Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.

The differential partitioning of Mg and Fe among
the mafic phases vary over a range of P–T conditions
according to two exchange reactions
16.19 Fe
3
Al
2
Si
3
O
12
KMg
3
AlSi
3
O
10
(OH)
2
almandine phlogopite
Mg
3
Al
2
Si
3
O
12
KFe
3
AlSi
3
O
10
(OH)
2
pyrope annite
16.20 Fe
3
Al
2
Si
3
O
12
Mg
5
AlSi
3
AlO
10
(OH)
8
almandine Mg-chlorite
Mg
3
Al
2
Si
3
O
12
Fe
5
AlSi
3
AlO
10
(OH)
8
pyrope Fe-chlorite
In an exchange reaction, there is only an exchange of
substituting cations, in this case Fe and Mg, between
two phases, garnet and biotite or garnet and chlorite,
without any modification in the modal proportions of
the phases. Because the same phases are involved in
these two exchange reactions they are not very sensitive
to P but the distribution coefficient, K
D
, is strongly de-
pendent on T (Figure 16.11). Hence, the partitioning
of Fe and Mg between garnet and biotite provides a
useful mineral thermometer that is explored near the
end of this chapter in Section 16.11.2.
The divariance of the continuous reaction 16.18 is
readily seen in a T–X diagram (Figure 16.12), where
the similarity to continuous reaction relations and
incongruent melting in the plagioclase system is obvi-
ous (Section 5.5.2). In this pseudo-binary diagram (so
designated because more than two components are rep-
resented), the univariant, pure Fe end-member reaction
16.17 occurs at a specific T that is lower than that of the
Mg end-member reaction. This behavior is typical of
mafic metamorphic mineral systems. As the T of an
assemblage of quartz muscovite chlorite (X
Mg
0.3)
is raised in Figure 16.12 it begins to react at T T
1
,
forming an Fe-rich biotite and a more Fe-rich garnet
(plus some water). With increasing T, progressively
more biotite and garnet are created and these product
phases, together with the reacting chlorite, become
more enriched in the Mg end-member. As in the sys-
tem of plagioclase crystals and melt, to maintain a mass
balance, the modal proportions of reactant and prod-
uct phases change (according to the lever rule) as they
become more magnesian with increasing T. Also, as in
the plagioclase system, two contrasting paths of product
crystallization are possible.
1. In the equilibrium crystallization path, every phase
remains homogeneous and maintains its equilib-
rium composition at every T during heating. As-
suming there are sufficient muscovite and quartz
reactants, the reaction is complete when all of the
chlorite is entirely consumed and the final products
are a relatively magnesian biotite and a more Fe-
rich garnet at T
2
(plus possible excess muscovite
and quartz).
2. In the fractional crystallization path, because of the
slow rates of diffusion in garnet, even up to mid-
grade temperatures, divalent cations cannot main-
tain equilibrium concentrations and proportions.
Metamorphic Mineral Reactions and Equilibria
487
0
Alm Ann H
2
O
Fe-Chl Qtz Ms
KFASH
Mg
Mg Fe
Garnet biotite H
2
O
Prp Phl H
2
O
Mg-Chl Qtz Ms
KMASH
Grt Bt
Qtz Ms
ChlH
2
O
Chlorite quartz
muscovite
T
2
T
T
1
0.3 1
X
Mg
C
h
l
o
r
i
t
e
B
i
o
t
i
t
e
G
a
r
n
e
t
16.12 Schematic pseudobinary T–X diagram for the K
2
O–FeO–MgO–Al
2
O
3
–SiO
2
–H
2
O (KFMASH) system at some fixed P showing reaction
loops for garnet, biotite, and chlorite. These loops enclose the divariant region where the six-phase assemblage of garnet biotite
quartz muscovite chlorite water is stable. Mg/(Mg Fe) X
Mg
in mafic phases in the divariant assemblage varies as a function
of T. The assemblage garnet biotite water is stable above the garnet loop and chlorite quartz muscovite is stable below the
chlorite loop. End-member reactions in the pure Fe (KFASH) and Mg (KMASH) systems indicated on the sides of the diagram. Redrawn
from Spear (1993).
Consequently, garnets in all but the highest grades
of metamorphic rocks are typically zoned, in like
manner as plagioclases in magmatic rocks (as a re-
sult of the very slow rates of coupled diffusion of
SiAl). Fractional crystallization of garnet produced
by reaction 16.18 can be predicted to yield a zoning
in which Mg/(Mg Fe) increases outward from
core to rim. Other garnet-producing reactions can
also create this same type of zoning (see Figure
16.34). Diffusivities of components in biotite and
chlorite are sufficiently fast so that their composi-
tions adjust continuously to changing temperatures
and result in growth of essentially homogeneous
grains.
The continuous reaction 16.18 can also be repre-
sented in an AFM diagram (Figure 16.13; these pro-
jections are described in Section 15.3.3). At a lower T,
a reference pelitic rock (filled circle) consists of biotite
chlorite ( quartz muscovite). As T increases,
the continuous sweep of the three-phase compatibility
triangle for garnet biotite chlorite toward more
magnesian and more stable compositions eclipses the
rock, causing production of garnet. At still higher T,
stable garnets become more magnesian, as do coexist-
ing biotites and chlorites.
16.6 SOLID–FLUID MINERAL REACTIONS
H, C, O—mainly in the form of H
2
O and CO
2
—and
lesser amounts of Cl, F, and S are essential constituents
in the atomic structures of crystalline phases in all
kinds of metamorphic rocks (Table 14.1). Reactions
liberating and consuming volatiles are the most com-
mon in metamorphic systems.
16.6.1 Fluids in the Crust of the Earth
Before moving onto metamorphic mineral reactions
involving volatile fluids, it is appropriate here to put
crustal volatile fluids into a global perspective.
The role of fluids in the crust of the Earth and how
they interact with the overlying hydrosphere are far
from trivial, as expounded in the classic work of Fyfe
et al. (1978). Ramifications are many and profound.
The 65,000-km long system of oceanic spreading ridges
focuses advective circulation of cold seawater through
hot basaltic rock, recycling the entire mass of the
world’s oceans once every 5 My (Special Interest Box
11.3). Devolatilization of subducting, fluid-rich oceanic
crust leads to generation of arc magmas in the over-
lying mantle wedge, with further contributions from
dehydration melting reactions in the deep contin-
ental crust. Fluid flow in the continental crust is now
acknowledged to play fundamental roles in all kinds of
transfers of matter and heat, in metamorphic mineral
reactions, in creating ore deposits and geothermal
resources (Section 4.3.3 and Figure 4.12), and in rock
deformation (Section 8.2.1; see also Section 17.2.5).
The amount of water in the crust is impressive (Fyfe
et al., 1978). Because the average amount of water is
about 4% of the mass of the crust, which is 2.3
10
25
g, then the mass of water lodged in the crust is
0.92 10
24
g. But this is the same order of magnitude
as the mass of water in the oceans, 1.4 10
24
g!
Crustal fluids are of three types and sources:
488 Igneous and Metamorphic Petrology
Grt
Cld
F
Chl
M
Bt
A
Grt
Cld
Increasing T
Ms Qtz H
2
O
F
Chl
M
Bt
16.13 AFM projections (Section 15.3.3) projected from muscovite showing shift of the three-phase triangle representing compatible garnet
chlorite biotite toward more magnesian compositions with increasing T as a result of the continuous reaction 16.18. A reference pelitic
rock (filled circle) in the biotite zone at low T is eclipsed by the sweeping triangle, causing the appearance of garnet at higher T.

1. Water from the hydrosphere of the Earth that is
held in openings in rocks. In the continental crust,
cracks in rocks such as granite and pore spaces
between sediment grains hold meteoric (ground)
water, while in oceanic regions saline seawater re-
sides in fractured mafic rocks at spreading ridges
and in seafloor sediment.
