Odekon M. Encyclopedia of paleoclimatology and ancient environments
Подождите немного. Документ загружается.

of events since that time, with some emphasis on the Younger
Dryas cold spell during the period of ice melting (Fairbanks,
1989). As noted by Olausson (1965) and many since, the
addition of large amounts of meltwater to the northern North
Atlantic interferes with the formation of deep water and thus
has many ramifications for the global thermohaline circulation
(Broecker and Denton, 1989).
The pioneer work in the reconstruction of the history of
the last million years was done on the cores obtained by the
Albatross during the Swedish Deep-Sea Expedition (1947–
1948, led by Hans Pettersson), by Gustaf Arrhenius, Cesare
Emiliani, Eric Olausson, Frances Parker, and Fred Phleger.
For the history of the last 100 million years, the pioneer studies
were done by a rapidly growing community of biostratigra-
phers and paleoceanographers on material recovered by the
drilling vessel Glomar Challenger, in the Deep Sea Drilling
Project (1968–1983). Among the contributors to the first of
the “Initial Reports or the Deep Sea Drilling Project” were
M. N. Bramlette, W. R. Riedel, W. A. Berggren, E. A. Pessagno,
J.D.Bukry,W.W.Hay,W.H.Blow,J.D.Hays,andA.G.Fischer.
Project Chief Scientist was M. N. A. Peterson. Just as the H.M.S.
Challenger symbolizes the beginning of modern oceanography ,
the Albatross and the Glomar Challenger areiconicforthebegin-
nings of paleoceanography. A third vessel, the JOIDES Resolution,
took over from 1985 and became the dominant tool for ocean
history exploration at that time.
The main result from the Albatross investigations was that
biogenic deep-sea deposits show cyclic sedimentation, a find-
ing that was linked to orbitally driven climate change and cor-
responding ice-age fluctuations. Much evidence has been found
since to support this notion, especially from the analysis of
oxygen isotopes of planktonic and benthic foraminifers, and
from time series analysis using Fourier methods (Hays et al.,
1976). The ice-age cycles are driven by changes in seasonal
contrast, much as initially suggested by the Serbian mathemati-
cian Milutin Milankovitch (1930). The dominant cycles reflect
the influence on seasonal contrast from changes in the obliquity
of Earth’s axis (41,000 years) and in the eccentricity of the
orbit (near 100,000 years) (Figure M6). Precession (the fact
that summer solstice migrates between positions close to and
far from the sun along the orbit) translates the eccentricity
signal into 23,000-year amplitude variations in climate, which
express themselves especially strongly in the productivity
cycles of upwelling regions. A striking change in response
of the climate system to the forcing occurs near 920,000 years
ago: a strong long-term cycle sets in as the amplitude of
the signal increases, first expressed as a doubling of the obli-
quity cycle, and then (near 700,000 year) moving into a
100,000-year cycle.
The main result from Glomar Challenger drilling concerned
the evolution of a cold ocean from a warm one, with all its
ramifications (Kennett, 1982). This general leitmotif governs
the evolution of marine organisms during the Cenozoic, from
nannoplankton to whales (which evolved blubber and baleen
to gather krill in cold upwelling regions).
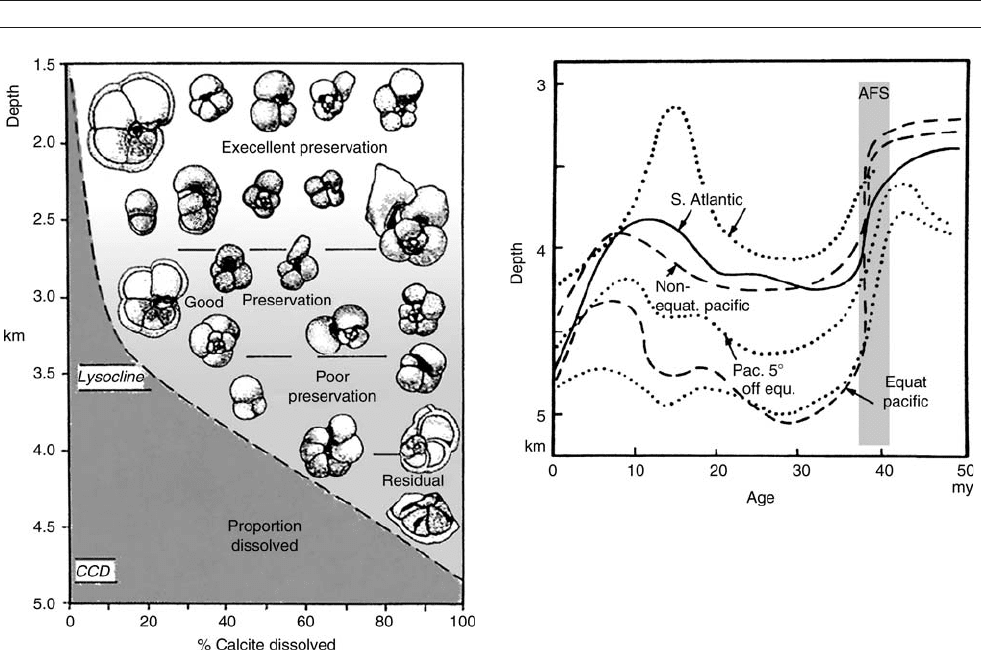
Figure M5 Reconstruction of CCD variations for various regions of the
ocean. Dotted lines: According to Berger and Roth ( 1975). Solid and
dashed lines: according to van Andel et al. (1977), as drawn in Seibold
and Berger (1993). AFS: Auversian Facies Shift (Berger and Wefer, 1996).
The AFS denotes an overall change in sedimentation patterns, whereby
calcareous ooze becomes dominant in deep-sea deposits (carbonate
deposition being shifted from shelf to deep sea) and opaline sediments
are no longer widespread, but become concentrated below upwelling
regions, including the sea around Antarctica.
Figure M4 Deterioration of preservation of calcareous fossils (here:
planktonic foraminifers) as a function of water depth, and definition of
lysocline and CCD. The color of the calcareous ooze becomes darker
with depth, as the proportion of carbonate decreases.
MARINE BIOGENIC SEDIMENTS 529
The chief drawback of early drilling was the mixing of sedi-
ment during drilling. This was largely eliminated during the
Ocean Drilling Program (1985–2003), by sending a piston cor-
ing advice ahead of the drill bit. The cores allow for high-
resolution studies, on a thousand-year scale, and this opened
the door for “tuning” of the sedimentary record to Milankovitch
forcing, which can be reconstructed rather precisely (Shackleton
and Crowhurst, 1994). As a result, detailed records of climate
change emerged, including the details and differences of ice-
age cycles in various regions of the ocean. Other topics that
have been studied in detail are Antarctic ice buildup and the
response of the ocean in terms of changing productivity and
deep-sea circulation, the nature of the climate change at the
Eocene-Oligocene boundary (Prothero and Berggren, 1992),
and the sequence of a strange carbon isotope excursion at the
end of the Paleocene (thought to involve large-scale release
of methane from submarine clathrates; Dickens et al., 1995).
The series of events surrounding the grand extinction event
65 million years ago attracted much interest, as did many other
examples of natural experiments that allow an assessment of
how the climate machine works and how the ocean responds
to disturbance.
