Odekon M. Encyclopedia of paleoclimatology and ancient environments
Подождите немного. Документ загружается.

proportion of
87
Sr is somewhat variable because it is a decay pro-
duct of
87
Rb. The decay constant l for
87
Rb is 1.42 10
–11
a
–1
,
resulting in a half-life T of 48.8 billion years (Ga).
The present-day quantity of isotope 87 (
87
Sr
p
) depends
on the initial stock (
87
Sr
0
) and the amount of radiogenic Sr
generated by decay of
87
Rb over time t
87
Sr
p
¼
87
Sr
0
þ
87
Rb e
l t
1
ð1 Þ
Because of the design of mass spectrometers, the above
quantities are measured as a ratio to the stable isotope
86
Sr
87
Sr
86
Sr
p
¼
87
Sr
86
Sr
0
þ
87
Rb
86
Sr
e
lt
1
ð2 Þ
The young Earth, at the time of its formation 4.55 Ga ago,
inherited the (
87
Sr/
86
Sr)
0
ratio of 0.699 from its chondritic
meteorite building blocks.
From Equation 2, it is evident that the term (
87
Sr /
86
Sr)
p
for
coeval rocks that originate from the same source and inherit
the same (
87
Sr/
86
Sr)
0
ratio depends only on their Rb/Sr ratio.
The latter is about six times larger for the fractionated rocks
that form the continents than for the basalts of the oceanic crust
(0.15 vs. 0.027). As a consequence, the average (
87
Sr/
86
Sr)
p
for continents is about 0.720 while for the basalts of the
oceanic crust it is 0.703 (Figure S32). The temporal evolution
of these two geologic entities is the principal control of chemi-
cal and isotopic composition of seawater, reflecting the relative
inputs from rivers and submarine hydrothermal circulation,
respectively.
Strontium isoto pic evolution of seawater
The present day
87
Sr/
86
Sr ratio of seawater is 0.7092 and is
uniform worldwide. This is because the average time that an
atom of Sr spends in seawater, the so-called residence time, is
somewhere between 2 and 5 million years, while the mixing
rate of the oceans is only thousands of years. Thus, from
the point of view of Sr, the seawater at any given time is
well mixed. Even marginal marine basins, such as the almost
brackish Hudson Bay of northern Canada, have a Sr isotopic
composition identical to that of the open sea. This is because
the Sr concentrations in riverine waters are 2–3 orders of
magnitude less than that of seawater, at 8 ppm, and any
dilution by river water, while lowering the Sr concentration,
will not impact the
87
Sr/
86
Sr ratio because almost the entire
Sr load originates from seawater.
Note that the
87
Sr/
86
Sr ratio of modern seawater is only
about 1/3 of the way up the scale between that of oceanic
and continental crust (Figure S32), despite the fact that modern
river input of Sr is about 6.5 times larger than its hydrothermal
flux (2.73 vs. 0.42 10
12
ga
–1
), and therefore dominates
the oceanic budget. This is because the average
87
Sr/
86
Sr of
river water, at 0.711, is much less radiogenic than that of
the average continental crust. The discrepancy is a reflection
of the fact that carbonate rocks almost always account for a
substantial proportion of the lithology in the watersheds of
major rivers. Carbonate rocks, being marine sediments, inherit
the Sr isotopic composition of seawater at the time of their for-
mation. The oceanic, and particularly the continental crust, was
less radiogenic in the early history of the Earth (Figure S33)
and this was reflected in the contemporaneous seawater and
in the sediments that precipitated from it. Subsequently, weath-
ering of ancient marine carbonates, their redeposition in the sea
and renewed uplift and erosion resulted in perpetual recycling
of this “ancient” Sr, thus “retarding” the
87
Sr/
86
Sr signal of
seawater relative to the development of the continental crust.
Unfortunately, we do not have samples of past seawater that
would enable us to measure directly its past chemical and iso-
topic composition. On the other hand, we do have sediments
that precipitated from seawater, either directly (salts, gypsum)
or with the help of biological intermediaries that precipitated
carbonate (phosphate, siliceous) minerals as shells or cry-
stallites. We can therefore utilize shells for about 540 Ma and
carbonate rocks (limestones, dolostones) for almost 4 Ga to
decipher the Sr isotopic composition of ancient seawater. The
matter is complicated by the fact that many of these shells
and rocks were post-depositionally (diagenetically) altered
and this often results in alteration of the
87
Sr/
86
Sr signal. Tech-
niques do exist for at least partial evaluation of the degree of
such alteration, but none is foolproof. Usually, the alteration
process shifts the
87
Sr/
86
Sr values upwards and it is common
practice to accept the lowest measured value for any suite of
samples as the best approximation of the
87
Sr/
86
Sr ratio of the
coeval ocean.
In the early stages of isotope geology it was believed that the
87
Sr/
86
Sr of seawater evolved linearly during geologic history
(Wickman, 1948) and it was hoped that such a linear trend could
be utilized for dating of (bio)chemical marine sediments. Due to
the low sensitivity of early mass spectrometers, this proposition
could only be tested some 20 years later, when it was realized
that we were dealing with a widely oscillating signal (Peterman
et al., 1970). This was subsequently documented by Veizer and
Compston (1974, 1976) and Burke et al. (1982). The latest edi-
tions of these curves for the entire 4 Ga of Earth history were
published by Shields and Veizer (2002)(Figure S33) and for
the Phanerozoic by Veizer et al. (1999)(Figure S34).
The most important features of the billion-year trend in
87
Sr/
86
Sr (Figure S33) are the departure of seawater from
mantle values somewhere around the Archean/Proterozoic
transition, 2.5 Ga ago; the relatively sharp rise until about
1.8 Ga ago; the flat trend to 900 Ma ago; and another steep
rise during the Neoproterozoic, followed by a trough-like struc-
ture during the Phanerozoic. The Precambrian portion of the
0.720
0.710
0.700
Continental
crust
Rivers
“Upper
mantle”
Erosion
?
?
?
?
?