2. Metamorphic fluids produced by devolatilizing
metamorphic reactions.
3. Magmatic fluids exsolved from evolving magmas
(Section 4.2.3). Common arc magmas of intermedi-
ate to silicic composition crystallizing biotite and
hornblende must have water concentrations of at
least 3–4 wt.% in order to stabilize these hydrous
phases. However, modal amounts of these hydrous
minerals are typically 30%; with 1–2 wt.% water
in hornblendes and 2–3 wt.% in biotites the excess
water in the magma system can be released into the
surroundings during crystallization.
Mixing from the three sources is likely in many crustal
fluids. Oxygen isotope ratios (Section 2.6 and Figure
2.24) can indicate dominant contributions.
During burial and compaction of marine sediment-
ary deposits along continental margins and in subduc-
tion zones, enormous volumes of entrapped aqueous
fluid are liberated. Sediments and loosely cemented
sedimentary rocks can have pore spaces occupying as
much as one-third the total rock volume to depths of
hundreds of feet but after burial to 5 km or so, 2
wt.% water remains in increasingly more isolated and
smaller pore spaces or perhaps as an intergranular fluid
along grain edges. In addition to compaction, pore vol-
ume diminishes as a consequence of recrystallization,
including grain boundary adjustments during pressure
solution (see Section 17.2.1), and precipitation of ce-
menting minerals from pore solutions. In rock bodies
with interconnecting openings (permeability) extend-
ing all the way to the surface, buoyant water will flow
upwards out of the body, commonly venting in springs.
After physical release of entrapped pore fluid, mostly
water, 5–10 wt.% H
2
O bound structurally in protolith
minerals can be driven out of rocks with rising T during
prograde dehydration reactions. These aqueous meta-
morphic fluids are released over virtually the whole
range of metamorphic conditions from widespread
hydrous solid solutions undergoing continuous break-
down (Figure 16.14; compare Table 14.2). In pelitic
rocks, for example, the generalized prograde reaction
sequence of clay minerals → chlorites → micas → an-
hydrous silicates consists entirely of solid solutions.
Walther and Orville (1982) calculated that the expelled
water (and smaller amounts of CO
2
) during devolatiliza-
tion of an average shale could instantaneously occupy
about 12% of the rock volume at 500°C and 5 kbar. To
put this in perspective, if all of the expelled water from
a 1000 km
3
block of dehydrated shale were to be col-
lected into a 100 km
2
lake it would be 1.25 km deep!
A similar situation prevails for metamorphism of
hydrous mafic rocks, where again, upwards of 5 wt.%
water can be liberated (Figure 16.14d). In the less
widespread calc–silicate rocks, decarbonation reactions
can potentially liberate vast amounts of CO
2
. On a
weight basis, there is 44 and 47% CO
2
in pure calcite
and dolomite, respectively. Most decarbonation reac-
tions are continuous over a range of P–T conditions
and the amount of liberated CO
2
depends on the fluid
composition and proportions of silicate- and carbonate-
bearing phases, as will be appreciated later. At lowest
metamorphic grades, methane (CH
4
) may also be ex-
pelled by reactions involving carbonaceous material in
shale and carbonate rock protoliths (Special Interest
Box 16.2).
Clearly, the amount of fluid in a metamorphic system
varies through time (Rubie, 1986). This is especially
true of polymetamorphic rocks. At a given depth and
P, the amount of fluid and its associated fluid pressure,
P
fluid
, is likely to change during the course of metamor-
phism. This means that P
fluid
does not always equal P,
as commonly assumed.
Virtually the only tangible, separate fluid phase re-
maining from metamorphic reactions in rocks collected
at the surface occurs as minute volumes entrapped in
fluid inclusions (Special Interest Box 16.3).
Metamorphic Mineral Reactions and Equilibria
489
Special Interest Box 16.2 Bleaching of
carbonate rocks around magmatic intrusions
In many contact metamorphic aureoles around
granitic intrusions, the most conspicuous change is
a bleaching of limestone or dolomite country rocks
whose original gray color is a result of very small
amounts of fine carbonaceous matter. The progres-
sive whitening of the protolith toward the magmatic
contact is generally not regular and concentric, but
is strongly influenced by bedding. Some relict beds
are more bleached than adjacent ones at the same
distance from the contact, suggesting a control re-
lated to bedding characterisitics such as permeabil-
ity rather than simple conductive heating. For the
Notch Peak aureole in western Utah, Todd (1990)
concluded that the bleaching agent was infiltrated
water that reacted with carbonaceous matter (mod-
eled as graphite, C) producing methane and carbon
dioxide according to 2C 2H
2
O CH
4
CO
2
.
The water-rich fluids cannot have come from
devolatilization reactions in the protolith because
they would have been CO
2
-rich. Isotopic data indic-
ate the infiltrating water that reacted with the rock
was of magmatic origin, not heated meteoric water.
Under most metamorphic conditions, above those
appropriate to the lower greenschist facies, mixtures
of CO
2
and H
2
O are completely miscible and form
a homogeneous supercritical fluid (Section 4.2.1).
However, dissolved salts (NaCl, KCl, and so on) ex-
tend a miscibility gap prevailing at low temperatures
to higher metamorphic temperatures (Labotka, 1991).
Immiscibility complicates phase equilibria but will be
ignored in this chapter. At high T and low P, say,
600°C and 2 kbar, CO
2
and H
2
O mix almost
ideally so that the proportion of one volatile component
in a mixture of the two can generally be represented
by its mole fraction, X (Section 3.5.1). Thus, under
ideal conditions for H
2
O in a fluid, X P /P
fluidH
2
OH
2
O
490 Igneous and Metamorphic Petrology
P (kbar)
Depth (km)
8
6
4
2
0
(a)
25
20
15
10
5
0
0 400200 600 800 1000
T (°C)
Ky
Sil
30°C/km
Prl
S
i
l
A
n
d
K
l
n
4
Q
t
z
P
r
l
2
H
2
O
Wt. % H
2
O
8
6
4
2
0
01020
Depth (km)
Rock Fluid
2Ky 6Qtz
2Sil 6Qtz
Kln 4Qtz
Prl
T (°C)
200
400
(b)
2
K
y
6
Q
t
z
4
H
2
O
Wt. % H
2
O
6
4
2
0
(c)
01020
1000
Depth (km)
200 400 600
T (°C)
Pelitic
rock
Clays
Chlorite–mica
Mica–garnet
Staurolite–garnet-mica
Sillimanite–K-feldspar
Migmatite
Partial
melts
Fluid
Wt. % H
2
O
6
4
2
0
(d)
0 102030
1000
Depth (km)
200 400 600
T (°C)
Mafic
rock
Partial
melts
Fluid
Zeolites
Prehnite–pumpellyite
Chlorite–epidote–
actinolite–albite
Hornblende–plagioclase
Hornblende–plagioclase–
pyroxene
Pyroxene–plagioclase
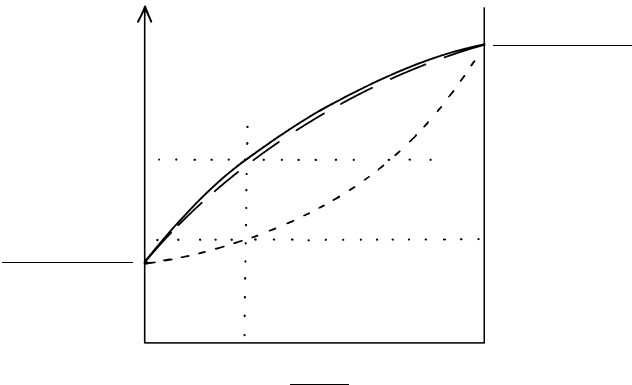
16.14 Water-release curves for hydrous mineral assemblages subjected to prograde dehydration reactions. Redrawn from Fyfe et al. (1978).