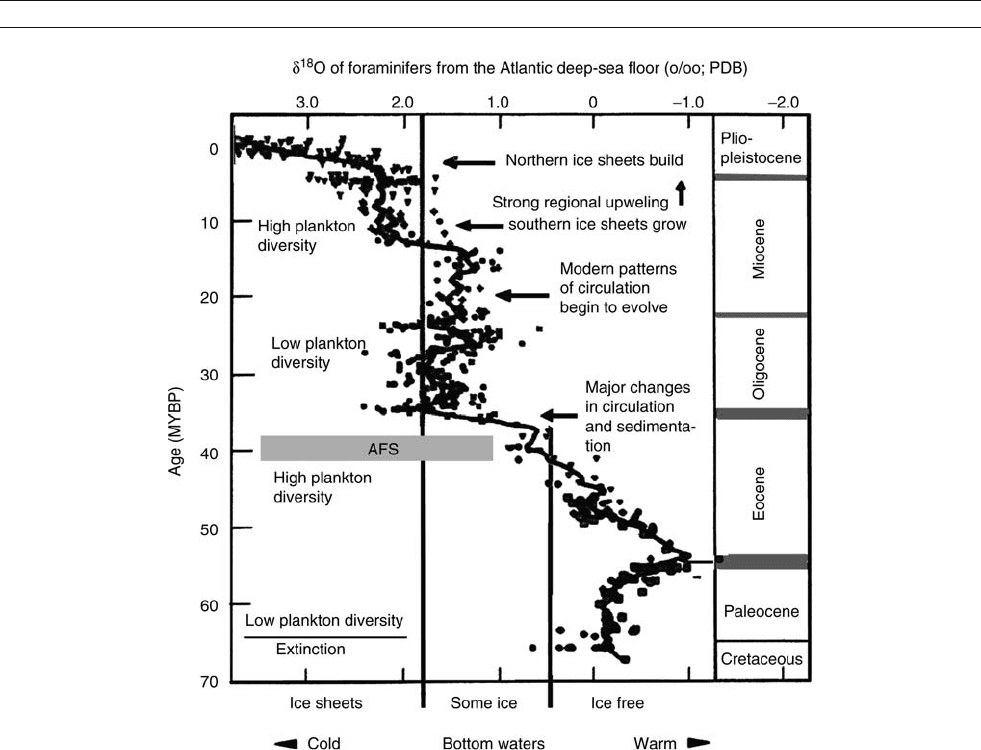
The great sustained stepwise cooling that characterizes glo-
bal climate history since the late Eocene (Auversian Facies
Shift, AFS, 40 million years ago) is well reflected in the oxy-
gen isotopes of benthic foraminifers from the deep-sea floor
of the Atlantic (Figure M7). The isotope index traces both ice
and temperature; thus, it illustrates both the cooling of deep
waters and the buildup of polar ice sheets, but at different
proportions depending on the time span considered. Ice on
Antarctica is thought to enter the picture around the time of
the AFS, followed by major changes in circulation and associ-
ated sedimentation patterns. However, great ice sheets have
presumably only covered the Antarctic continent since the
middle Miocene. At the Pacific equator, the first indications
for a narrowing of the zone of upwelling appear after the
AFS, in the shape of a band of opaline sediments (that is, sedi-
ments rich in radiolarians and diatoms). These sediments are
significant as a clue to the rise of zonal winds. An asymmetry
of zonal wind activity about the equator would have been
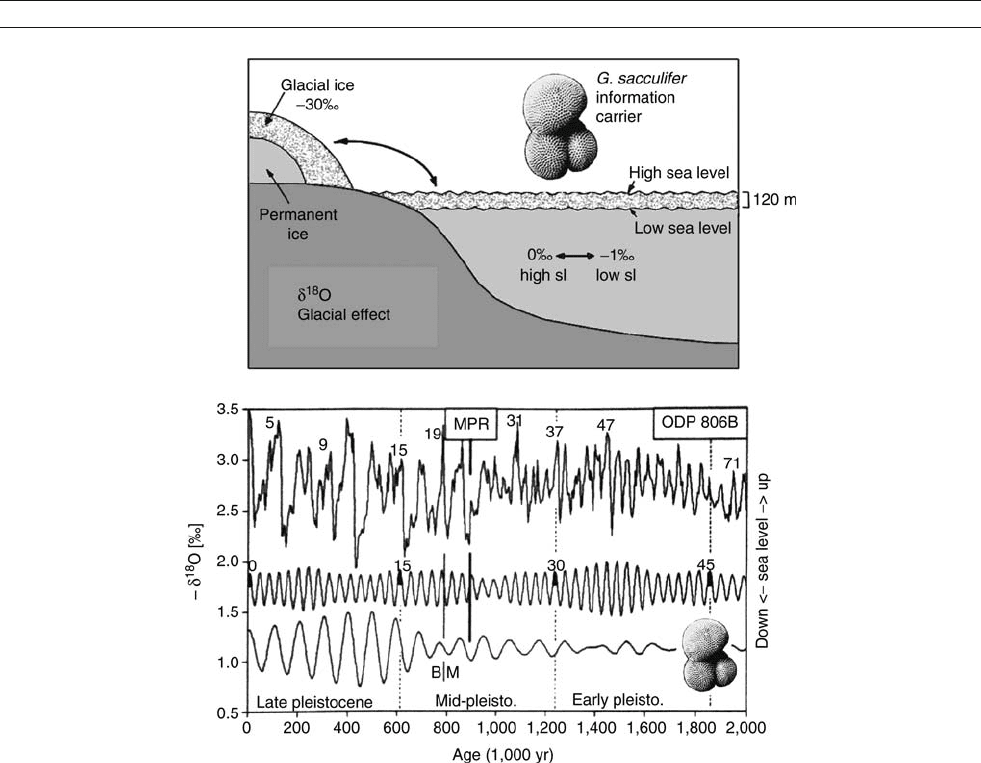
Figure M6 Oxygen isotope stratigraphy of Quaternary biogenic sediments from the western equatorial Pacific. Upper: Diagram showing how
ice-mass changes are translated into variations of oxygen isotope composition of planktonic foraminifers (concept introduced by C. Emiliani, 1955).
Lower: Stratigraphy of a sequence of cores from Ontong Java Plateau (modified after Berger and Wefer, 1992). Numbers: Emiliani isotope
stages. MPR: Mid-Pleistocene Revolution (climate shift to long cycles with large amplitudes). Top: Original isotope sequence. Below: 41-kyr cycle
contained in the series, and 100-kyr cycle, based on Fourier decomposition of the original record.
530 MARINE BIOGENIC SEDIMENTS
present, similar to that found today but more pronounced. The
southern trades helped to drive equatorial upwelling. A strong
asymmetry presumably persisted until well into the late Neo-
gene, when ice buildup in the Northern Hemisphere provided
a counterweight to southern polar ice fields (Flohn, 1985).
The low plankton diversity during the Oligocene is puz-
zling. The rapid increase of diversity in the early Miocene, with
the evolution of deep-living and shallow-living plankton, has
been linked to the development of a thermocline. Certainly,
the existence of a thermocline within the sunlit zone is a defin-
ing feature of the modern ocean. Photosynthesizing organisms
must stay in the sunlit zone sufficiently long, on average, to
produce more substance than they use up in respiration. When-
ever the mixed layer is substantially thicker than the sunlit
layer, this basic condition (Sverdrup’s criterion of net photo-
synthesis) is not likely to be met. A deep and/or ill-defined
thermocline will allow a thick mixed layer. This makes the
pelagic realm an unfavorable habitat for production. In addi-
tion, the lack of opportunity for migrating up and down through
the different environments defined by a thermocline precludes
diversification. We may conclude, therefore, that in the Oligo-
cene the thermocline was largely ineffective as an element
in structuring the pelagic environment; that is, the habitat of
plankton.
Strong coastal upwelling, based on silica and organic matter
content of hemipelagic sediments, is well documented for the
last 10 million years or so, especially in the Southern Hemi-
sphere (Summerhayes et al., 1992; Christensen and Giraudeau,
2002). Presumably, the resulting high abundance of plankton
and associated herring-like fish favored the rapid evolution of
warm-blooded predators, including sea birds, both with and
without flight, and marine mammals.
A direct comparison of warm oceans (pre-AFS) and cool or
cold oceans (post-AFS) is complicated by the fact that the
former are associated with large shelf seas and the latter are
not. Furthermore, the configuration of continents was quite
different, with a broad seaway once connecting the Pacific,
Indian and Atlantic Oceans in tropical latitudes – the Tethys
(Barron and Peterson, 1989; Barrera and Johnson, 1999). Any
efforts to relate differences or similarities in life habitat condi-
tions and biodiversity to the warm-cold contrast may well be
compromised by the presence or absence of large shelf seas
and the Tethys seaway (The Tethys persisted well into the Cen-
ozoic; Haq, 1981). There are some features of the warm ocean,
Figure M7 Stepwise cooling in the Cenozoic, since 40 million years ago (AFS, Auversian Facies Shift). The first step implies production of cold deep
water (seen in the extinction of benthic foraminifers); the second, buildup of Antarctic ice caps; the third, buildup of ice in the northern polar
regions (modified after a diagram in Miller et al., 1987).