Seawater
Sediments
Diagenesis
Subduction
Dissolved
load
of
rivers
Weathering
volcanism
hydrothermal alteration
87
Sr
86
Sr
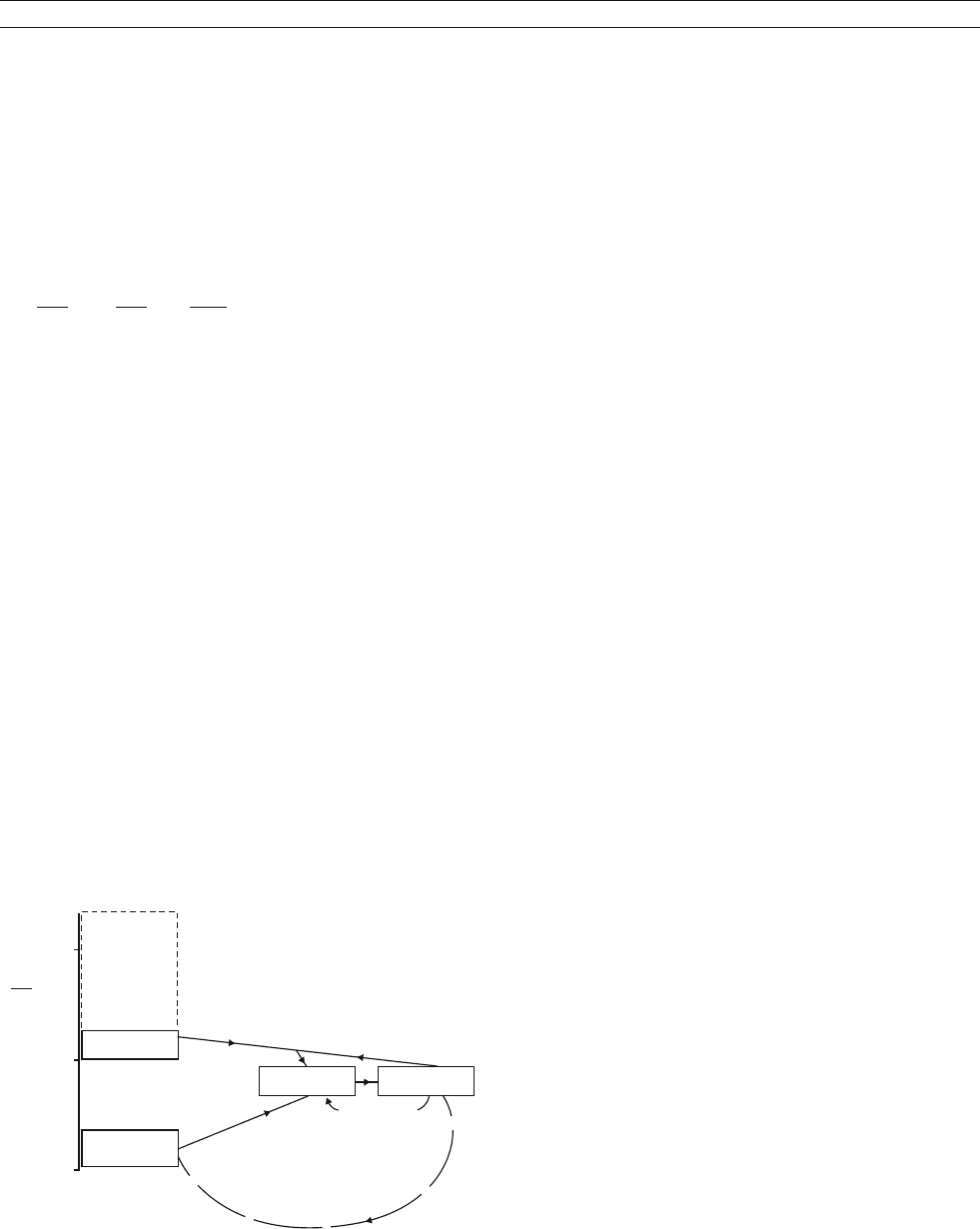
Figure S32 Schematic presentation of Sr isotopic surficial cycle
(modified after Wadleigh, 1982).
924 STRONTIUM ISOTOPES
curve is in all likelihood composed of higher order oscillations
similar to those of the Phanerozoic, but we do not yet have the
geochronological resolution to decipher it. For this reason, we
can discuss only the lower bound of the trend. The mantle-
like values of seawater during the early Archean indicate that
either the river input was negligible, or the hydrothermal flux
was much stronger than today, or both. The geological interpre-
tation is that the continents could have been much smaller
and seawater circulation via submarine hydrothermal systems
(in order to dissipate heat from the hotter mantle) more vigor-
ous. This was followed by the major interval of generation
of continental crust, between 2.7 and 1.8 Ga ago, the cumu-
lative size of continents reached near-modern extent towards
the end of this time interval. Simultaneously, the chemical
and isotopic composition of the oceans evolved from being
“mantle-buffered” to “river-buffered,” and this development
n = 5059
4,000 3,000 2,000 1,000 0
A
g
e (Ma)
0.710
0.708
0.706
0.704
0.702
0.700
Barites
Mantle evolution
Seawater evolution
87
Sr/
86
Sr
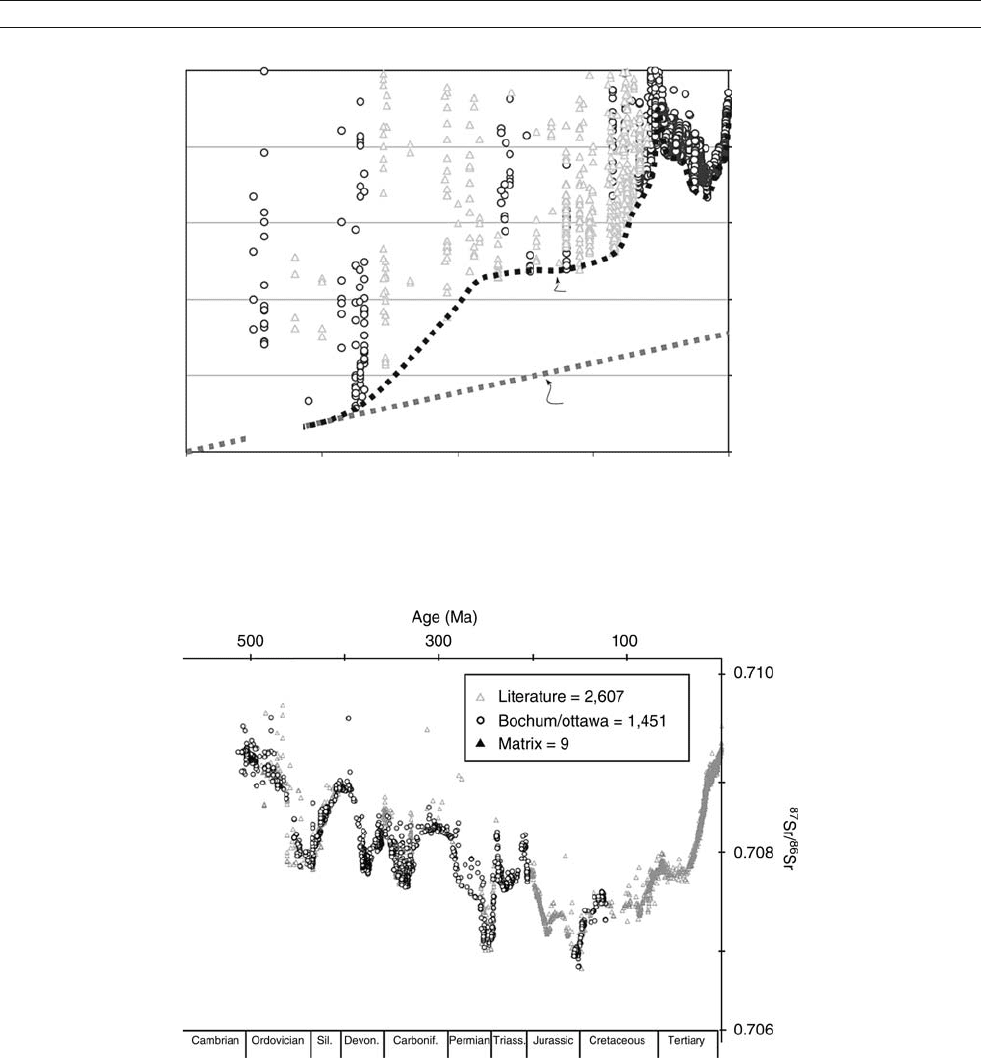
Figure S33 Strontium isotope record of carbonate rocks (seawater) during geologic history. Full symbols: chronologically well constrained; empty
symbols: poorly constrained samples (reproduced from Shields and Veizer, 2002).