(a) Stability fields and univariant breakdown curves for some low-grade hydrous aluminosilicates. Note the metastability of andalusite
and kyanite at temperatures below about 400°C where kaolinite and pyrophyllite are more stable in systems that contain sufficient
water (a
water
1). (b) Steplike fluid-release line (heavy line) for a model pelitic rock that consists of kaolinite 4 quartz along a 30°C/km
geotherm from (a). This assemblage contains 9.5 wt.% water bound structurally in kaolinite. At a depth of about 12 km, T reaches
370°C where this assemblage reacts to produce pyrophyllite 2H
2
O. On a wt.% basis this liberated water is 4.5 wt.%; 5 wt.%
remains structurally bound in pyrophyllite. With increasing T, pyrophyllite breaks down at 425°C to 2 kyanite 6 quartz 4 H
2
O.
All of the water has now been released from solid phases. At 550°C, kyanite should convert to sillimanite. Because kaolinite and
pyrophyllite are here considered to be pure phases with no solid solution, release of water occurs sharply at their univariant dehydration
curves. (c) Generalized fluid-release curve for a shale along a 30°C/km geotherm. Water release is continuous with increasing P and T
because of the complex crystalline solid solutions constituting pelitic rocks that progressively break down by continuous reactions.
Changes in slope of the release curve are related to beginning and completion of dehydration of major hydrous silicates. Depending upon
the exact composition of the system, especially the remaining water concentration, partial melting may begin somewhere near 700°C to
form migmatite. (d) Generalized fluid-release curve for mafic rock along a 30°C/km geotherm, assuming rock is initially dry and then hy-
drates to a zeolite facies assemblage with 5 wt.% water. (On the seafloor palagonitization could produce a more hydrous rock at lower
T.) Note temperatures for partial melting and migmatite formation.

P /(P P ), where P and P are
partial pressures. At lower T and higher P, volatile
mixtures are nonideal. For thermodynamic calculations,
this nonideality requires use of fluid activities or fugac-
ities (Spear, 1993, p. 228).
Among natural fluids, water has unusually high
solvent capacity, especially for ionic compounds such
as NaCl, because of the strongly polar nature of its
molecule and lone-pair electrons (e.g. Brimhall and
Crerar, 1987).
The separate volatile phase in metamorphic systems
has been referred to as a vapor, gas, liquid, fluid, and
supercritical fluid. However, because of the low P and
T of the critical points of H
2
O and CO
2
, above which
there is no sharp distinction between gas (vapor) and
liquid, a separate phase of these volatiles can simply
be called a fluid (Section 4.2.1). In most metamorphic
systems the density, or specific volume, of the fluid
varies continuously as a function of T and P. But as
both increase with increasing depth, the fluid expan-
sion resulting from increasing T is nearly compensated
by compression from increasing P. For example, along
a geothermal gradient of 15°C/km to a depth of 35 km
the specific volume of water deviates only slightly
from its familiar value at atmospheric conditions of
1.0 cm
3
/g (Figure 4.3). For carbon dioxide along the
same geotherm the specific volume ranges between
about 0.8 and 1.2 cm
3
/g (Spear, 1993, p. 229).
16.6.2 Fundamental Concepts of Solid–Fluid Reactions
The general form of a prograde, volatile-liberating re-
action is
16.21 Heat volatile-bearing crystalline phase
→ volatile-poor (or -free) crystalline phase
volatile fluid
Hence, most devolatilization reactions are endothermic;
they consume heat, “dry out” mineral assemblages, and
moderate otherwise rising temperatures during meta-
morphic heating. In the reverse sense, hydration and
carbonation reactions are exothermic. Conversion of
volatile-free rocks, such as dry basalt, into volatile-
bearing greenschist or zeolite facies assemblages pro-
duces heat; temperatures can increase several tens of
degrees if all the heat in the rock is conserved. Provid-
ing CO
2
and H
2
O are continuously available, exother-
mic reactions might be thermally self-accelerating and
cause an initially volatile-free magmatic protolith to
move rapidly through the lowest metamorphic grades.
Entropy–Molar Volume Relations. Under most meta-
morphic conditions, a volatile phase has a large molar
entropy compared to the partial molar entropy of the
same volatile structurally bound in a hydrous silicate or
carbonate mineral. Consequently, the separate volatile
CO
2
H
2
OCO
2
H
2
OH
2
O
Metamorphic Mineral Reactions and Equilibria
491
Special Interest Box 16.3 Fluid inclusions
Metamorphic minerals, typically quartz, commonly
contain minute fluid inclusions (Crawford and
Hollister, 1986). They originate as more or less
randomly distributed pockets of fluid entrapped
during growth of the host grain (primary inclu-
sions) or as healed fluid-filled microcracks formed
after the host grain crystallized (secondary inclu-
sions; Figure 16.25). Inclusions are generally
0.03–0.05 mm in diameter but larger ones can
be found in veins formed from fluid precipitates
in rock fractures. Vein quartz in metamorphic
terranes is typically snow-white because of an
estimated 10
9
fluid inclusions per cm
3
(Roedder,
1984, p. 2). Inclusions can provide important
insights into the composition of the fluid present
when the host mineral grain formed and P–T con-
ditions of crystallization.
In addition to a homogeneous fluid, or locally
two immiscible fluids, inclusions also may contain
a bubble of low-density vapor and one or more
crystalline daughter phases (usually halite, but also
sylvite, calcite, and various fluorides) that appar-
ently precipitated as the fluid cooled from high
temperatures.
Laser-excited Raman microspectroscopy dis-
criminates among fluids of SO
4
, CH
4
, CO
2
, and
H
2
O within inclusions. More indirect information
is provided by observations on a heating–freezing
stage. Pure H
2
O fluid freezes at 0°C but dissolved
salts depress the freezing T, to as much as 23°C
at the NaCl–H
2
O eutectic. Identifiable daughter
crystal may help constrain the fluid composition.
Pure CO
2
freezes at 56.6°C, but mixed with H
2
O
raises the freezing T towards 0°C while mixtures
with CH
4
(methane) lower it towards about
184°C.
Distinguishing inclusions formed during pro-
grade and particularly peak metamorphic condi-
tions from secondary inclusions formed during
cracking accompanying uplift and cooling of a
metamorphic terrane is not easy. Moreover, inclu-
sions can be perturbed in many ways after initial
entrapment, such as by leakage and reaction with
the host crystal. Caution must, therefore, be taken
in the interpretation of P–T–X data based on fluid
inclusions.
Inclusion studies reveal that fluids in low-grade
metamorphic rocks are dominantly methane, pre-
sumably derived from carbonaceous material,
those in lower greenschist- to middle amphibolite-
facies rocks fluids are essentially aqueous, while in
granulite-facies rocks CO
2
dominates (Spear, 1993,
p. 669).