MARINE BIOGENIC SEDIMENTS 531
however, that are quite different from those of a cold one on
a fundamental level. Foremost among these is a dearth of oxy-
gen in deep waters, which largely derives from the fact that
oxygen is less soluble in warm water than in cold. This, pre-
sumably, greatly contributed to the fact that anaerobic condi-
tions, reflected in the accumulation of black mud (now shale),
were widespread during Cretaceous periods, and especially so
during certain privileged intervals spanning the Albian-Aptian,
Cenomanian-Turonian and Santonian-Campanian boundaries.
The cause for extended anaerobic conditions, it is thought, is
a greatly increased effusion of volcanic magma to the surface
of the planet, bringing with it a corresponding increase of car-
bon dioxide in the atmosphere (Arthur et al., 1985). The system
responds by burying organic carbon, which counteracts the
geochemical imbalance. The burial of organic carbon preferen-
tially removes C-12 from the carbon cycle, driving up the ratio
between C-13 and C-12, which is then reflected in the stable
isotope composition of calcareous fossils deposited at the time
(Figure M8). The origin of much of the retrievable petroleum
on the planet is linked to anaerobic conditions in the middle
Cretaceous seas. The paleoceanographic implications of oxy-
gen stress are vast, including widespread denitrification, which
would have greatly impacted the level of productivity (For
background on productivity in the sea, and reading the record
of productivity in biogenic sediments, see Falkowski and
Woodhead, 1992).
The great concepts of orbitally driven climate cycles
during the Quaternary, of general cooling during the Ceno-
zoic, and of warm oxygen-stressed seas in the Cretaceous,
along with the revolutionary insights from plate tectonics,
have fundamentally changed the way geologists look at the
environmental history of the planet in the second half of the
twentieth century. However, perhaps the single most important
clue to the nature of Phanerozoic history, and especially
regarding evolution, has been the recognition of the impor-
tance of the end-of-Cretaceous (“K-T”) impact of a celestial
body with Earth, producing widespread extinction (for discus-
sions, see Berggren and van Couvering, 1984). It was within
biogenic deposits that the extinction event was first clearly
documented, in particular in calcareous shallow-water and
pelagic deposits (e.g., Berggren, 1962; Bramlette, 1965). The
evidence for impact was found in a famous pelagic carbonate
section near Gubbio in Italy (Alvarez et al., 1980), where the
exact horizon of extinction of planktonic foraminifers had
been previously established (Luterbacher and Premoli-Silva,
1964).
Studies of the K/T boundary have greatly contributed to the
reconstruction of the exact sequence of events, and especially
to elucidating the early response of the system to stress (invol-
ving strange plankton blooms: Thierstein and Okada, 1979)
and the course of recovery from the event in terms of biodiver-
sity (in a time-span counted in millions of years).
Wolfgang H. Berger and Gerold Wefer
Bibliography
Alvarez, L.W., Alvarez, W., Asaro, F., and Michel, H.V., 1980. Extraterres-
trial cause for the Cretaceous-Tertiary extinction. Science, 208,
1095–1108.
Arrhenius, G.O.S., 1952. Sediment cores from the east Pacific. Rep. Swed.
Deep-Sea Exped. 1947–1948, 5(1), 1–227.
Arthur, M.A., Dean, W.E., and Schlanger, S.O., 1985. Variations in the glo-
bal carbon cycle during the Cretaceous related to climate, volcanism,
and changes in atmospheric CO
2
. In Sundquist, E.T., and Broecker,
W.S. (eds.), The Carbon Cycle and Atmospheric CO
2
: Natural Varia-
tions Archean to Present. Washington, DC: American geophysical
union. AGU Geophysical Monograph, vol. 32, pp. 504–529.
Barrera, E., and Johnson, C.C. (eds.) 1999. Evolution of the Cretaceous
Ocean-Climate System. Boulder, CO: Geological Society of America.
GSA Special Paper 332, 445pp.
Barron, E.J., and Peterson, W.H., 1989. Model simulation of the Cretaceous
ocean circulation. Science, 244, 684–686.
Berger, W.H., and Roth, P.H., 1975. Oceanic micropaleontology: Progress
and prospects. Rev. Geophys. Space Phys., 13(3), 561–585 and 624–635.
Berger, W.H., and Wefer, G., 1992. Klimageschichteaus Tiefseesedimenten –
Neues vom Ontong-Java-Plateau (Westpazifik). Naturwissenschaften, 79,
541–550.
Berger, W.H., and Wefer, G., 1996. Expeditions into the past: Paleoceano-
graphic studies in the South Atlantic. In Wefer, G. et al., (eds), The
South Atlantic: Present and Past Circulation. Berlin: Springer,
pp. 363–410.
Berggren, W.A., 1962. Some planktonic foraminifera from the Maestrich-
tian and the Danian stages of southern Scandinavia. Stockholm Univ.
Contrib. Geol., 9,1–106.
Berggren, W.A., and van Couvering, J.A. (eds.) 1984. Catastrophes and
Earth History. Princeton, NJ: Princeton University Press, 464pp.
Berggren, W.A., Kent, D.V., Flynn, J.J., and Van Couvering, J.A., 1985.
Cenozoic Geochronology. Geol. Soc. Amer. Bull., 96, 1407–1418.
Bolli, H.M., Saunders, J.B., and Perch-Nielsen, K. (eds.) 1985. Plankton
Stratigraphy. Cambridge, UK: Cambridge University Press, 1032pp.
Bramlette, M.N., 1965. Massive extinctions of biota at the end of Mesozoic
time. Science, 148, 1696–1699.
Broecker, W.S., and Denton, G.H., 1989. The role of ocean-atmosphere
reorganizations in glacial cycles. Geochim. Cosmochim. Acta, 53,
2465–2501.
Bukry, D., and Bramlette, M.N., 1969. Coccolith age determinations – Leg
1, Deep Sea Drilling Project. Initial Rep. Deep Sea Drilling Project, 1,
369–387.
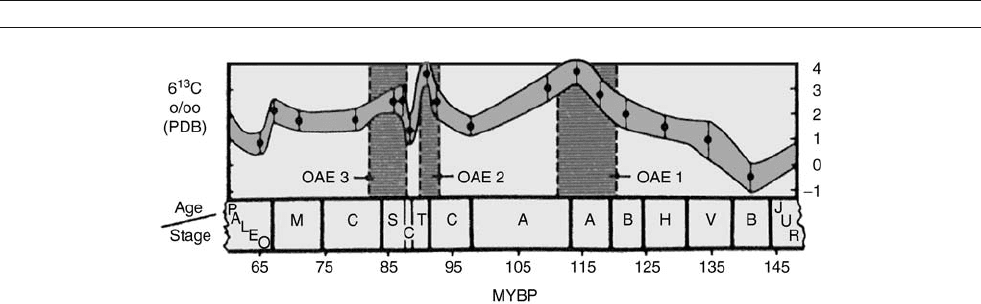
Figure M8 General trends of carbon isotope stratigraphy (pelagic marine limestones) in the Cretaceous, suggesting the removal of organic carbon
during times of low oxygen availability (that is, during deposition of black mud) (modified from Arthur et al. 1985). OAE: Oceanic anoxic event.
532 MARINE BIOGENIC SEDIMENTS
Butcher, S.S., Charlson, R.J., Orians, G.H., and Wolfe, G.V. (eds.) 1992.
Global Biogeochemical Cycles. London/ San Diego,CA: Academic
Press, 379pp.
Christensen, B.A., and Giraudeau J. (eds.) 2002. Neogene and quaternary
evolution of the Benguela coastal upwelling system. Mar. Geol., 180
(Special Issue), 1–276.
CLIMAP Project Members, 1976. The surface of the ice-age Earth.
Science, 191, 1131–1137.
Daly, R.A., 1934. The Changing World of the Ice Age. New Haven: Yale
University Press, 271pp.
Dickens, G.R., O’Neil, R.R., Rea, D.K., and Owen, R.M., 1995.
Dissociation of oceanic methane hydrate as a cause of the carbon
isotope excursion at the end of the Paleocene. Paleoceanography, 10,
965–971.
Emiliani, C., 1955. Pleistocene temperatures. J. Geol., 63, 538–578.
Fairbanks, R.G., 1989. A 17,000-year glacio-eustatic sea level record:
Influence of glacial melting rates on the Younger Dryas event and
deep-ocean circulation. Nature, 342, 637–642.