Figure S34 Sr isotope record for Phanerozoic seawater based on 4,055 samples of calcite shells (reproduced from Veizer et al., 1999).
STRONTIUM ISOTOPES 925
may also have been the cause of other contemporary phenom-
ena, such as the transition from oxygen-poor to oxygen-rich
atmospheric composition. The steep rise in
87
Sr /
86
Sr in the
Neoproterozoic may be related to another pronounced phase
of continent formation, the Pan African orogeny.
Th e o rigin of t he troug h-like P han er ozoic struct ure
( Figure S34) with its superimposed oscillations is at this
stage enigmatic. Attempts to correlate the details of this struc-
ture with tectonic development of the Earth have not led to
any unequivocal conclusions. A great deal of attention was
paid particularly to the steep Tertiary rise of
87
Sr /
86
Sr values,
that was assumed to be a reflection of the collision between
India and Asia, with the resulting uplift of the Himalayas.
The corollary of such an interpretation was a potential role that
this uplift could have played in increased global erosion that, in
turn, would have resulted in atmospheric CO
2
drawdown and
the overall Tertiary cooling of the globe (Ruddiman, 1997).
However, the modeling simulations do not appear to support
the validity of such a scenario.
Isoto pe stratigrap hy
Despite the fact that the
87
Sr /
86
Sr trend for seawater is not
linear, the sections of this oscillating trend that show a particu-
larly steep slope can be utilized for dating and correlation
of marine (bio)chemical sediments, the approach known as iso-
tope stratigraphy (McArthur, 1994). In particular, the Cenozoic
portion of the trend ( Figure S34) enabled correlations at levels
comparable to biostratigraphic techniques.
Ján Veizer
Bibliogra phy
Burke , W. H., D enison, R.E., H etherington, E .A., K oepnick, R .B ., Ne ls on, H.F. ,
and O tto, J .B., 1982. Va ri ation of s eawater
87
Sr /
86
Sr throughout
Phane rozoic time. Geology, 10 , 516–519.
Faure, G., 1986. Principles of Isotope Geology. New York: Wiley, 589pp.
McArthur, J.M., 1994. Recent trends in strontium isotope stratigraphy.
Terra Nova, 6, 331–358.
Peterman, Z.E., Hedge, C.E., and Tourtelot, H.A., 1970. Isotopic composi-
tion of strontium in seawater throughout Phanerozoic time. Geochim.
Cosmochim. Acta, 34, 105– 120.
Ruddiman, W.F., 1997. Tectonic Uplift and Climate Change. New York:
Plenum Press, 535pp.
Shields, G., and Veizer, J., 2002. Precambrian marine carbonate isotope
database: Version 1.1. Geochem. Geophys. Geosyst., 3(6), 12 ( http://
g-cubed.org /gc2002 / 2001GC000266).
Veizer, J., and Compston, W., 1974.
87
Sr /
86
Sr composition of seawater
during the Phanerozoic. Geochim. Cosmochim. Acta, 38, 1461–1484.
Veizer, J., and Compston, W., 1976.
87
Sr /
86
Sr in Precambrian carbonates as
an index of crustal evolution. Geochim. Cosmochim. Acta, 40,
905– 914.
Veizer, J., Ala, D., Azmy, K., Bruckschen, P., Buhl, D., Bruhn, F.,
Carden, G.A.F., Diener, A., Ebneth, S., Goddéris, Y., Jasper, T.,
Korte, C., Pawellek, F., Podlaha, O.G., and Strauss, H., 1999.
87
Sr /
86
Sr,
d
13
C and d
18
O evolution of Phanerozoic seawater. Chem. Geol.,
161,59–88.
Wadleigh, M.A., 1982. Marine Geochemical Cycle of Strontium. M.Sc.
Thesis, University of Ottawa, ON, 187pp.
Wickman, P.E., 1948. Isotope ratios: A clue to the age of certain marine
sediments. J. Geol., 56,61–66.
Cross- refere nces
Atmospheric Evolution, Earth
Mountain Uplift and Climate Change
Stable Isotope Analysis
SULFUR ISOTOPES
Sulfur isotope systematic s
The element sulfur has four stable isotopes
32
S,
33
S,
34
S and
36
S
with the following natural abundances: 95.02%, 0.75%, 4.21%,
and 0.02% (Hoefs, 1997). Most commonly, the two major iso-
topes (
32
S,
34
S) are being measured and results expressed as
d
34
S, defined by the following equation
d
34
S %½¼
34
S
32
S
Sample
34
S
32
S
Standard
1
0
B
@
1
C
A
1 ; 000 ð1 Þ
and reported as per mil difference with respect to the V-CDT-
standard (defining the Zero-Point of the sulfur isotope scale).
Due to analytical improvements, the minor sulfur isotopes
33
S
and
36
S can now be measured routinely with their delta values
defined analogous to equation 1.
Sulfur is present in the atmosphere, hydrosphere, biosphere
and lithosphere ( Figure S35). In addition to natural processes,
the present day sulfur cycle is strongly affected by anthropo-
genic sulfur inputs. The transfer of sulfur between the different
reservoirs is mediated through abiological and biological pro-
cesses. Such processes are generally associated with a change
in the redox state and an isotopic fractionation of variable
magnitude, depending on the mode of isotope exchange (i.e.,
equilibrium vs. kinetic isotope effect). From the geological per-
spective, research focuses on sulfate as the most oxidized and
sulfide as the most reduced form of sulfur. Respective pro-
cesses are the precipitation of evaporitic sulfate from seawater
and the bacterial cycling of sulfur in sediments.