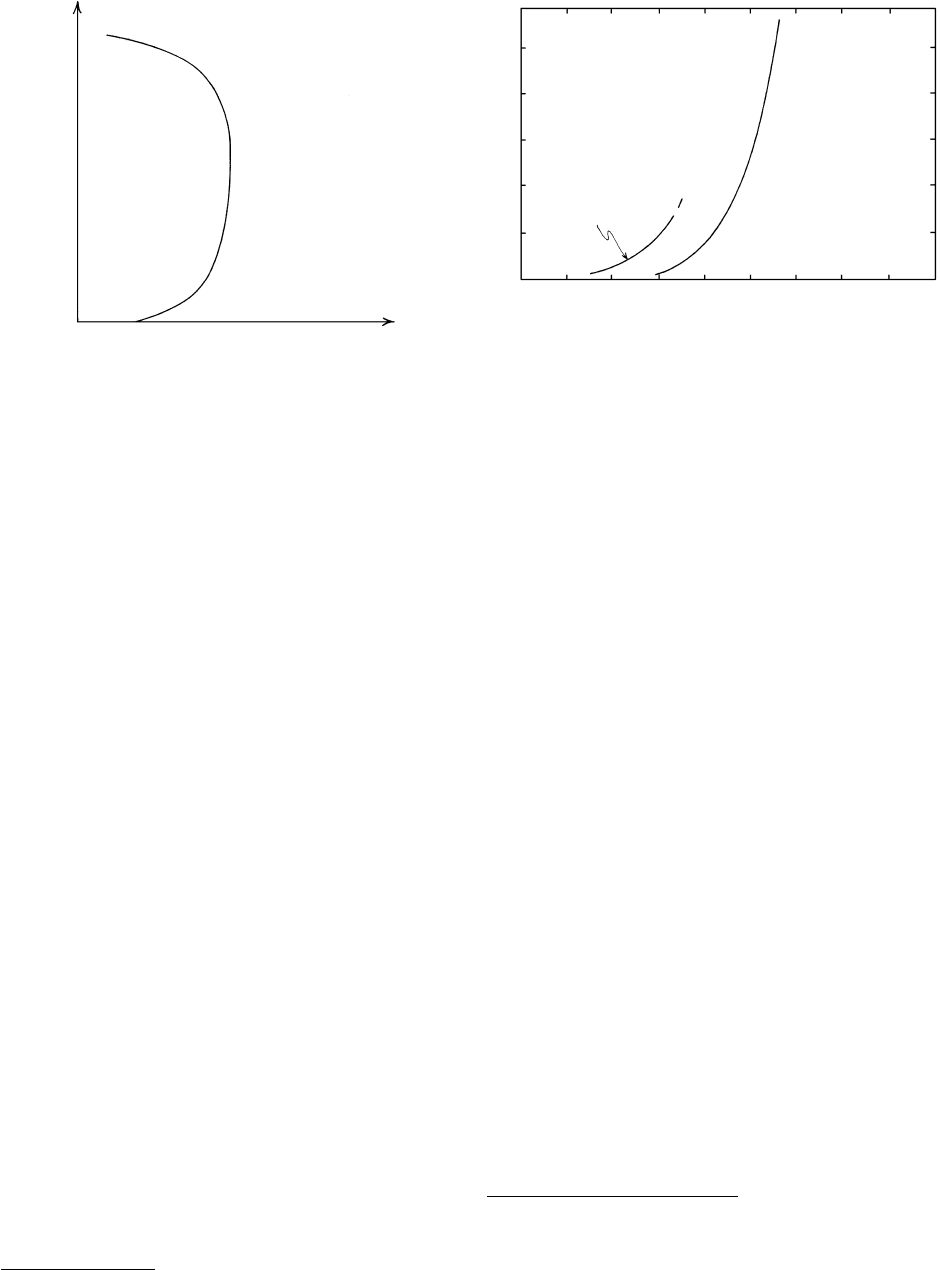
fluid phase is almost always on the higher T side of the
reaction because for that assemblage to be stabilized
with lower free energy, there must be a positive T and
a positive S so that G (ST) is negative.
All mineral equilibria involving a volatile phase are
governed by the volatile partial pressure, P
volatile
, as
well as P and T. In systems where P
volatile
P, the equi-
librium pressure increases with increasing T so that the
reaction line in P–T space has a positive slope (Figure
16.15; see also Figure 5.31). Unlike the straight or
nearly straight equilibrium line for solid-solid reactions
(Figure 16.4), the univariant equilibrium line in P–T
space for solid-fluid net-transfer reactions (16.21) is
markedly curved. The changing slope is governed by
the relative magnitude of changes in molar volume and
entropy expressed in the Clapeyron equation, dP/dT
S/V. In metamorphic systems where P 2–3 kbars
but T is high, as in contact aureoles around shallow
crustal magmatic intrusions, the univariant reaction
curve typically has a low positive slope. Relative to the
S of the reaction, V is large because molar volumes
of volatile phases are large (see Figure 4.3 for water).
But as V for reaction 16.21 diminishes with increas-
ing P because of the greater compressibility of fluids
than solids (Section 8.3.1), the slope of the reaction
curve steepens. Steep devolatilization reaction curves
that typify many regional metamorphic systems indic-
ate a stronger dependency on T than on P. Ultimately,
increasing P may sufficiently compress the fluid so that
V 0. As S is still positive, the reaction curve bends
back on itself with negative dP/dT slope. Depending
on the specific reaction, this bendback can occur in the
continental crust or the upper mantle.
Coupled Reactions. Like solid–solid reactions (Figure
16.4), the maximum thermal stability of a volatile-bearing
mineral is always that of the mineral alone. In the
presence of other reactable phases the stability field
shrinks. An important example of this concept is found
in the stability of calcite. Pure calcite breaks down
according to the reaction
16.22 CaCO
3
CaO CO
2
calcite lime
at temperatures in excess of 1200°C, which is almost
invariably greater than that attained during meta-
morphism. Hence, calcite alone, as in pure marbles, is
stable throughout the realm of metamorphism and lime
does not occur naturally as a mineral. However, calcite
commonly coexists with quartz in sandy limestones,
allowing the coupled reaction (Figure 16.16)
16.23 CaCO
3
SiO
2
CaSiO
3
CO
2
calcite quartz wollastonite
The free energy change at, say, 600°C and 1 atm for
this reaction is 54,000 J/mol in contrast to 33,000
J/mol for reaction 16.22, the breakdown of pure calcite
(Robie and Hemingway, 1995). Hence, wollastonite
CO
2
is stable at 600°C, whereas lime CO
2
is not. The
coupled reaction with quartz substantially lowers the T
of the breakdown of calcite. In the presence of another
phase, such as rutile, in addition to quartz, calcite
breaks down at still lower T.
Similar depression of the T of a dehydration reac-
tion occurs in the presence of an additional phase in
silicate systems, such as where quartz can react in
coupled manner with muscovite (Figure 14.31).
Equilibria Where P
volatile
P. In systems where the
volatile partial pressure is less than the confining P the
equilibria can also be shifted to lower T. This follows
from Le Chatelier’s principle: A reduction, at some
fixed P and T, in the amount of water in, for example,
the muscovite quartz K-feldspar Al
2
SiO
5
H
2
O equilibrium causes a shift to the right to produce
492 Igneous and Metamorphic Petrology
P
T
Volatile-poor or
volatile-free
mineral assemblage
volatile fluid
∆V 0
∆V 0
∆V 0
Volatile-bearing
mineral
or
mineral
assemblage
16.15 Idealized univariant devolatilization curve for reaction 16.21.
P P
fluid
P
CO
2
(kbar)
Calcite quartz
rutile
titanite CO
2
3
2
1
0
200 400 600 800 1000
T (°C)
W
o
l
l
a
s
t
o
n
i
t
e
C
O
2
C
a
l
c
i
t
e
q
u
a
r
t
z
16.16 Univariant decarbonation curves for calcite-bearing assem-
blages where the fluid is pure CO
2
(X 1) and P
fluid
P.
Redrawn from Greenwood (1976).