Falkowski, P.G., and Woodhead, A.D. (eds.) 1992. Primary Productivity
and Biogeochemical Cycles in the Sea. New York: Plenum Press,
550pp.
Fischer, G., and Wefer, G. (eds.) 1999. Use of Proxies in Paleoceanogra-
phy. Berlin: Springer, 735pp.
Flohn, H., 1985. Das Problem der Klimaänderungen in Vergangenheit und
Zukunft. Darmstadt: Wissenschaftliche Buchgesellschaft, 228pp.
Funnell, B.M., and Riedel, W.R. (eds.) 1971. The Micropalaeontology of
Oceans. Cambridge, UK: Cambridge University Press, 828pp.
Haq, B.U., 1981. Paleogene paleoceanography: Early Cenozoic oceans
revisited. Oceanologica Acta, 4 (Supplement), 71–82.
Hay, W.W., 1988. Paleoceanography: A review for the GSA centennial.
Geol. Soc. Am. Bull., 100, 1934–1956.
Hays, J.D., Imbrie, J., and Shackleton, N.J., 1976. Variations in the Earth’s
orbit: Pacemaker of the ice ages. Science, 194, 1121–1132.
Kennett, J.P., 1982. Marine Geology. Englewood Cliffs, NJ: Prentice-Hall,
813pp.
Kennett, J.P. (ed.) 1985. The Miocene Ocean: Paleoceanography and bio-
geography. Geol. Soc. Am. Memoir, 163
,1–337.
Luterbacher, H.P., and Premoli-Silva, I., 1964. Biostratigrafia del limite
Cretaceo-Terziario. Riv. Ital. Paleontol., 70,67–128.
Milankovitch, M., 1930. Mathematische Klimalehre und astronomische
Theorie der Klimaschwankungen. Handbuch der Klimatologie, Bd 1,
Teil A, Berlin, Bornträger, 176pp.
Miller, K.G., Fairbanks, R.G., and Mountain, G.S., 1987. Tertiary oxygen
isotope synthesis, sealevel history, and continental margin erosion.
Paleoceanography, 2,1–19.
Olausson, E., 1965. Evidence of climatic changes in North Atlantic deep-
sea cores, with remarks on isotopic paleotemperature analysis. Prog.
Oceanogr., 3, 221–252.
Parker, F.L., 1962. Planktonic foraminiferal species in Pacific sediments.
Micropaleontology, 8(2), 219–254.
Prothero, D.R., and Berggren, W.A. (eds.) 1992. Eocene-Oligocene Climatic
and Biotic Evolution. Princeton, NJ: Princeton University Press, 566pp.
Purdy, E.G., 1974. Reef configurations: Cause and effect. In Laporte, L.F.
(ed.), Reefs in Time and Space. Tulsa, OK: Society of Economic
Paleontologists and Mineralogists. SEPM Special Publication 18,
pp. 9–76.
Reading, H.G. (ed.) 1986. Sedimentary Environments and Facies, 2nd edn.
Oxford: Blackwell Scientific, 615pp.
Seibold, E., and Berger, W.H., 1993. The Sea Floor. 3rd edn. Berlin:
Springer, 356pp.
Shackleton, N., and Crowhurst, S., 1994. Details that make the difference.
Oceanus, 36(4), 45–48.
Summerhayes, C.P., Prell, W.L., and Emeis, K.C. (eds.) 1992. Upwelling
Systems: Evolution since the Early Miocene. Piccadilly: Geological
Society of London. Geological Society of London Special Publication
64, 519pp.
Thierstein, H.R., and Okada, H., 1979. The Cretaceous/Tertiary boundary
event in the North Atlantic. Initial Rep. Deep Sea Drill. Proj., 43, 601–616.
van Andel, Tj.H., Thiede, J., Sclater, J.G., and Hay, W.W., 1977. Deposi-
tional history of the South Atlantic Ocean during the last 125 million
years. J. Geol., 85, 651–698.
Vincent, E., and Berger, W.H., 1981. Planktonic foraminifera and their use
in paleoceanography. In Emiliani, C. (ed.),“The Sea, vol. 7, the Oceanic
Lithosphere.” New York: Wiley-Interscience, pp. 1025–1119.
Wefer, G., and Berger, W.H., 1991. Isotope paleontology: Growth and com-
position of extant calcareous species. Mar. Geol.,
100, 207–248.
Winter, A., and Siesser, W.G., 1994. Coccolithophores. Cambridge, UK:
Cambridge University Press, 242pp.
Cross-references
Astronomical Theory of Climate Change
Carbonate Compensation Depth
CLIMAP
Coccoliths
Coral and Coral Reefs
Cretaceous/ Tertiary (K-T) Boundary Impact, Climate Effects
Deep Sea Drilling Project (DSDP)
Diatoms
Foraminifera
Ocean Anoxic Events
Ocean Drilling Program (ODP)
Paleocene-Eocene Thermal Maximum
Quaternary Climate Transitions and Cycles
Radiolaria
MARINE CARBON GEOCHEMISTRY
Introduction
The ocean contains approximately 50 times as much dissolved
CO
2
as is contained in the atmosphere, and the carbon cycle in
the ocean controls the pCO
2
of the atmosphere on time scales
of thousands of years. The atmospheric CO
2
concentration
today is rising because of human activities, such as fossil fuel
combustion and deforestation. The CO
2
rise is faster than the
century-scale ocean/atmosphere equilibration time, but the
ocean will ultimately absorb most of the CO
2
that is released.
In the past, changes in the carbon cycle of the ocean drove
changes in atmospheric pCO
2
synchronously with the glacial/
interglacial climate cycles. The orbital forcing which paces
the glaciations (called Milankovitch cycles, see Astronomical
Theory of Climate Change) is asynchronous across the equator;
extra warmth in the north is balanced by decreased heating in
the south. However, because of the rapid mixing of the atmo-
sphere, the glacial CO
2
cycles tend to warm or cool the entire
planet synchronously, and are largely responsible for the global
synchrony of the glaciations. The carbon cycle of the ocean is
therefore a primary determiner of the climate of the Earth, in
the past and in the future.
The carbon cycle in the ocean begins with gas fluxes across
the sea surface into the atmosphere. These depend on wind
speed, temperature, and the chemistry of the water at the sea
surface. Seawater chemistry of CO
2
is complicated by the pre-
sence of the carbonate pH buffer system pH. Only a tiny frac-
tion of the dissolved carbon is present as the uncharged
dissolved gas that can interact with the atmosphere. Because
of this chemistry, a slight change in the pH of seawater can
result in an enormous change in the concentration of dissolved
CO
2
, and in the equilibrium pressure of CO
2
in the atmosphere.
The solubility of dissolved CO
2
depends on temperature and to
a lesser extent salinity, so that a warming parcel of seawater
will tend to push more CO
2
into the atmosphere. The CO
2
con-
centration of surface ocean waters is also affected by the
growth and sinking of phytoplankton in the surface ocean.
The carbon cycle of the ocean is therefore determined by a
MARINE CARBON GEOCHEMISTRY 533

combination of chemical, physical, and biological mechanisms
(see also Carbon cycle).
The physical chemistry of carbon in seawater
Carbon is a unique element in the biosphere, in part because of
its extremely varied chemistry. The oxidized form, CO
2
, is the
dominant form in the atmosphere. In seawater, oxidized carbon
undergoes hydration and pH reactions leading to generation of
bicarbonate (HCO
3
) and carbonate (CO
3
2
) ions. These species
are collectively called “dissolved inorganic carbon” or DIC.
The most reduced form of carbon is methane, CH
4
, while most
biological carbon exists at intermediate oxidation states analo-
gous to carbohydrates, which follow the approximate stoichio-
metry CH
2
O. Reduced forms of carbon are collectively called
“organic carbon.” Production of organic carbon from inorganic
requires an input of energy, such as the light energy used in
photosynthesis, or chemical energy used in chemosynthesis.