The pure precipitation of sulfate minerals from evaporating
seawater is only associated with a small, almost negligible iso-
tope effect (Claypool et al., 1980). As a consequence, marine
evaporites and sulfate incorporated into marine chemical and
biological precipitates are regarded as proxy signals for the iso-
topic composition of seawater sulfate (e.g., Strauss, 1999;
Kampschulte and Strauss, 2003).
In contrast, variable but generally substantial isotopic fractio-
nation occurs during bacterial sulfate reduction (e.g., Canfield,
2001). At the expense of sedimentary organic matter, sulfate
is reduced to hydrogen sulfide, which subsequently reacts with
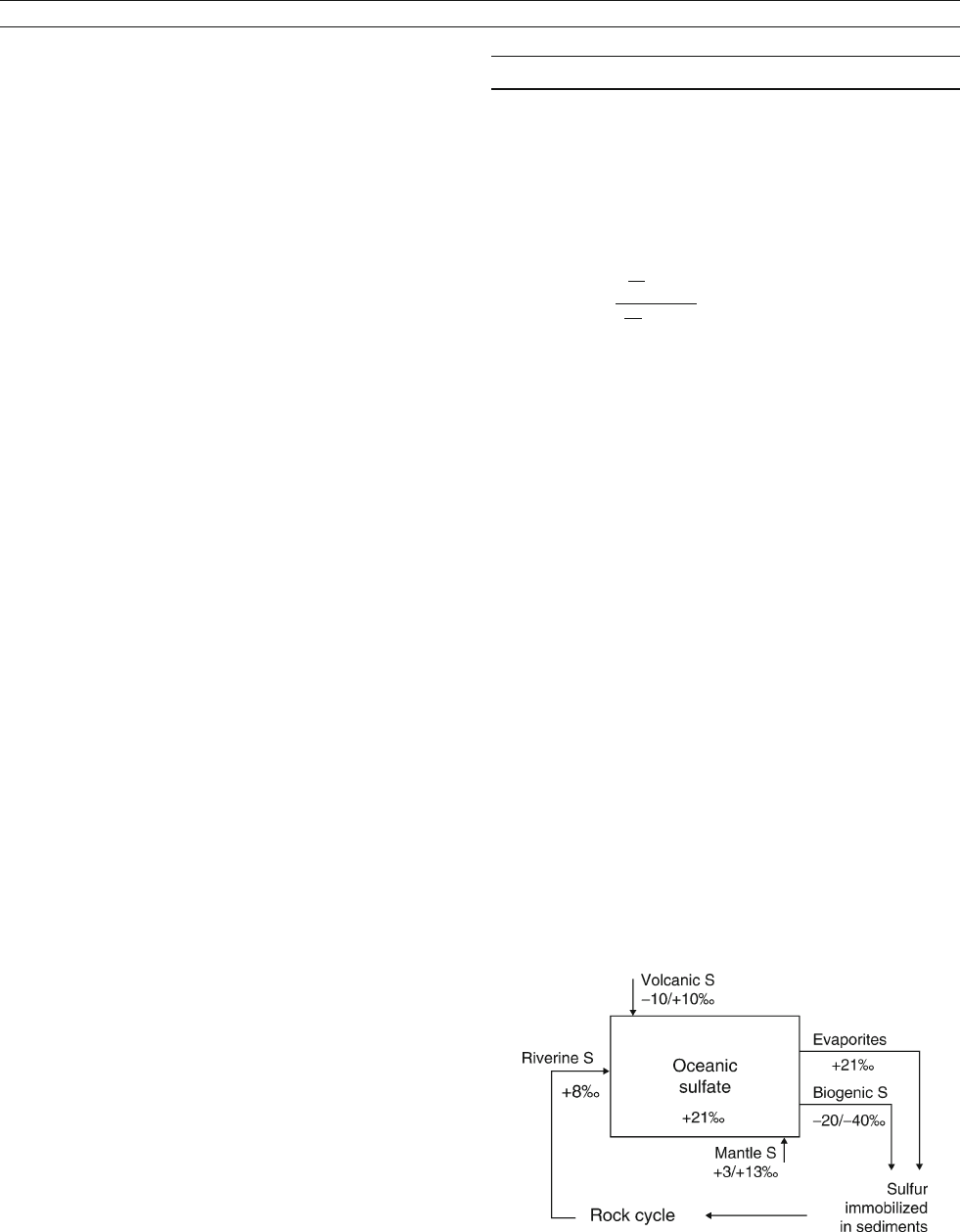
Figure S35 Schematic view of the sulfur cycle showing d
34
S values for
modern reservoirs.
926 SULFUR ISOTOPES
Fe to form sedimentary pyrite (FeS
2
). The resulting sulfide
is depleted in
34
S with respect to ambient sulfate due to the
preferential utilization of
32
S by sulfate-reducing bacteria.
The size of this isotope effect is dependent on the type of organ-
ism, the reaction rate, and the availability of sulfate and reac-
tive organic matter (Canfield, 2001). Experimental data from
pure cultures indicate an isotopic fractionation of 2–42% (e.g.,
Detmers et al., 2001), whereas natural populations display an iso-
tope effect of 19–43% (Habicht and Canfield, 2001). For mod-
ern marine sediments, however, the isotope difference between
porewater sulfate and sedimentary pyrite amounts to 31–56%
(Habicht and Canfield, 2001). This additional isotopic frac-
tionation accompanies the disproportionation of partly oxidized
intermediate sulfur compounds: elemental sulfur, thiosulfate,
and sulfite (e.g., Canfield and Thamdrup, 1994; Habicht et al.,
1998). Measurements in recent marine sediments indicate that
41–85% of the apparent isotopic fractionation between seawater
sulfate and sedimentary sulfide are the result of bacterial sulfate
reduction, with the remaining part being due to disproportiona-
tion reactions (Habicht and Canfield, 2001 ).
Progressive bacterial sulfate reduction under sulfate-limiting
conditions (e.g., porewaters in deep sediments) results in a con-
tinuous depletion of
32
S. As a consequence, the d
34
S becomes
increasingly more positive. This explains part of the sulfur iso-
tope record for reduced sedimentary sulfur.
The oxidative weathering of sulfur-bearing minerals and
subsequent riverine delivery as dissolved sulfate from the
continents to the ocean is not associated with an isotope effect.
The long-term evolution of the global sulfur cycle can be
evaluated via simple isotope mass balance calculations. Con-
sidering the two principal output functions as oxidized (sulfate)
and reduced (sulfide) sulfur (see Figure S35), this mass balance
is defined as:
d
Input
¼ f
Sulfide
d
Sulfide
1 f
Sulfide
ðÞd
Sulfate
ð2Þ
with d
Input
representing the average isotopic composition of
crustal sulfur (assumed constant at + 2%, Holser et al., 1988)
and f
Sulfide
d
Sulfide
and (1–f
Sulfide
)d
Sulfate
being the fraction and
isotopic composition of sulfide and sulfate sulfur. Fluctuations
in the sulfur isotopic composition of seawater sulfate can then
be interpreted as a consequence of temporal variations in the
fractional burial of reduced (sulfide) sulfur.