CO
2
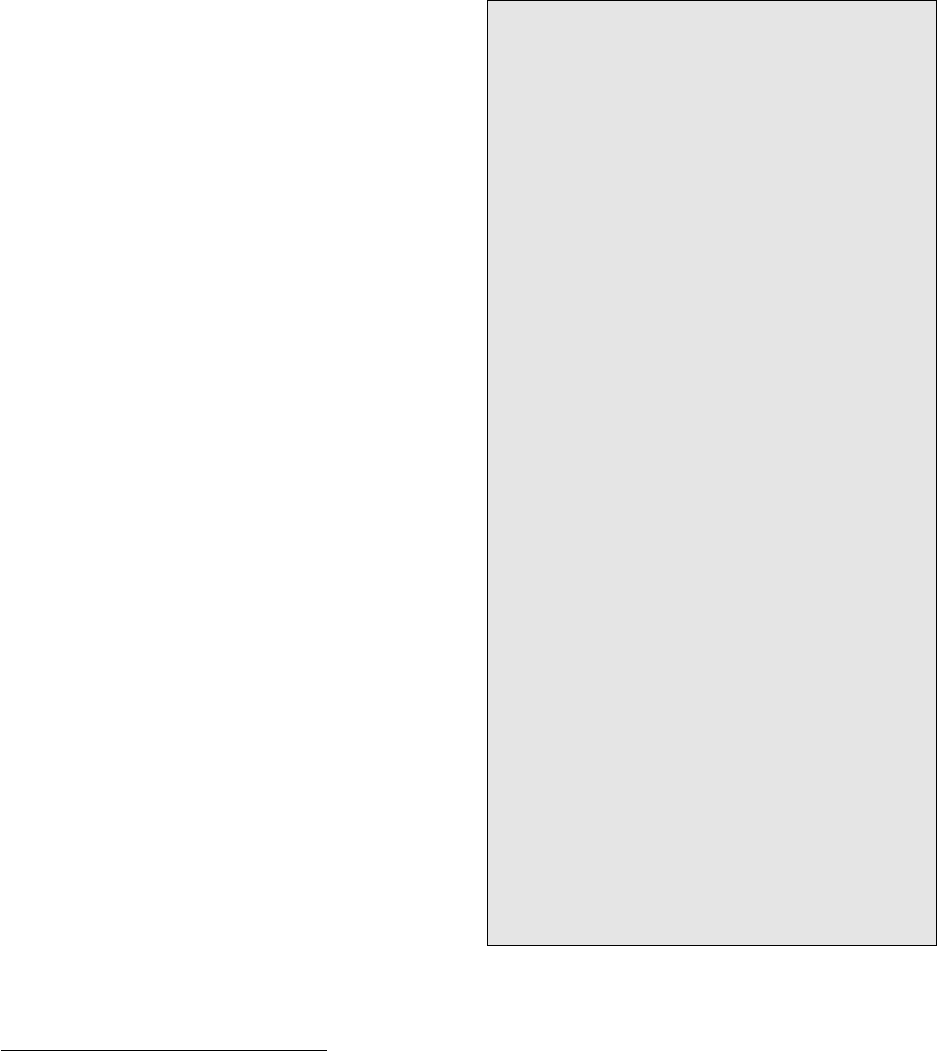
more water ( K-feldspar Al
2
SiO
5
) to compensate
for the reduction. Consequently, the univariant equilib-
rium line in P–T space shifts to lower temperatures
(Figure 14.31).
Situations where P
volatile
P can arise in two ways.
First, there may be little or no separate water phase
in the rock—it is “dried out.” Second, the total fluid
pressure may be the same as the confining pressure
(P
fluid
P) but because of the presence of an additional
volatile, such as CO
2
, the partial pressure of water,
P , is reduced. P
fluid
P P . In terms of con-
centration, there is a dilution of water by the additional
volatile. In terms of the activity, a 1. This situation
might arise where CO
2
liberated from decarbonation
reactions in nearby calc–silicate rocks invades pelites
undergoing dehydration reactions. These mixed-volatile
fluids are of paramount significance in metamorphic
reactions, warranting the following section.
16.6.3 Equilibria with Mixed-Volatile Fluids
The fact that wollastonite is common in contact meta-
morphosed sandy limestones near magmatic intrusions
but is rare in similar protoliths in regional terranes
could be explained by the stabilization of wollastonite
at the lower pressures (Figure 16.16) that prevail in
many contact metamorphic aureoles. However, it turns
out that there is another explanation that also has a
bearing on paradoxes in devolatilization reactions
noted by early workers (Special Interest Box 16.4). It
turns out that the composition of the volatile fluid is
an explicit governing variable in mineral equilibria, in
addition to P and T. Fundamental concepts regarding
the influence of fluid composition in mixed-volatile
fluid equilibria were established by H. J. Greenwood
(see 1976). Kerrick (1974) and Spear (1993) also review
the thermodynamics.
Although calc–silicate rocks are not as widespread
in metamorphic terranes as mafic and pelitic rocks,
mixed-fluid mineral reactions in them provide signific-
ant insights into the advective flow of volatile fluids
during metamorphism and serve as monitors of the
volume of transported fluid.
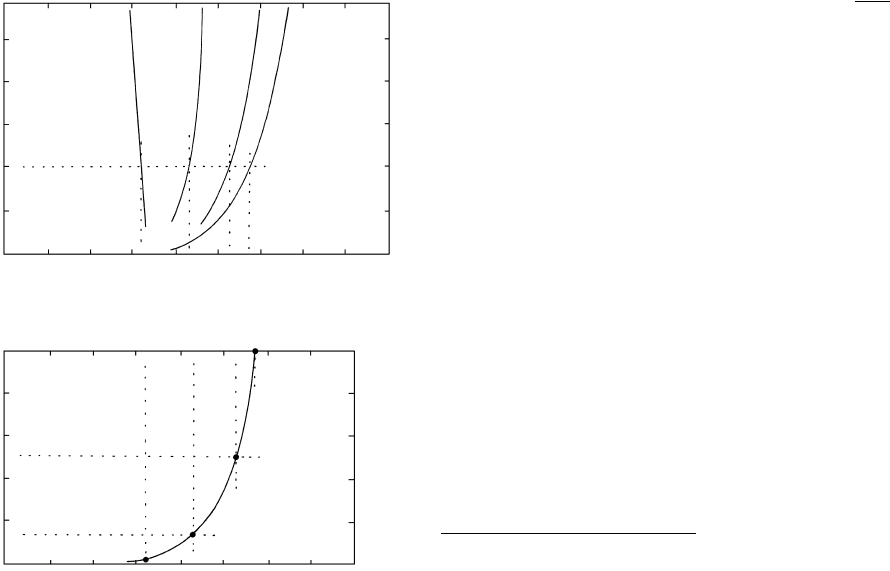
Calcite–Quartz–Wollastonite Equilibria. In a closed
CaO–SiO
2
–CO
2
system consisting of the univariant as-
semblage calcite quartz wollastonite the equilib-
rium CO
2
pressure increases as T increases, creating a
positively sloping reaction curve. If for any reason the
amount of CO
2
is perturbed to be less than this equi-
librium value at a particular T, in other words, P
P
fluid
or X 1, more calcite will tend to liberate
CO
2
by reacting with quartz, shifting the equilibria in
favor of wollastonite. Accordingly, the univariant reac-
tion curve is shifted to lower temperatures in Figure
16.17a. Hence, as a general rule, where the partial pres-
sure or mole fraction of a particular volatile species is
CO
2
CO
2
H
2
O
CO
2
H
2
OH
2
O
P
fluid
or 1, respectively, in a mixed-volatile fluid
phase the stability field of a phase that contains the
volatile shrinks to lower T.
An alternative way of viewing such equilibria stems
from the concept of a perfectly mobile component.