In the air, the CO
2
abundance can be defined as a concentra-
tion (molecules per volume), a proportion or “mixing ratio”
(molecules of CO
2
per molecule total), or as the proportion of
the total pressure that can be attributed to CO
2
. This last quan-
tity is called the “partial pressure,” and at sufficiently low total
pressures, the ideal gas law tells us that the partial pressure of a
gas like CO
2
is very close to the total pressure of the mixture
multiplied by the fraction of the molecules that are CO
2
. The
atmospheric CO
2
abundance is commonly expressed either as
a mixing ratio in units of parts per million (ppm), or as a partial
pressure in units of microatmospheres (10
6
atm = matm). The
numerical value of either of these measures in the atmosphere
is close to 370 in both cases (and is presently rising by approxi-
mately 1.5 ppm or matm per year). The abbreviation “pCO
2
”
stands for partial pressure (and is unfortunately easy to confuse
with the meaning of pH, defined as the negative logarithm
of the concentration of H
þ
). For a detailed discussion of the
physical chemistry of inorganic carbon in seawater, see Stumm
and Morgan (1981).
A convenient way to think about CO
2
equilibrium between
air and water is to take the chemistry of a water sample,
and calculate how much CO
2
would exist in the air above it
in equilibrium. In this way, we can define the pCO
2
of a water
sample. This tells us nothing about the real physical pressure
that a diver, for example, would feel in the water, but tells us
what the composition of a bubble would be after it has had a
chance to equilibrate. In nature, water and gas phases are not
always in equilibrium; in fact, for CO
2
at the surface of the
ocean, disequilibrium is the rule. If the pCO
2
of a water sample
is higher than the pCO
2
of the air it is in contact with, then CO
2
will tend to evaporate from the water into the air, lowering
the pCO
2
of the water and raising the pCO
2
of the air as the
system approaches equilibrium (see Carbon dioxide, dissolved
(ocean)).
The solubility of any gas in water depends on tem-
perature, with increasing solubility as the water cools down.
CO
2
gas is no exception. At a given concentration of dissolved
CO
2
, the equilibrium partial pressure in the gas phase there-
fore decreases as the temperature drops (increasing solubility
means less remains in the gas phase). The surface ocean is
warm in the tropics and cold at the poles. Therefore, one com-
ponent of the carbon cycle in the ocean is a tendency for
CO
2
to escape to the atmosphere in the warm tropics, where
it is less soluble, and to invade the ocean in the colder high
latitudes.
Partioning of carbonate species in seawater
CO
2
gas proper is but a minor participant in a larger chemical
system called the carbonate buffer system. CO
2
gas combines
with water (is “hydrated”) to form carbonic acid (H
2
CO
3
). Car-
bonic acid undergoes two proton dissociation reactions (loss of
H
þ
) to form the charged (ionic) species bicarbonate (HCO
3
)
and carbonate ion (CO
3
2
). The hydration reaction is
CO
2
þ H
2
O Ð H
2
CO
3
ð1Þ
In practice, it is analytically difficult to distinguish hydrated
from unhydrated CO
2
, so these two species are lumped
together into a single category that for simplicity we will just
call dissolved CO
2
in the discussion that follows. The equili-
bration time of this reaction is roughly a minute, although it
can be accelerated in biological systems by the enzyme carbo-
nic acid anhydrase.
The dissociation reactions are governed by equilibrium expres-
sions, denoted K
1
for the loss of the first proton and K
2
for the sec-
ond. The dissociation constant K
1
can be written to determine the
ratio of the products to the reactants of the reaction
H
2
CO
3
Ð HCO
3
þ H
þ
ð2Þ
while the second, K
2
, applies to
HCO
3
Ð CO
2
3
þ H
þ
ð3Þ
The ratios are taken between the activities of each of the
solutes, which can be approximated by the concentrations in
moles per liter, denoted by square brackets:
K
1
¼½HCO
3
½H
þ
=½CO
2
and
K
2
¼½CO
2
3
½H
þ
=½HCO
3
Values of the dissociation constants are given by Dickson
and Millero (1987).
Built upon these seemingly simple relations is a chemical
system of rather counter-intuitive behavior. One example is
the response of [CO
3
2
] to changes in the CO
2
concentration
of the solution (Equation 1). Carbonate ions determine whether
CaCO
3
from coral reefs or planktonic shells dissolves (see
Carbonate compensation depth). The response of an equili-
brium reaction to an addition of a species on one side of the
equilibrium is a tendency to shift the equilibrium toward the
opposite direction, to “mop up” the added species. The res-
ponse of the first dissociation (Equation 2) in isolation would
be an increase in HCO
3
and H
þ
(an increase in H
þ
is equiva-
lent to a decrease in pH). The response of the second dissocia-
tion reaction might seem to be another shift to the right,
increasing the concentration of CO
3
2
(and increasing the stabi-
lity of CaCO
3
). We could call this the “rising tide floats all
boats” response.
However, this simple expectation is wrong. The flaw in our
reasoning was to ignore the protons, which exist in much lower
concentration than any of the carbon species. The rightward
shift in Reaction 2 above generates a much more profound
change in H
þ
, relative to its concentration, than the change in
HCO
3
.H
þ
therefore dominates the response of Reaction 3,
which shifts to the left rather than to the right as we would
assume. The behavior of the system can be seen more intuitively
by combining both reactions, eliminating the troublesome
534 MARINE CARBON GEOCHEMISTRY

protons (and thereby conceptually forbidding them from
changing their concentrations at all – a better approximation).
This new reaction is:
H
2
CO
3
þ CO
2
3
Ð 2HCO
3
ð4Þ
When the reaction is written in this way, an addition of car-
bonic acid (or, equivalently, CO
2
) can be seen to consume
CO
3
2
, provoking the dissolution of coral reefs, rather than their
growth. In spite of the fact that CaCO
3
contains carbon, it can-
not act as a sink for CO
2
unless a source of base is added.
The buffer chemistry of seawater has an immediate implica-
tion for the interaction of seawater with rising atmospheric
pCO
2
, even in the absence of solid CaCO
3
. For a simple unbuf-
fered gas like oxygen, for example, the solubility of the gas is
expressed by Henry’s law
K
h;O
2
¼ pO
2
=½O
2
which tells us that the concentration of the gas in solution goes
up proportionately to a rising partial pressure in the gas phase.
The relation also holds for CO
2
,as
K
h;CO
2
¼ pCO
2
=½CO
2
but the total amount of CO
2
that the seawater can absorb is
affected by the buffer chemistry as expressed in Reaction 4
above. Dissolved carbonate ion (CO
3
2
) reacts with added
CO
2
, effectively hiding it from the atmosphere and from
Henry’s law. The buffer works by reacting most of the invading
CO
2
with CO
3
2
to form HCO
3
, so the strength of the buffer is
determined by the ratio of the CO
2
and CO
3
2
concentrations.
Globally, the average ratio of CO
3
2
to CO
2
in surface waters
is about 10, so seawater can hold 10 times as much CO
2
as it
would without the buffer system. As atmospheric CO
2
rises,
however, sea surface CO
3
2
concentrations will drop, and the
buffer capacity of seawater will decline.
When the gas concentration in the surface ocean is out of
equilibrium with the atmosphere, it relaxes toward equilibrium
by a process known as gas exchange. The rate of gas exchange
depends on the wind speed, surface roughness, bubbles and
foam, the presence or absence of a surface microlayer, or skin
of oils, and the diffusivity of the gas. A typical gas exchange
rate is 3–5md
1
, meaning that a typical mixed layer of 100 m
thick at the ocean surface will approach equilibrium with
an e-folding time scale of 100 m/5 m d
1
= 20 days (see
Wanninkhof, 1992). For CO
2
, the factor of 10 buffering would
increase the time scale to about 200 days. For the ocean as a
whole, the rate at which gases invade the deep ocean is limited
by the circulation time scale of the ocean, which is about a
thousand years. For CO
2
, because the ocean is the larger reser-
voir, the atmosphere largely responds to ocean forcing, rather
than the other way around, on the time scale of ocean overturn-
ing circulation.
The balancing of the various dissolved carbon species across
the equilibrium reaction makes it somewhat more complicated
to think about the carbon cycle in the ocean. For example,
when seawater with one set of concentrations is mixed with a
different seawater sample with a different set of concentrations,
the mixed carbonate buffer system adjusts to re-establish pH
balance. That is to say, if we combine one solution with
10 mMCO
2
with equal parts of another that starts with 20 mM
CO
2
, we do not necessarily obtain a solution that contains
15 mMCO
2
, because some of the CO
2
reacts with CO
3
2
in
the resulting mixture. This makes life complicated for oceano-
graphers, who would prefer that their chemical quantities be
“conservative to mixing.” The solution is a pair of derived
quantities for seawater that do have the required conservation
properties. One is the total concentration of all of the CO
2
spe-
cies; this is called the “total CO
2
,” or SCO
2
. If we mix a solu-
tion that contains 1,800 mM SCO
2
with equal parts of another
that contains 2,200 mM SCO
2
, we will obtain a solution that
contains 2,000 mM SCO
2
, with no tricky business.