Sulfur isotopic evolution of seawater
The average sulfur isotopic composition of dissolved oceanic
sulfate lies at + 21% (e.g., Rees et al., 1978). Lateral and
vertical homogeneity of this value is a consequence of
a long residence time for sulfur in the ocean (3 million
years) in comparison to the short mixing time for the ocean
(1,000 years). Only marginal seas, such as the eastern Mediter-
ranean or the Baltic Sea, represent exceptions. Their isotopic
composition is more strongly affected by the riverine input of
dissolved sulfate (on average + 8 to + 10%). However, part
of this input stems from anthropogenic sources (e.g., Grinenko
and Krouse, 1992).
Despite its homogeneity at any given point in time (e.g.,
Nielsen, 1989), empirical data clearly show that the sulfur iso-
topic composition of seawater sulfate has fluctuated widely
throughout four billion years of Earth’s history (e.g., Strauss,
2002). However, the existing isotope record is highly variable
in its temporal resolution and becomes increasingly fragmen-
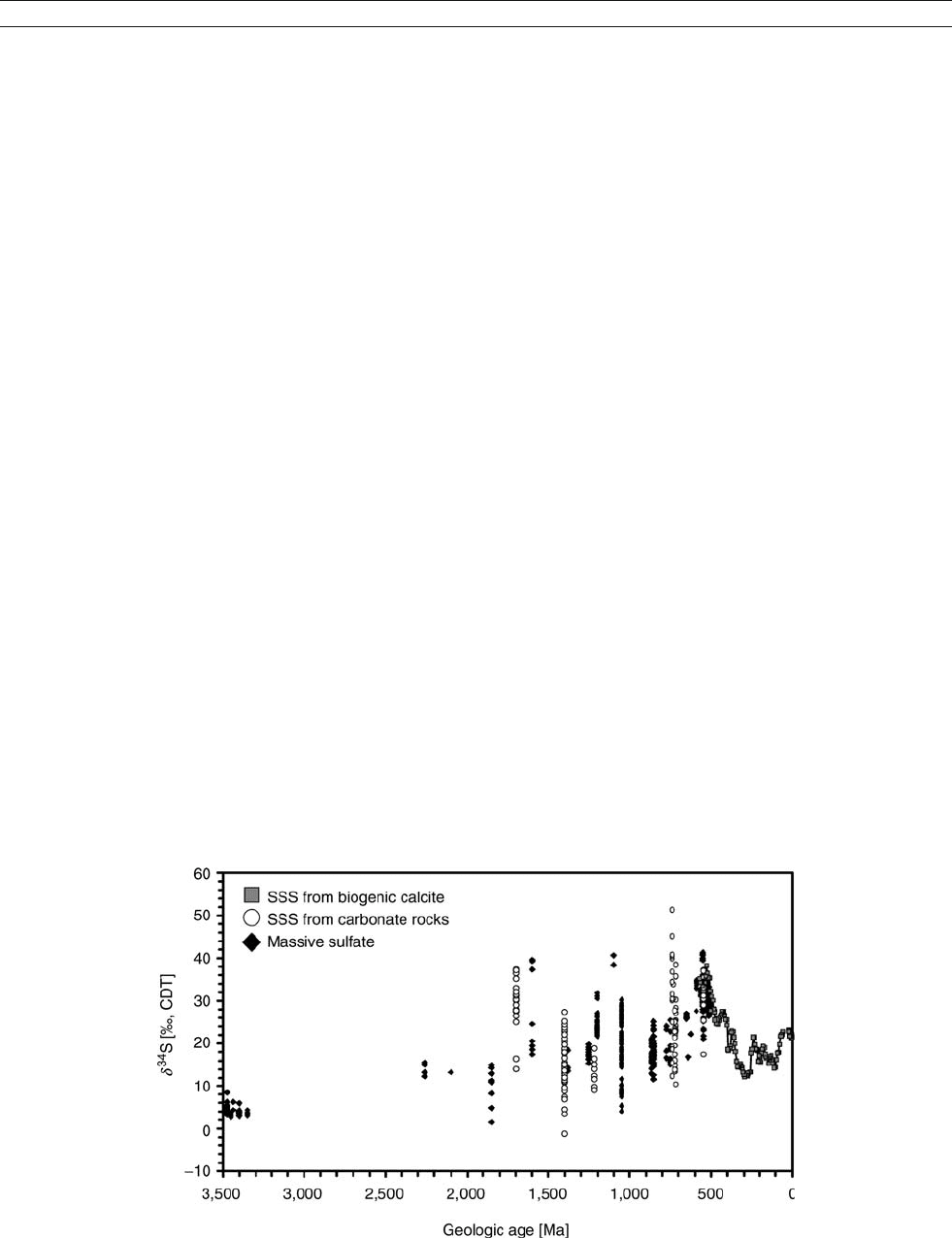
tary for the early part of Earth’s history (Figure S36).
Early Archean (3.5 billion years ago) barite occurrences from
Australia, South Africa and India constrain our knowledge
about the sulfur isotopic composition of seawater sulfate from
the early part of Earth’s history at a value around þ 4% (Strauss,
2002). Sulfate abundance in the global ocean is considered to
have been very low (<1 mM, Canfield et al., 2000). This inter-
pretation is supported by a lack of substantial sulfur isotopic
fractionation between sulfate and sedimentary sulfide (e.g.,
Strauss, 2002). Still, contrasting views of an Archean high-
sulfate ocean have been proposed (e.g., Ohmoto, 1997).
The sulfur isotopic composition of seawater sulfate
increases throughout the Precambrian. Due to the fragmentary
record, it is unclear whether this increase is linear or episodic.
A significant the sulfur isotopic composition of seawater sul-
fate is considered to occur between 2.4 and 2.2 billion years
ago. It is thought to coincide with a substantial increase in
the oxygen abundance in the atmosphere, termed the Great
Oxidation Event (Holland, 2002). This would have initiated
Figure S36 Sulfur isotopic evolution of seawater sulfate.
SULFUR ISOTOPES 927
the oxidative weathering on the continents, delivering dis-
solved sulfate to the ocean.
The resulting increase in oceanic sulfate abundance would
have triggered an increase in sulfur cycling via bacterial sulfate
reduction (Canfield and Raiswell, 1999). Supporting evidence
stems from the observation that large sulfur isotopic fractiona-
tions between sulfate and sedimentary sulfide commence at that
time (e.g., Strauss, 2002). These are comparable in size to those
recorded from younger times and modern marine sediments.