This is an ideal fluid, including possible dissolved ions,
such as Na
and Cl
, that can move freely into and out
of an open rock as a result of its high permeability. Any
reaction requiring the perfectly mobile components
can be freely accomplished. The thermodynamic prop-
erties of these mobile components, such as their chem-
ical potentials, , are determined in some unlimited
reservoir external to the rock. In the inventory of com-
ponents in the rock system, mobile components are
Metamorphic Mineral Reactions and Equilibria
493
Special Interest Box 16.4 N. L. Bowen,
pioneer metamorphic petrologist
Special Interest Box 5.1 indicated that N. L. Bowen
is generally considered to be the founder of modern
igneous petrology. Although his work in metamor-
phic petrology was much more limited, consisting
of essentially only two journal articles, in one of
these Bowen (1940) laid some of the groundwork
for modern metamorphic petrology. A product of
his brief tenure at the University of Chicago, this
one article was written “with the purpose of pre-
senting to students as a logical whole, with a con-
necting thread of fundamental law, a subject that
otherwise is bare” (p. 227). With typical thorough-
ness and clarity, Bowen not only described the
nature of mineral assemblages produced by pro-
grade metamorphism of calc–silicate rocks but in so
doing established the use of the now-standard (1)
graphical techniques for depiction of mineral assem-
blages and bulk rock compositions in triangular
diagrams and (2) petrogenetic grids wherein phase
assemblages are linked to intensive variables (P and
T). Bowen also recognized a conundrum in the
metamorphism of siliceous dolostones. In some
metamorphic terranes periclase appears at an appar-
ent lower grade than wollastonite, whereas in
others the appearances are reversed. He also noted
that dolomite quartz in sandy dolomite rocks
should react to tremolite calcite but subsequent
petrologists found that the products of this reaction
are talc calcite or diopside (Ferry, 1994). These
inconsistencies were resolved in the late 1960s and
1970s as experimental work and thermodynamic
calculations (see Greenwood, 1976) revealed that
mineral equilibria are governed by the composition
of C–O–H fluids, in addition to P and T that were
deemed the sole relevant intensive variables by
Bowen.
ignored. In a system of CaO and SiO
2
where CO
2
is a
perfectly mobile component, equilibria are dictated by
the univariant line labeled X 1 in Figure 16.17a.
However, if CO
2
is not perfectly mobile and its amount
in a rock is restricted then during a retrograde process
of decreasing T from, say, 800°C and 1 kbar, there may
be insufficient CO
2
to react with all of the wollastonite
in the rock below this univariant line; that is, the excess
wollastonite remains stable at declining temperatures,
together with some calcite and quartz. As a general
rule, then, in a system where there is insufficient of a
volatile to react with some volatile-free solid phase, the
unreacted excess of that solid remains stable into the
P–T field where the volatile-bearing product assemblage
would otherwise be stable. Implications for widespread
anhydrous magmatic rock protoliths undergoing meta-
morphism under water-deficient conditions should be
clear.
In systems involving a volatile fluid phase, such as
CO
2
, equation 16.3 takes the form
CO
2
16.24
where the V
s
term refers only to the solid phases in an
equilibria. f is the fugacity of CO
2
(Section 3.5.1)
and the last term in the expression takes into account
the contribution of the fluid phase to changes in free
energy. Philpotts (1990, p. 335) discusses application of
this expression to calcite–quartz–wollastonite equilib-
ria and indicates how f can be related to the mole
fraction X in Figure 16.17a.
The dependence of the calcite–quartz–wollastonite
equilibria on fluid composition (X or X ) and T
at fixed P 1 kbar is shown in Figure 16.17b. The
maximum thermal stability of calcite ( quartz) occurs
in systems in which the fluid is pure CO
2
, providing an
optimum condition where the volatile can be lodged in
calcite. At low X where the fluid is practically pure
water, calcite is stable only at relatively low tempera-
tures. Because carbonates are not stable in systems
where X 0, the equilibrium curve never reaches
this value, only becomes asymptotic to it.
Other Mixed-Volatile Reactions. A generalized mixed-
volatile reaction can be written as
16.25 A B
where A and B are solid phases and n and n are
the amounts, or stoichiometric coefficients, of H
2
O
and CO
2
. These coefficients are positive if they are on
the right-hand side of the reaction as products or neg-
ative if they are on the left-hand side as reactants. n
and n can have opposite sign.
Six types of reactions based on reaction 16.25 are
listed below as prograde reactions with increasing T
and entropy. Corresponding univariant reaction curves
are shown in T–X
fluid
space in Figure 16.18.
1. Solid–solid reaction, such as grossular quartz
wollastonite anorthite (compare Figure 16.4).
Because none of the solid phases contain volatiles,
the equilibrium T is not governed by fluid composi-
tion and the univariant reaction line is a straight
isotherm.
2. Decarbonation reaction, such as calcite quartz
wollastonite CO
2
(reaction 16.23 and Figure
16.17). The equilibrium T for the reaction is high-
est where X 1 and decreases as the fluid in the
system is diluted by increasing H
2
O.
3. Dehydration reaction, such as muscovite quartz
alkali feldspar Al
2
SiO
5
H
2
O (reaction 14.1
and Figure 14.31). The equilibrium T for the reac-
tion is highest where X 1 and decreases as the
fluid in the system is diluted by increasing CO
2
.
This is a mirror image of type 2.
4. Reaction liberates both H
2
O and CO
2
, such as
tremolite 3 calcite 2 quartz 5 diopside
H
2
O
CO
2
CO
2
H
2
O
CO
2
H
2
O
n
H
2
O
H
2
O n
CO
2
CO
2
CO
2
CO
2
H
2
OCO
2
CO
2
CO
2
CO
2
G
T
ref
,P
ref
V
s
(P
eq
P
ref
) RT ln
f
CO
2
P
ref
494 Igneous and Metamorphic Petrology
X
CO
2
X
H
2
O
1.0
0.8
0.6
0.4
0.2
0.0
(b)
(a)
0.0
0.2
0.4
0.6
0.8
1.0
400200 600 800
T (°C)
P
fluid
(kbar)
Calcite
quartz
fluid
Calcite
quartz
fluid
P
fluid
1 kbar
(P
CO
2
1 atm)
X
CO
2
1 0
~0 0
0 13
0 5
Wollastonite
fluid
Wollastonite
fluid
3
2
1
0
200 400 600 800 1000
T (°C)
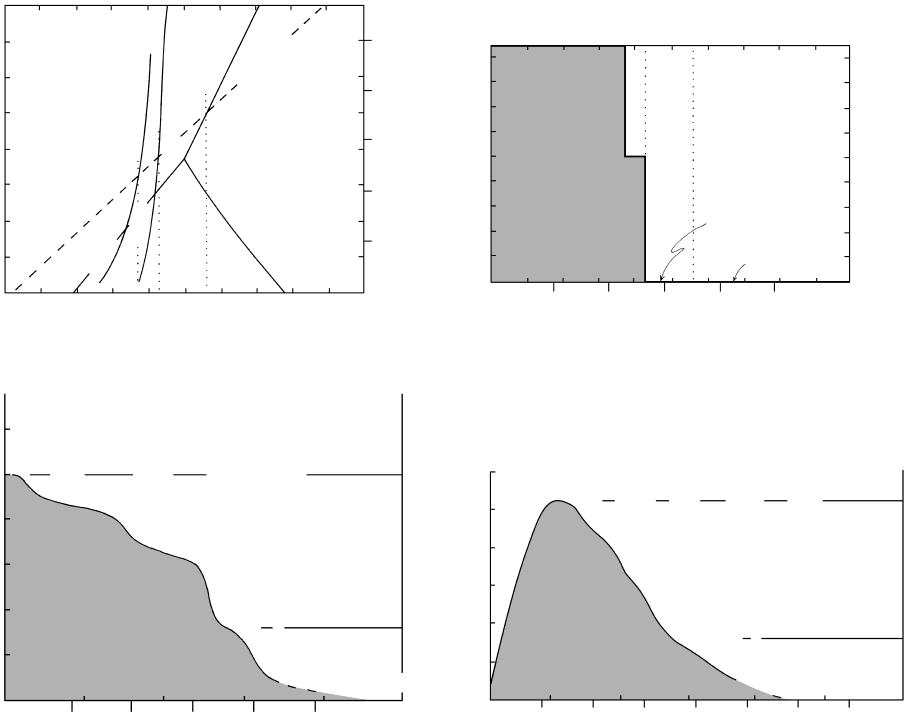
16.17 Univariant decarbonation curves for reaction 16.23 as a func-
tion of composition of the fluid. Compare Figure 16.16.