The other derived quantity is called the alkalinity, which is
defined as the sum of all charges of the salts of weak acids in
solution. One way to think about alkalinity is that if we were
to add H
þ
in an amount equal to the alkalinity, then all of
the carbon in solution would be in the form of CO
2
, free to
exchange with the atmosphere. Ignoring contributions from
borate and other minor species, the “carbonate alkalinity” of
seawater can be written as
alk ¼ HCO
3
þ 2CO
2
3
ð5Þ
where the factor of two in front of CO
3
2
reflects the two pro-
tons it would take to produce carbonic acid from carbonate.
The crucial point is that Reaction 4, above, has the same alka-
linity on both sides of the reaction. As pH equilibrium redistri-
butes carbon species toward one side or the other of Reaction
3, the alkalinity is unchanged. Therefore, if we combine two
water parcels with different alkalinities, the alkalinity of the
resulting mixture is a simple combination of the two. We say
that both alkalinity and total CO
2
are conservative to mixing,
and this simplifies our thinking about all kinds of processes
that occur in the ocean carbon cycle.
How can we relate the alkalinity and total CO
2
to the equi-
librium pCO
2
of surface waters, and exchange of CO
2
with the
atmosphere? As we have already seen, dissolved CO
2
gas itself
is only a minor part of the total carbon inventory of seawater,
and the fraction of carbon in the form of CO
2
depends on the
pH of the solution. Together, the alkalinity and the total CO
2
concentration can tell us everything there is to know about the
carbon chemistry of the seawater. At a constant alkalinity, if
we increase the total CO
2
concentration, the concentration of dis-
solved CO
2
and the equilibrium pCO
2
of the water sample will
go up. This is easy to imagine, as an increase in the number of
carbons without any change in the number of charges must lead
to an increase in the number of uncharged carbons, that is CO
2
.
In a similar way, if we maintain a constant total CO
2
and
increase the alkalinity, then the number of charges per carbon
atom goes up, and the proportion of uncharged CO
2
molecules
goes down.
A useful property of the alkalinity is that it does not care
about fluxes of CO
2
gas. An invasion of CO
2
from the atmo-
sphere will increase the total CO
2
of the seawater, and alter
the proportions of the various carbon species according to
Reaction 3, but since shifts along Reaction 3 have no effect
on the alkalinity, the alkalinity will be unchanged in response
to gas exchange. In an analogous way, if we were to add strong
acid to seawater somehow, this would change alkalinity while
leaving the total CO
2
unchanged. This latter is a hypothetical
example; natural fluxes of strong acid such as acid rain are gen-
erally negligible in the carbon chemistry of the surface ocean.
The biological pump
The other major mechanism governing the fluxes of CO
2
across the sea surface, nearly equal in magnitude to the effect of
MARINE CARBON GEOCHEMISTRY 535

temperature discussed above, is the growth and sinking of phyto-
plankton in the surface ocean. Geochemically, the effect of
phytoplankton growth is to convert dissolved carbon into particu-
late carbon. Particles can sink, generating a net flux of carbon
downwards in the ocean. Some of the phytoplankton (plants of
the sea) sink on their own, while another fraction of the sinking
carbon arises from the fecal pellets excreted by zooplankton gra-
zers. This process has been called the “biological pump” (see
Broecker and Peng, 1982). The atmosphere only sees the surface
ocean, so the effect of the biological pump is to change the ocean
chemistry so that the atmosphere sees, and therefore changes, the
chemistry of the atmosphere itself.
It is helpful to divide the biological pump into two components,
because these components have opposite effects on the CO
2
concentration and pCO
2
of the surface ocean. The first, and domi-
nant, component is the organic carbon pump, comprised of the
proteins, lipids, and other structural biochemical machinery of
the cell. This is called the “soft tissue” pump. Only a small fraction
of the plankton production is exported to depth; in very productive
regions, the export fraction may reach half, while in the open
ocean only 5–10% of the production is exported. The rest of
the biomass produced is respired in the surface ocean, returning
to dissolved carbon. Organic carbon production depletes the total
CO
2
concentration of the seawater , but has only a small effect on
the alkalinity. Therefore, the effect of the soft tissue pump is to
decrease the pCO
2
of surface waters.
The second component of the biological pump is the pro-
duction and sinking of the calcium carbonate (CaCO
3
) shells
of some plankton species. This process is called the “hard
tissue” pump. The formation of CaCO
3
takes place by the
reaction
Ca
2þ
þ CO
2
3
Ð CaCO
3
ð6Þ
Because one mole of CO
3
2
accounts for one mole of total
CO
2
but two equivalents of alkalinity (see Equation 5), the
effect of the CaCO
3
pump is a 2:1 change in alkalinity versus
total CO
2
. A decrease in alkalinity leaves a greater proportion
of uncharged carbon (CO
2
), so the net effect of the CaCO
3
pump on the pCO
2
of surface waters is an increase in pCO
2
.
When these two components of the biological pump are
combined in the surface ocean, the rate of the soft tissue pump
exceeds that of the hard tissues, so the overall effect of biologi-
cal activity in the ocean is to decrease the p CO
2
of the atmo-
sphere. CaCO
3
secreting plankton are favored in tropical
surface waters, but even here the rate of organic carbon sinking
from the surface ocean exceeds that of CaCO
3
by a factor of up
to 10. In polar waters colder than 10
C, CaCO
3
production
becomes even less important. If the biota of the ocean were
killed and the contents of the ocean allowed to redistribute
themselves in response to this, the pCO
2
of the atmosphere
would perhaps double.
Photosynthesis in the ocean is limited by a number of fac-
tors that determine the extent to which biology affects ocean
chemistry and atmospheric pCO
2
(Longhurst, 1998). The first
factor is light; production of biomass requires energy, which
is derived by sunlight via the photosynthetic apparatus.
Although open ocean waters are clearer than any freshwater
lakes on Earth, the incident sunlight is absorbed and scattered
by phytoplankton, other particulate and dissolved material,
and by the water itself. Although the absorption of light
depends on wavelength (red is absorbed most quickly, which
is why undersea scapes appear blue), in general the absorption
of intensity of “photosynthetically available radiation” (PAR)
decreases over a depth scale of about 100 m. Below this depth,
the metabolic costs of respiration exceed the available energy
from photosynthesis, and phytoplankton are unable to thrive.
The surface sunlit zone, capable of supporting phytoplankton,
is called the “euphotic zone.” The production of CaCO
3
does
not require energy in the same way as photosynthesis does,
and there is some secretion of CaCO
3
by zooplankton such as
foraminifera below the euphotic zone, but most CaCO
3
produc-
tion is associated with phytoplankton called coccolithophorids
in the euphotic zone.
A second factor influencing the availability of light to the
phytoplankton is mixing. Winds tend to mix the surface waters
of the ocean into a distinct mixed layer ranging from a few to a
thousand meters thick. The thickness of the mixed layer
depends on the contrast between the temperature (and to a les-
ser extent the salinity) of the surface ocean compared with the
deep ocean. In the tropics, warm surface waters are more buoy-
ant than the colder waters below. This density difference inhi-
bits mixing of waters across the stratification boundary, and
the mixed layer tends to be thin. Phytoplankton suspended in
the surface euphotic zone tends to remain in the euphotic zone.
However, at high latitudes, or in subpolar winter, the cooler
surface waters mix more readily with subsurface waters, allow-
ing the wind to create a deep mixed layer. The phytoplankton
spend at least some of their time at depths deeper than the
euphotic zone, limiting their growth. The “spring bloom” in
the North Atlantic is a clear demonstration of the effect of mix-
ing. As soon as spring warming stabilizes a shallow mixed
layer, the phytoplankton concentrations and photosynthesis
rates increase dramatically.