Towards the end of the Precambrian (600–500 million years
ago), the average sulfur isotopic composition of seawater
sulfate reaches its all-time maximum at + 35%. This high
d
34
S value continues into the Phanerozoic.
Substantial fluctuations in the sulfur isotopic composi-
tion of seawater sulfate characterize the Phanerozoic (the last
545 million years). The high d
34
S values at the beginning of this
era are followed by a long-term decrease to a minimum value
of + 11% in the Permian (some 250 million years ago) and a sub-
sequent rise to the present day value. A detailed isotope record
with good temporal resolution, based on structurally substituted
sulfate (SSS) in biogenic carbonates, reveals additional higher
order oscillations (Kampschulte and Strauss, 2003).
Following the simple isotope mass balance (Equation ( 2))
these temporal variations in d
34
S of seawater sulfate reflect var-
iations in the fractional burial of reduced sedimentary sulfur.
Potential causes for such variations include habitat size (near
shore shelf area) and availability of reactive organic matter.
Evolution of biological sulfur cycling
The observation of a large sulfur isotope effect associated with
bacterial sulfate reduction has prompted researchers to utilize
this as a proxy for biogenicity in their studies of sedimentary
pyrite. The resulting sulfur isotope record (Figure S37) reveals
a temporal evolution that parallels the evolution of seawater
sulfate in many respects.
Early Archean sedimentary pyrite generally displays a very
limited variation in d
34
S near 0% and only a small difference
to the sulfur isotopic composition of barite sulfur (e.g., Strauss,
2002). Lack of a large isotope effect suggests that bacterial sul-
fur cycling played only a minor role in sedimentary systems.
Starting in the late Archean/early Proterozoic (about 2.8–2.4
billion years ago) a substantial spread in d
34
S for sedimentary
sulfide indicates sizeable sulfur isotopic fractionations. These
are attributed to the activity of sulfate-reducing bacteria.
Throughout the Proterozoic (2.5–0.5 billion years ago), the sul-
fur isotopic composition of sedimentary pyrite remains highly
variable. Maximum isotopic fractionation between oceanic
sulfate and sedimentary pyrite ranges between 30 and 40%,
reflecting bacterial sulfate reduction. The Neoproterozoic
(1,000–545 million years ago) witnesses not only large spreads
in d
34
S but also strongly positive,
34
S enriched sulfur isotope
values for sedimentary sulfide. Considering minimum d
34
S
values for sedimentary pyrite and d
34
S values of + 35% for
Neoproterozoic seawater sulfate results in a maximum isotopic
fractionation of 60%. Such a large apparent isotope fractiona-
tion reflects the isotope effect associated with disproportiona-
tion reactions in addition to bacterial sulfate reduction.
The Phanerozoic (the last 545 million years) sulfur isotope
record for sedimentary sulfur displays comparable large varia-
tions in d
34
S and an apparent isotopic fractionation that reflects
sulfate reduction and disproportionation. The sulfur isotope
record for reduced sulfur parallels roughly the respective record
for seawater sulfate (Figure S36): high d
34
S values in the early
Phanerozoic, followed by a decrease towards the Permian and a
subsequent rise during Mesozoic and Cenozoic. The compar-
able temporal evolution of both sulfur isotope records at least
during the last 600 million years suggests that bacterial sulfate
reduction and disproportionation of intermediate sulfur com-
pounds have been the key processes in biological sulfur cycling
in sedimentary environments.
Multiple sulfur isotopic evidence for an atmospheric
impact on the early sulfur cycle
The measurement of multiple sulfur isotopes (
32
S,
33
S,
34
S,
36
S)
reveal that all physical, chemical and biological processes on
Earth result in mass-dependent fractionation (MDF) of these
isotopes. Hence, mass-dependent relations can be defined as:
d
33
S
TFL
¼ 0:515 d
34
S ð3Þ
60
50
40
30
20
10
0
−10
−20
−30
−40
4,000 3,500 3,000 2,500 2,000
Geolo
g
ic a
g
e [Ma]
d
34
S [‰, CDT]
1,500 1,000 500 0
Figure S37 Sulfur isotopic composition of reduced sedimentary sulfur.
928 SULFUR ISOTOPES
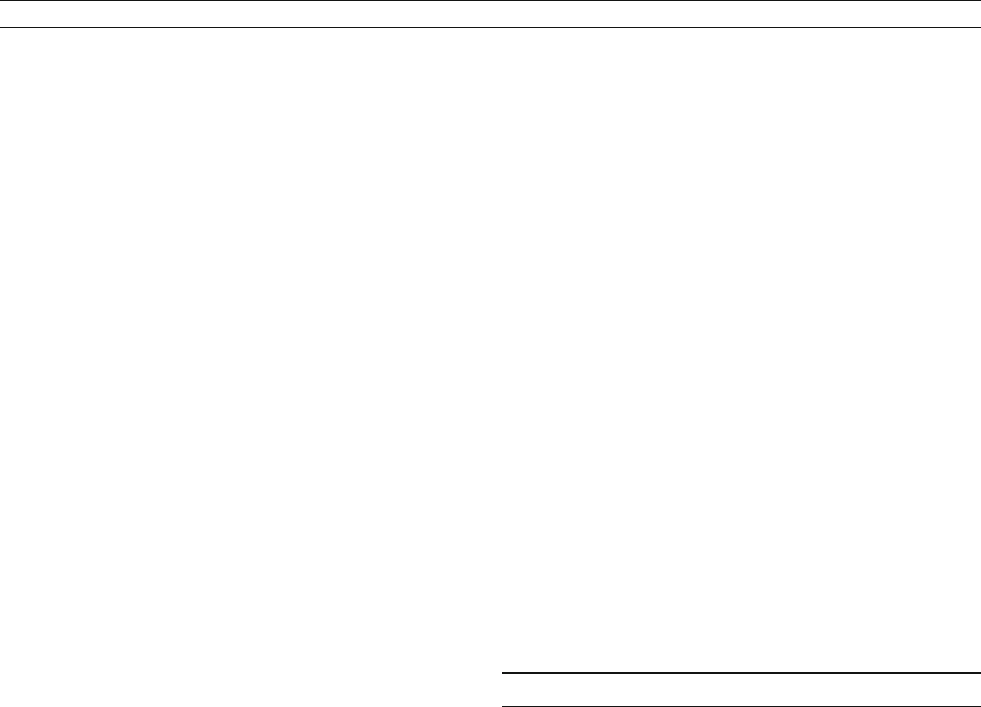
d
36
S
TFL
¼ 1:9 d
34
S ð4Þ
with data falling on a respective Terrestrial Fractionation Line
(TFL) in a three-isotope-plot. Multiple sulfur isotope analyses
of sedimentary sulfide and sulfate of Archean and early Paleo-
proterozoic age (>2350 million years) yielded clear deviations
from these mass-dependent relationships (e.g., Farquhar et al.,
2000). Such mass-independent fractionation (MIF) in a mea-
sured sample can be quantified as:
D
33
S ¼ d
33
S
measured
d
33
S
TFL
ð5Þ
D
36
S ¼ d
36
S
measured
d
36
S
TFL
ð6Þ
Based on experimental and modelling data, this mass-indepen-
dent isotope fractionation is believed to result from photoche-
mical reactions in the atmosphere involving gaseous sulfur
species, in particular sulfur dioxide. Furthermore, respective
reactions require the absence of an effective UV shield (like
ozone). Consequently, the presence of MIF-S has been taken
as evidence for the absence of free oxygen in the Archean
and early Paleoproterozoic atmosphere (e.g., Farquhar et al.,
2000; 2007). Modelling evidence suggests that the maximum
concentration of atmospheric oxygen had to be <10
5
of the
present atmospheric level (PAL) in order to yield MIF-S. Sedi-
mentary sulfur younger than 2350 million years displays only
mass-dependent sulfur isotopic fractionation, indicating that
the atmospheric oxygen level had increased to >10
2
PAL
(e.g., Pavlov and Kasting, 2002).