Dotted lines show correspondence between specific values
of T, P, and composition in the two diagrams. (a) P
fluid
P P and X X 1. (b) Reaction curve in
T–X (X ) space at P
fluid
1 kbar. The four data points
defining the curve are taken from (a). From experimental
work of H. J. Greenwood.
H
2
OCO
2
H
2
OCO
2
CO
2
H
2
O
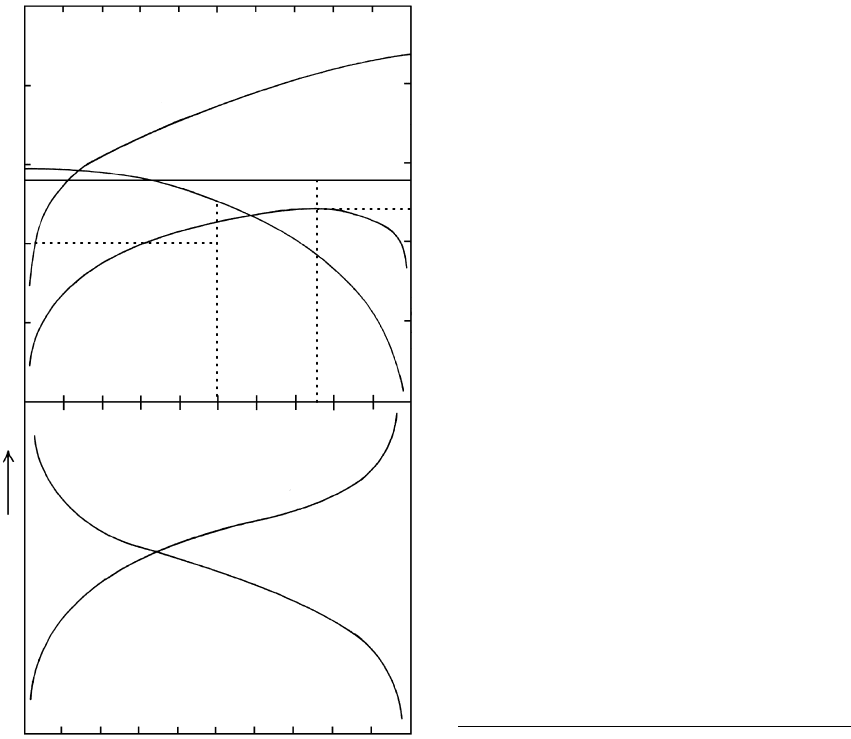
H
2
O 3CO
2
. The univariant reaction curve has a
maximum T at a fluid composition equivalent to
that liberated in the reaction (one mole of H
2
O
for every 3 moles of CO
2
) and diminishes in T
for increasing concentrations of H
2
O and CO
2
.
The peak T lies at X n /(n n )
3/(1 3) 0.75. The curve cannot intersect either
the pure CO
2
axis or H
2
O axis because that would
mean that a hydrous mineral is stable in a pure CO
2
fluid and vice versa, which is impossible.
CO
2
H
2
OCO
2
CO
2
5. Reaction consumes CO
2
and liberates H
2
O, such
as 2 zoisite CO
2
3 anorthite calcite H
2
O.
6. Reaction consumes H
2
O and liberates CO
2
, such as
6 dolomite 8 quartz 2 H
2
O talc 6 calcite
6 CO
2
.
Reaction curves 5 and 6 are mirror images of one
another and both have substantially decreasing tem-
peratures as the concentration of the product volatile is
decreased and the reactant volatile increased. In another
sense, the T of reaction is decreased if the ambient fluid
in the rock differs from that given off by the reaction.
16.6.4 Local versus External Control of Fluid
Composition during Devolatilization Reactions
The CO
2
/H
2
O ratio in pore fluids can vary in space
and time during the course of metamorphism depend-
ing upon the mobility and amount of the fluid, rock
permeability, the nature of reactions, especially their kin-
etic rates, and the assemblage of reacting solid phases.
This variability introduces additional complexity into
mixed-volatile reactions as just described. Two ideal
end-member processes can be envisaged—closed ver-
sus open system behavior. These can also be described
as locally internally buffered versus externally controlled
infiltration reactions. These contrasting processes are
described using as a model the isobaric univariant re-
action curve in Figure 16.19 for tremolite 3 calcite
2 quartz 5 diopside H
2
O 3CO
2
. The three re-
actant phases are found in some low-grade calc–silicate
rocks.
Internally Buffered Devolatilization Reactions. In a
closed rock system suppose there is only a very small
amount of an ambient fluid possibly entrapped in iso-
lated pore spaces and whose composition is X
X 0.5. Because permeability is nil and essentially
no fluid leaves or enters, the fluid is not perfectly
mobile. As the rock is heated from, say, 400°C in Fig-
ure 16.19a nothing happens until the vertical isopleth
intersects the univariant curve at about 525°C. Here,
or actually overstepping this equilibrium value by some
amount to promote the reaction, diopside begins to
crystallize by reaction between tremolite, calcite and
quartz. As T continues to rise, the closed system is con-
strained to move along the univariant curve so long as
these four solids coexist and because the composition
of the liberated fluid becomes more enriched in CO
2
;
three times as much of it is evolved from the reaction
as is H
2
O, easily changing the composition of the small
volume of initial ambient fluid. In this isobaric univari-
ant equilibrium, the four stably coexisting solids in
concert with the one independent variable of T buffers
the fluid composition. Should one or more of the reac-
tant phases—tremolite, calcite and quartz—be entirely
consumed at any T, the rock system will leave the
curve, lose its buffering capability, and resume a vertical
CO
2
H
2
O
Metamorphic Mineral Reactions and Equilibria
495
T (°C)
T
800
700
600
500
400
0.0 0.2 0.4 0.6 0.8 1.0
1.0
(a)
P 2 kbar
(b)
0.8 0.6 0.4 0.2 0.0
X
CO
2
X
H
2
O
W
o
C
O
2
5
D
i
3
C
O
2
H
2
O
T
r
3
C
a
l
2
Q
t
z
K
f
s
A
l
2
S
i
O
5
H
2
O
T
l
c
6
C
a
l
6
C
O
2
3
A
n
C
a
l
H
2
O
2
Z
o
C
O
2
6
D
o
l
8
Q
t
z
2
H
2
O
M
s
Q
t
z
C
a
l
Q
t
z
2Wo An
Grs Qtz
16.18 Petrogenetic grid showing univariant curves for select mixed-
volatile fluid reactions in T–X (X ) space. Note the
change in coordinate axes from Figure 16.17b so that here, by
convention, the T is represented on the y-axis and X on the
x-axis. The Cal Qtz Wo CO
2
curve is common to both
and can be compared in the two figures. Each univariant
reaction curve is drawn for its own unique compositional
system. Fluid is pure water on the left-hand vertical axis and
pure CO
2
on the right-hand axis. Curves are schematic in (b).
Redrawn from Kerrick (1974) and Blatt and Tracy (1996).