The end of the spring bloom in the North Atlantic (and in
other regions) is caused by another factor that limits phyto-
plankton growth: nutrient ions such as NO
3
and PO
4
3
, which
are essential to the manufacture of the biochemical machinery
of the cell. Nitrogen is used in the amino acid building blocks
of proteins, and phosphorus is used in DNA and in an energy
storage molecule called ATP. The elemental ratio of C:N:P in
phytoplankton is relatively constant, and has been named the
“Redfield ratio.” An astonishing fact about ocean chemistry is
that the ratio of N:P in seawater is close to that of phytoplank-
ton, so that to a first approximation, both elements get used up
at the same time. Carbon, in contrast, exists in much greater
abundance in seawater, so that the nutrients are always depleted
first. The mean concentration of PO
4
3
in the ocean is 2.2 mM;
when this value is multiplied by a revised C:P Redfield ratio
of 135 we estimate that approximately 200 mM of the roughly
2,000 mM SCO
2
concentration in the ocean (N10%) travels
with PO
4
3
as “metabolic” CO
2
. The metabolic CO
2
represents
the maximum impact of biology on the chemistry of the
surface ocean.
The effect of the soft-tissue biological pump in the ocean is
to deplete NO
3
and PO
4
3
in surface waters by exporting them
to the deep ocean. Primary production in the ocean is therefore
tied intimately to upwelling or mixing of nutrient-rich subsur-
face waters into the surface euphotic zone. One place where
this occurs is in near-shore waters, where winds drive offshore
currents, pulling subsurface waters up from below. Another
such place is along the equator, where the change of direction
of the Coriolis force pulls surface waters away from the equa-
tor, leaving a void that must be filled by upwelling subsurface
waters. A third mechanism for bringing nutrients to the surface
takes us back to the spring bloom example above; deep winter-
time mixing entrains nutrient-rich deep water into the euphotic
536 MARINE CARBON GEOCHEMISTRY

zone, recharging it with nutrients for the next spring bloom.
After the nutrients are used up by the spring bloom, phyto-
plankton concentrations and photosynthesis rates decrease
for the rest of the summer.
In contrast to the North Atlantic, some regions of the surface
oceans, such as the Equatorial Pacific, the North Atlantic, and
the Southern Ocean, maintain high concentrations of nutrients
in the surface euphotic zone all year round, with no spring
bloom. One of the more exciting discoveries of the past decade
is that the phytoplankton may be limited by the availability
of another nutrient, iron (Fe) (see Iron and climate change).
Phytoplankton require iron in the minute ratio of approximately
1:10
5
Fe:C. The oxic form of iron, Fe
3+
, is relatively insoluble
in water, and so seawater tends to become depleted in iron rela-
tive to their stocks of NO
3
and PO
4
3
. When this water upwells,
it must await the delivery of iron by deposition as dust from the
atmosphere. The North Atlantic, according to this theory, is
rich in dust deposition, so supports a spring bloom, while the
equatorial Pacific is more remote, and is dust and iron limited.
There is a general correspondence between the temperature
of the surface ocean and its nutrient content, although this rela-
tionship is not absolute. In part, this is because colder surface
waters mix more readily with nutrient-rich subsurface. Deeper
mixing also creates a situation of light limitation for phyto-
plankton, as explained above. At any event, the net effect of
this correspondence is to set temperature and sea surface nutri-
ents in opposition to each other in their effects on atmospheric
pCO
2
. The cold temperatures in the Southern Ocean, for exam-
ple, tend to lower the pCO
2
of surface water, pulling CO
2
into
the ocean. However, the high concentration of nutrients in
Southern Ocean surface waters indicate that the SCO
2
concen-
tration is higher there than it would be if nutrients were
depleted, tending to increase atmospheric pCO
2
in the opposite
direction from the thermal forcing. On the other hand, the region
of the highest CO
2
flux from the ocean to the atmosphere is the
equatorial Pacific, where nutrient-rich waters are warmed by
the tropical sun, in other words where both temperature and
nutrient forcing are working in the same direction.
Most of the organic matter that sinks out of the euphotic
zone in the surface ocean never reaches the sea floor, typically
4 km below. It is subject to microbial degradation and to graz-
ing by zooplankton in the mid-water column. The sinking flux
of particles can be measured using a device called a sediment
trap, which consists of a large funnel or cylinder with a carou-
sel of sealable sample chambers that can sequentially store the
falling debris (Honjo, 1996). Several traps are typically tied to
a mooring, allowing an assessment of the sinking flux of car-
bon as a function of depth. These fluxes are generally found
to decrease drastically over the top 500 m of the water column.
Different chemical components of the debris are removed
selectively as they sink. The flux of organic carbon decreases
more quickly with depth than does the flux of CaCO
3
or SiO
2
(another type of shelly hard part, the mineral opal is secreted
by the phytoplankton diatoms) (see Diatoms). Nitrogen and
phosphorus are removed more quickly than carbon, as indicated
by increasing C:N and C:P ratios in sediment traps. The flux of
CaCO
3
appears rather uniform with depth in sediment traps, but
budgets for CaCO
3
production and deposition on the sea floor,
and for alkalinity in the water column, seem to indicate a sub-
stantial dissolution of CaCO
3
in the mid-water column. The
discrepancy with sediment trap results may be caused by biases
in trap efficiency in shallow waters (Yu et al., 2001)orby
CaCO
3
dissolution (Betzer et al., 1984) within the traps.
Organic carbon by itself is not sufficiently dense to sink
very quickly through seawater, if at all, and so attention has
focused on the role of CaCO
3
, SiO
2
, and wind-blown continen-
tal material as “ballast” (Armstrong et al., 2002; Klaas and
Archer, 2002). This theory is consistent with the sediment trap
data in that while the fluxes of the various constituents vary
widely in space and time, the ratio of organic carbon to ballast
in the deep traps, and in particular to CaCO
3
, seems much more
stable. Only a small fraction of the organic matter produced in
the surface ocean makes it to the deep sea, so the ballast model
predicts that the absolute flux of organic carbon to depth might
be determined by the fluxes of ballast. However, the ballast
model does not explain the trap data in the top kilometer of
the water column, where the organic carbon to ballast ratio is
higher and more variable than in the deep sea.
CaCO
3
compensation and the pH of the ocean
We have seen how the biological pump affects the pCO
2
of the
atmosphere, by pumping carbon and nutrients from the surface
ocean to the deep. In addition to this direct effect, the biological
pump has an indirect effect on pCO
2
, called carbonate compen-
sation (Broecker and Peng, 1987). CaCO
3
is produced biologi-
cally, mostly in the surface ocean. Some fraction of this CaCO
3
reaches the sea floor, and its chemical constituents (Ca
2+
, one
SCO
2
, and two alkalinities) are permanently removed from
the ocean as it is buried. This output is balanced by input from
the dissolution of rocks, mostly sedimentary CaCO
3
, a process
called weathering on land. The idea is that the pH of the ocean
adjusts; acting as a stable negative feedback to insure that the
output of CaCO
3
by burial balances the input from weathering.
As we have seen, a change in seawater pH will have a large
impact on the speciation of carbon, and therefore on pCO
2
(see Paleo-ocean pH ).
The input of dissolved CaCO
3
to the ocean derives from the
chemical weathering of rocks on land (see Weathering and
climate). The saturation state for CaCO
3
dissolution is given
by the reaction
CaCO
3
! Ca
2þ
þ CO
2
3
; K
sp
¼½Ca
2þ
½CO
2
3
ð7Þ
If the product of the reactants, Ca
2+
and CO
3
2
, is less than
the value of K
sp
then the solution is undersaturated and CaCO
3
will tend to dissolve. In practice, the CO
3
2
combines with CO
2
and water to yield the overall reaction
CaCO
3
þ CO
2
þ H
2
O ! Ca
2þ
þ 2HCO
3
ð8Þ
where the Ca
2+
and HCO
3
reflect the dominant ion pair in most
river water. The reactant CO
2
is derived ultimately from the
atmosphere, but CO
2
partial pressures in soil gas are elevated
to an order of magnitude higher than atmospheric values by
the action of plants and the microbial respiration of soil carbon.
River water, reflecting its origin as soil water, is typically
strongly supersaturated in CO
2
, and close to saturation with
respect to CaCO
3
at that pCO
2
. In areas where the rate of fresh-
water runoff is extremely high, the dissolution reaction may not
have time to keep up with the flux of distilled rainwater, and
the solution concentrations will drop. The lithology, or rock
type, of a region also has an impact on the chemistry of rivers.