Harald Strauss and Ján Veizer
Bibliography
Canfield, D.E., 2001. Biogeochemistry of sulfur isotopes. In Valley, J.W.,
and Cole, D.R. (eds.), Stable Isotope Geochemistry. Rev. Mineral. Geo-
chem., 43, 607–636.
Canfield, D.E., and Raiswell, R., 1999. The evolution of the sulfur cycle.
Am. J. Sci., 299, 697–723.
Canfield, D.E., and Thamdrup, B., 1994. The production of
34
S-depleted
sulphide during bacterial disproportionation of elemental sulphur.
Science, 266, 1973–1975.
Canfield, D.E., Habicht, K.S., and Thamdrup, B., 2000. The Archean sulfur
cycle and the early history of atmospheric oxygen. Science, 288,
658–661.
Clark, I., and Fritz, P., 1997. Environmental Isotopes in Hydrogeology.
Boca Raton, FL: CRC Press, 328pp.
Claypool, G.E., Holser, W.T., Kaplan, I.R., Sakai, H., and Zak, I., 1980.
The age curve of sulfur and oxygen isotopes in marine sulfate and their
mutual interpretation. Chem. Geol., 28, 190–260.
Detmers, J., Brüchert, V., Habicht, K. S., and Kuever, J., 2001. Diversity of
sulphur isotope fractionations by sulphate-reducing prokaryotes. Appl.
Environ. Microbiol., 67, 888–894.
Farquhar, J., Bao, H. M., and Thiemens, M., 2000. Atmospheric influence
of Earth's earliest sulfur cycle. Science, 289, 756–758.
Farquhar, J., Peters, M., Johnston, D.T., Strauss, H., Masterson, A.,
Wiechert, U., and Kaufman, A.J., 2007. Isotopic evidence for
Mesoarchaean anoxia and changing atmospheric sulphur chemistry.
Nature, 449, 706–709.
Grinenko, V., and Krouse, H.R., 1992. Isotope data on the nature of river-
ine sulfates. Mitt. Geol.-Paläont. Inst. Univ. Hamburg, 72,9–18.
Habicht, K.S., and Canfield, D.E., 2001. Isotope fractionation by sulphate-
reducting natural populations and the isotopic composition of sulphides
in marine sediments. Geology, 29, 555–558.
Habicht, K.S., Canfield, D.E., and Rethmeier, J., 1998. Sulphur isotope
fractionation during bacterial sulphate reduction and disproportionation
of thiosulphate and sulfite. Geochim. Cosmochim. Acta, 62,
2585–2595.
Hoefs, J., 1997. Stable Isotope Geochemistry. Berlin: Springer, 201pp.
Holland, H.D., 2002. Volcanic gases, black smokers, and the great oxida-
tion event. Geochim. Cosmochim. Acta, 66, 3811–3826.
Holser, W.T., Schidlowski, M., Mackenzie, F.T., and Maynard, J.B., 1988.
Geochemical cycles of carbon and sulfur. In Gregor, C.B., Garrels, R.M.,
Mackenzie, F.T., and Maynard, J.B. (eds.), Chemical Cycles in the Evolu-
tion of the Earth. New York: Wiley, pp. 105–173.
Kampschulte, A., and Strauss, H., 2003. The sulphur isotopic evolution of
Phanerozoic seawater based on the analysis of structurally substituted
sulphate in carbonates. Chem. Geol.
, 204(3–4), 255–286.
Nielsen, H., 1989. Local and global aspects of the sulphur isotope age
curve of oceanic sulphate. In Brimblecombe, P., and Lein, A.Y. (eds.),
Evolution of the Global Biogeochemical Sulphur Cycle. New York:
Wiley, pp. 57–64.
Ohmoto, H., 1997. When did the Earth’s atmosphere become oxic? The
Geochemical News, 93-Fall,12–28.
Pavlov, A.A., Kasting, J.F., 2002. Mass-independent fractionation of sulfur
isotopes in Archean sediments: Strong evidence for an anoxic Archean
atmosphere. Astrobiology, 2,27–41.
Rees, C.E., Jenkins, W.J., and Monster, J., 1978. The sulphur isotopic com-
position of ocean water sulphate. Geochim. Cosmochim. Acta, 42,
377–381.
Strauss, H., 1999. Geological evolution from isotope proxy signals –
sulphur. Chem. Geol., 161,89–101.
Strauss, H., 2002. The isotopic composition of Precambrian sulphides –
seawater chemistry and biological evolution. Spec. Publ. Int. Assoc.
Sediment., 33,67–105.
Cross-references
Atmospheric Evolution, Earth
Carbon Isotope Variations over Geologic Time
Isotope Fractionation
Stable Isotope Analysis
SUN-CLIMATE CONNECTIONS
Introduction
All energy distributed in the climate system originates from
the Sun. Earth’s surface temperature is maintained by a balance
between incoming and outgoing radiation. Irradiance is the most
important parameter related to solar variability, as the relationship
between irradiance and climate in radiative balance is simple and
direct. The Earth currently receives an average of 1,367 W m
2
,
integrated over all wavelengths. Climate variability directly
associated with the radiative imbalance caused by changes in
solar radiation is “solar forced.” In contrast, a “solar influ-
enced” or “solar triggered” climate change depends mostly on
atmospheric or oceanic feedback mechanisms for the effect.
The Sun also generates strong magnetic fields. Through
their interaction with Earth’s magnetic field and charged parti-
cles such as cosmic rays, magnetic fields are central to both
observational and proxy records establishing solar variability.
