phasize that crossing tie-lines imply contrasting conditions (temperatures) of equilibration of coexisting phases. Redrawn from Spear
(1993).
(Compare K
D
with the partition coefficient in magmatic
systems, Section 2.5.1). For the garnet–biotite pair
16.16 K
This distribution is displayed in Figure 16.11, where it
can be seen that for any Mg/Fe ratio in biotite the
ratio in a coexisting garnet is smaller so that K
1.
Continuous reactions in mafic phases are con-
veniently broken down into Fe and Mg end-members.
A reaction of this type that is of considerable signific-
ance in low-grade pelitic rocks of regional Barrovian
zones can be expressed for Fe end-members as
16.17 Fe-chlorite quartz muscovite
almandine annite water.
(It may be disconcerting that we are introducing a con-
tinuous reaction that also involves a fluid, which has
not been discussed yet. However, such continuous
solid–fluid reactions are very common and, for the pur-
poses of this section, the presence of water can simply
be ignored.) This reaction, which also happens to be of
the net transfer type, is univariant because there are
five components (K
2
O, FeO, Al
2
O
3
, SiO
2
, and H
2
O)
and six phases, F 2 5 6 1. With addition of
another component, MgO, to the reaction system, it
becomes divariant and the general continuous reaction
is written as
16.18 chlorite quartz muscovite
garnet biotite water.
D
Grt–Bt
D
Grt–Bt
(Mg/Fe)
Grt
(Mg/Fe)
Bt