To a first approximation, however, we expect that the total flux
of dissolved CaCO
3
to the ocean depends primarily on the
total rate of fresh water runoff to the oceans, and secondarily
on temperature, soil biological activity ( pCO
2
), terrain type,
MARINE CARBON GEOCHEMISTRY 537

and other variables. Once the Ca
2+
and alkalinity are delivered
to the ocean, they are used to produce CaCO
3
according to the
reaction
Ca
2þ
þ 2HCO
3
! CaCO
3
þ H
2
O þ CO
2
ð9Þ
returning the atmospheric CO
2
that weathering borrowed back
to the atmosphere.
CaCO
3
production occurs in shallow ocean waters and in
surface waters overlying the deep sea (Milliman, 1993). In
many cases, in coral reefs for example, the production rate of
CaCO
3
seems to mirror the rate of organic carbon production,
as though the organisms calcified in order to shift the pH bal-
ance back toward the acidic, ensuring a readily available supply
of dissolved CO
2
. Some fraction, roughly half, of the CaCO
3
produced in surface waters dissolves in the water column,
and the rest reaches the sea floor to be redissolved or buried.
The fate of CaCO
3
landing on the sea floor depends strongly
on depth in the ocean, because the solubility of CaCO
3
increases with pressure (Archer, 1996). This is because the dis-
solution reaction entails a small decrease in volume as CaCO
3
dissolves; an increase in pressure encourages the system to find
the volumetrically smaller, dissolved, form. In addition, there is
a surface/deep contrast in the concentration of CO
3
2
, resulting
from the biological pump. We have already seen that the effect
of the biological pump, dominated by the soft-tissue pump, on
the carbon chemistry of the water column is to deplete the sur-
face waters in SCO
2
. As a result of the pH equilibrium reaction
3, dissolved CO
3
2
is increased over the mean ocean value. The
concentration of CO
3
2
goes down with depth, but the amount
of CO
3
2
required in equilibrium goes up with depth. These
factors combine to create a “saturation horizon ” in the water
column, above which CaCO
3
is supersaturated, and below
which it is undersaturated. Some fraction of the CaCO
3
raining
to the sea floor lands on topographic highs, in sediments over-
lain by supersaturated water, and tends to be buried. CaCO
3
landing in abyssal sediments tends to dissolve. The distribution
of CaCO
3
on the sea floor resembles snow-capped mountains,
with preservation on the mountaintops and none in the valleys.
The depth of the “snow line,” where CaCO
3
is no longer
preserved in sediments, has been called the carbonate compen-
sation depth, or CCD. In practice, sedimentary CaCO
3
dissolu-
tion is complicated by the rate of diffusive contact of the pore
water with overlying water, and by other sediment reactions
such as organic carbon respiration. Not all of the CaCO
3
depos-
ited above the saturation horizon is buried, and some of the
CaCO
3
flux to undersaturated sediments may be preserved.
The CCD (a property of the solid sediment) is not the same
as the saturation horizon (a property of the water column). In
most parts of the ocean, the CCD is 0.5–1.5 km deeper than
the saturation horizon (see Carbonate compensation depth;
Carbon dioxide, dissolved (ocean)).
The pH-determining mechanism of the ocean, called carbonate
compensation (beware of the unfortunate overlap with the sepa-
rate concept of the carbonate compensation depth or CCD), arises
from the need for the ocean to balance the input and burial rates of
CaCO
3
. The way the ocean regulates burial to balance weathering
is through the pH of the ocean. The input of CaCO
3
from weather-
ing tends to drive the ocean towards the basic, as a source of excess
alkalinity. The neutralization of the primary CO
3
2
dissolution pro-
duct by atmospheric CO
2
to produce HCO
3
in Reaction 8 is a con-
sumption of the acid from the atmosphere and ocean by CaCO
3
,a
base. If this source of base were unbalanced by CaCO
3
burial
in sediments, the ocean would get progressively more basic over
time. This would increase the proportion of the dissolved carbon
that is CO
3
2
, increasing the depth range over which CaCO
3
is stable and increasing the burial rate of CaCO
3
, until ultimately
the burial rate would balance the weathering rate. The time
scale over which this relaxation takes place is 5–10 kyr
(Archer et al., 1997).
Numerous factors can drive CaCO
3
compensation. An
increase in chemical weathering would drive the CCD deeper,
lowering atmospheric pCO
2
. Today, a significant fraction of
CaCO
3
deposition occurs on continental shelves and carbonate
platforms, which may have been exposed during times of low-
ered sea level such as the Last Glacial Maximum (LGM). A
decrease in shallow-water burial would have required an
increase in deep sea burial to compensate, also lowering
pCO
2
. A decrease in the production rate of CaCO
3
, driven per-
haps by ocean chemistry or climate change, would require that
a greater proportion of the CaCO
3
produced be buried, requir-
ing a deeper saturation horizon and CCD, resulting in lower
pCO
2
. Finally, a CaCO
3
compensation response in the future
will ultimately neutralize much of the fossil fuel CO
2
that
humankind is releasing to the atmosphere. The CO
2
invasion
will acidify the ocean, lowering [CO
3
2
] and decreasing CaCO
3
burial. Excess CaCO
3
source to the ocean will drive the ocean
toward the basic, neutralizing the excess CO
2
into the form of
dissolved HCO
3
in the ocean water column.
David Archer
Bibliography
Archer, D.E., 1996. An atlas of the distribution of calcium carbonate in
sediments of the deep sea. Glob. Biogeochem. Cycles, 10, 159–174.
Archer, D., Kheshgi, H., and Maier-Riemer, E., 1997. Multiple timescales
for neutralization of fossil fuel CO
2
. Geophys. Res. Lett., 24, 405–408.
Armstrong, R.A., Lee, C., Hedges, J.I., Honjo, S., and Wakeham, S.G.,
2002. A new, mechanistic model for organic carbon fluxes in the ocean
based on the quantitative association of POC with ballast minerals.
Deep Sea Res. II , 49, 219–236.
Betzer, P.R., Byrne, R.H., Acker, J.G., Lewis, C.S., and Jolley, R.R., 1984
The oceanic carbonate system: A reassessment of biogenic controls.
Science, 226, 1074–1077.
Broecker, W.S., and Peng, T.H., 1982. Tracers in the Sea. Palisades, NY:
Eldigio Press.
Broecker, W.S., and Peng, T.H., 1987. The role of CaCO
3
compensation in
the glacial to interglacial atmospheric CO2 change. Glob. Biogeochem.
Cycles, 1,15–29.
Dickson, A.G., and Millero, F.J., 1987. A comparison of the equilibrium
constants for the dissociation of carbonic acid in seawater media. Deep
Sea Res., 34, 1733–1743.
Honjo, S., 1996. Fluxes of particles to the interior of the open oceans. In
Ittekkot, V., Aschauffer, P., Honjo, S., and Depetris, P. (eds.), Particle
Flux in the Ocean. New York: Wiley.
Klaas, C., and Archer, D.E., 2002. Association of sinking organic matter
with various types of mineral ballast in the deep sea: Implications for
the rain ratio. Glob. Biogeochem. Cycles, 16, 10.1029/ 2001GB001765.
Longhurst, A., 1998. Ecological Geography of the Sea. San Diego, CA:
Academic Press, 398pp.
Milliman, J.D., 1993. Production and accumulation of calcium carbonate in
the ocean: Budget of a non-steady state. Glob. Biogeochem. Cycles, 7,
927–957.
Stumm, W., and Morgan, J.J., 1981. Aquatic Chemistry. New York: Wiley,
780pp.
Wanninkhof, R., 1992. Relationship between wind speed and gas exchange
over the ocean. J. Geophys. Res., 97(C5), 7373–7382.
Yu, E.-F., Francois, R., Bacon, M.P., Honjo, S., Fleer, A.P., Manganini, S.J.,
Loeff, M.M.R.v.d., and Ittekot, V., 2001. Trapping efficiency of
bottom-tethered sediment traps estimated from the intercepted fluxes
of
230
Th and
231
Pa. Deep Sea Res. I, 48, 865–889.
538 MARINE CARBON GEOCHEMISTRY