H
2
OCO
2
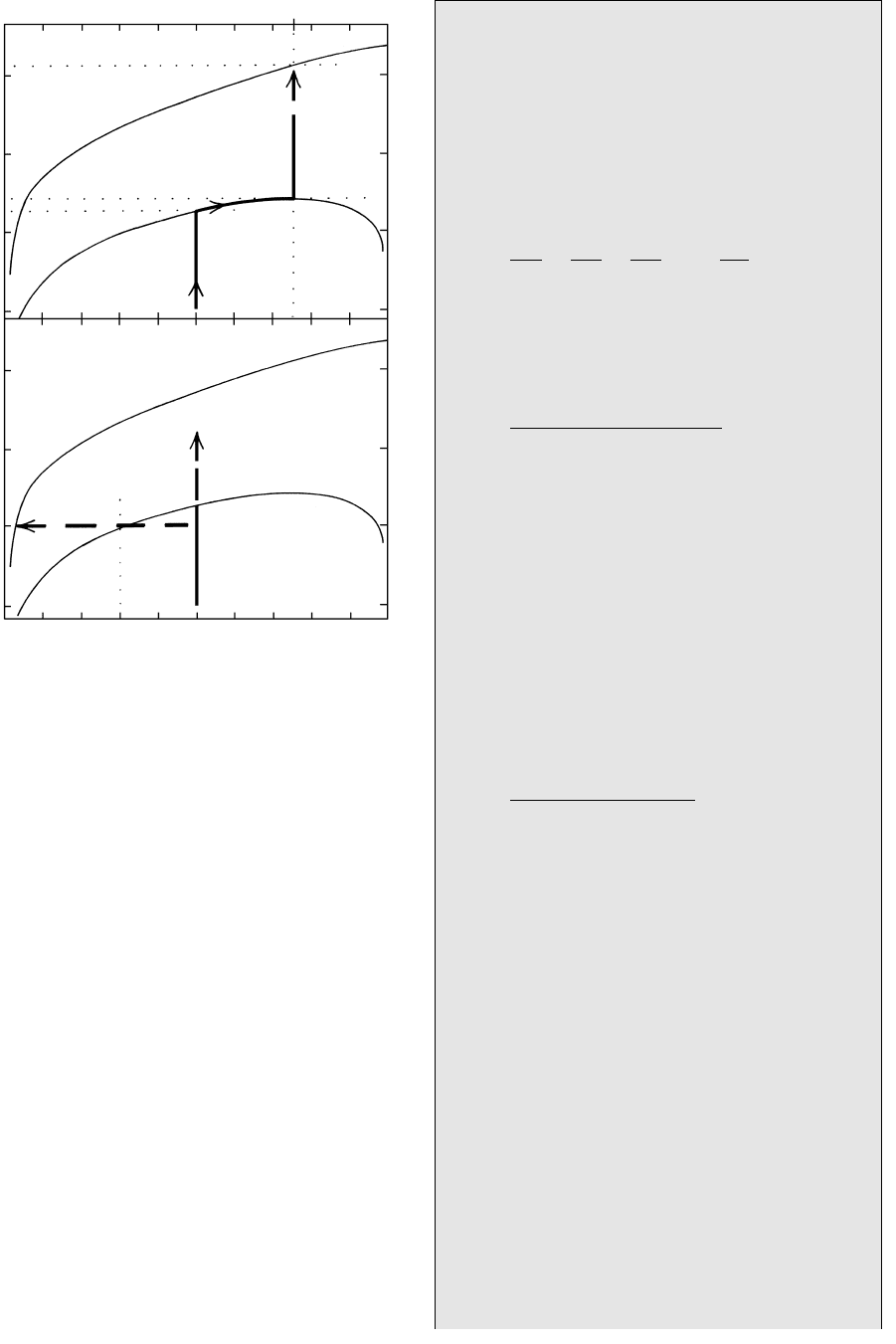
trajectory. However, if none of the reactant phases is
completely consumed the prograding buffered system
produces more diopside and a changing fluid composi-
tion that is ever richer in CO
2
via continuous reactions
that consume the reactants. Eventually the system may
reach the maximum T on the univariant curve at about
540°C where the fluid composition is X 0.75,
the exact ratio of the fluid liberated from the reaction
(3CO
2
: 1H
2
O). Additional input of heat into the sys-
tem cannot raise its T until one or more of the reactant
phases has been consumed. (This behavior is reminis-
cent of peritectic behavior in the Mg
2
SiO
4
–SiO
2
sys-
tem in Figure 5.8.) Once this happens, a heating system
can leave the curve and its T continue to rise.
Mixed-volatile reactions during metamorphism of
calc-silicate and impure carbonate rocks can be mon-
itored by a reaction progress variable, , that provides
quantitative information on changes in the modal and
bulk chemical composition of the rock and its volume
as well as the compositions and amounts of evolved
fluids and thermal budget (Advanced Topic Box 16.5).
Even with large changes in T and fluid composition
CO
2
496 Igneous and Metamorphic Petrology
T (°C) T (°C)
525
400
500
(b) INFILTRATION
IN OPEN SYSTEM
INTERNALLY BUFFERED
CLOSED SYSTEM
540
710
0.0 0.2 0.3 0.4 0.6
I
II
0.8 1.0
X
CO
2
X
CO
2
0.75(a)
W
o
C
O
2
C
a
l
Q
t
z
W
o
C
O
2
C
a
l
Q
t
z
5
D
i
3
C
O
2
H
2
O
T
r
3
C
a
l
2
Q
t
z
5
D
i
3
C
O
2
H
2
O
T
r
3
C
a
l
2
Q
t
z
16.19 Contrasting metamorphism of siliceous dolostone under
closed- and open-system conditions in the presence of a
mixed-volatile fluid based on two reaction curves from Figure
16.18a. See text for discussion.
Advanced Topic Box 16.5 Evaluation of the
progress variable during metamorphism of
impure carbonate rocks
The progress variable, , quantifies changes in modal
mineral and fluid composition of a rock system during
mixed-volatile metamorphism (Ferry, 1983; Spear,
1993, pp. 459–66 and 678–81). There are two basic
equations based on mass balance considerations:
where
i
is the stoichiometric coefficient of species
M
i
in the reaction and n is the number of moles
of species i that is produced (positive
i
) or con-
sumed (negative
i
) and
Two examples show the use of the progress variable.
Consider internal buffering in the 5 kbar reac-
tion (Figure 16.24c)
5 dolomite 8 quartz H
2
O
tremolite 3 calcite 7CO
2
starting at 400°C with an initial fluid composition
of X 0.4 and the final fluid composition at the
invariant point at 625°C of X 0.95. In this
5 kbar reaction, the stoichiometric coefficients
of H
2
O and CO
2
are v 1 and v 7.
Hence, the second basic equation above becomes
To evaluate n it is set equal to (porosity
volume of rock)/molar volume of fluid. Assum-
ing a liberal porosity of 1% and molar volume
of the fluid from Spear (1993, Figure 7.21) of
28.2 cm
3
/mol, n 0.0355/100 cm
3
of rock so
that 0.423 0.0355 0.015 moles (per 100 cm
3
of rock). The change in the molar abundances of
phases during the course of the reaction is
n
Cal
33 0.015 0.045 moles per
100 cm
3
of rock
n 70.105 moles per 100 cm
3
of rock
n 10.015 moles per 100 cm
3
of rock
These molar abundances can be converted into
volumetric changes, as follows (using again Spear,
1993, Figure 7.21 for the volatiles):
V
Cal
0.045 moles 36.934 cm
3
/mole
1.66 cm
3
per 100 cm
3
of rock
H
2
O
CO
2
total
i
total
i
n
total
i
(0.95 0.40)
0.95(–1) 0.057
0.423n
total
i
CO
2
H
2
O
f
CO
2
CO
2
i
n
total
i
(X
CO
2
f
X
CO
2
i
)
(X
CO
2
f
H
2
O
X
H
2
O
f
CO
2
)
M
i
n
M
1
1
n
M
2
2
n
M
2
1
. . .
n
M
i
i