A current challenge in Sun-climate research is the interpreta-
tion of long-term proxy records related to the Sun’s magnetic
activity. It will only be possible to confirm a Sun-climate
connection for past climates when the innermost workings of
the Sun are understood well enough to quantify the relation
between magnetic proxies and irradiance.
On millennial and shorter time scales, there is evidence
that small changes in irradiance affect climate. It has proven
difficult to identify the physical mechanisms by which small
observed and reconstructed irradiance changes (a few tenths
of a percent) could produce observed and reconstructed climate
changes, up to 2
C within 50 years (Bond et al., 2001). Over
SUN-CLIMATE CONNECTIONS 929
the past ten years, researchers have discovered that the atmos-
phere and ocean can undergo abrupt re-organizations when per-
turbed only slightly. This can cause large regional climate
change while global average changes are small. Positive feed-
back systems have been proposed involving the upper and
lower atmosphere, oceans, and polar ice. These can amplify
the effects of small irradiance changes, and are likely critical
for Sun-climate connections unless past solar variability was
much larger than present estimates.
Solar variability
Solar cycles and sunspots
Perhaps the best-known indirect index of solar variability is the
11-year sunspot cycle. Over this cycle, the number of dark
spots visible on the Sun’s surface ranges from 0 to over 200.
Sunspots are associated with strong local magnetic activity
inhibiting surface convection, leading to cooling and darkening
relative to the rest of the visible solar surface. Bright regions
called faculae surround sunspots, causing a net increase in total
solar irradiance (TSI) of about 0.1% during sunspot peaks rela-
tive to sunspot minima. Sunspot numbers from 1610 to present
are shown in Figure S38, with the relationship to TSI measured
by satellites since 1978 shown in Figure S39.
Though measured irradiance and sunspot number are highly
correlated, direct comparison is only possible for the past three
solar cycles. The relation between long-term trends in sunspot
numbers (Figure S38) and irradiance is unclear. Sunspot num-
bers return nearly to 0 during cycle minima, while during the
Maunder Minimum, from 1645–1715, hardly any sunspots
were observed in 50 years. If irradiance varied over this period,
it must be reconstructed from records of solar activity other
than sunspot numbers.
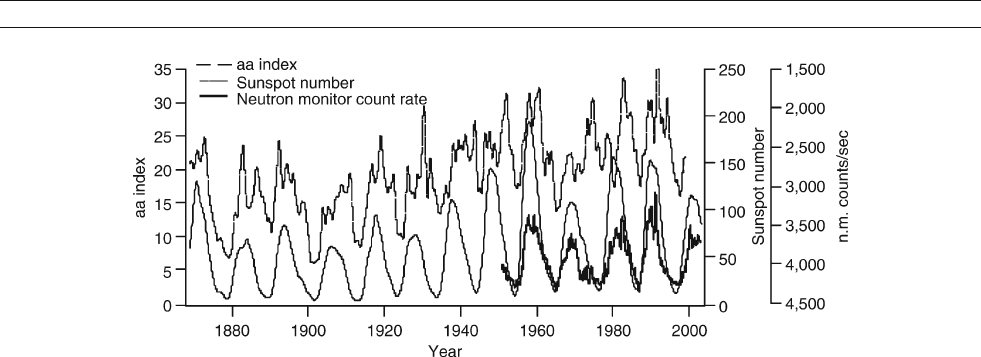
Solar magnetic activity and the geomagnetic field
Solar magnetic activity is evident on Earth due to interaction
with the geomagnetic field. The aa index (Figure S40)isa
measure of the Earth’s magnetic field constructed from surface
magnetometers.
A portion of the solar magnetic field is carried through
the solar system and beyond by the solar wind. This is the
interplanetary magnetic field (IMF), which influences Earth’s
magnetic field. Increasing IMF strength over the past 100 years
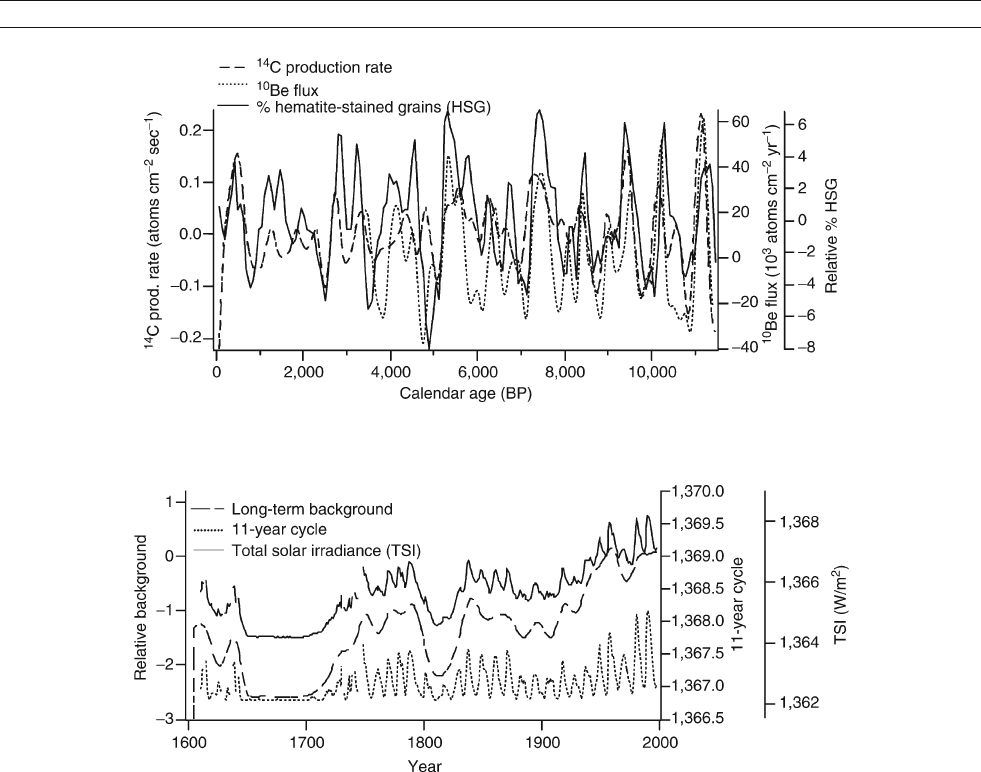
Figure S38 Sunspot numbers and global radiocarbon production rate show coherent variability. Carbon-14 production from nuclear weapons
testing limits comparison with sunspots to before 1950. Note the lack of sunspots during the Maunder Minimum, and the increasing amplitudes
of the solar cycles (data from National Geophysical Data Center, USA).
Figure S39 Total solar irradiance and sunspots are closely associated for the past three solar cycles (data from National Geophysical Data
Center, USA).
930 SUN-CLIMATE CONNECTIONS
is responsible for the long-term upward trend of the aa index
(Lockwood et al., 1998). Though there is an 11-year cycle in aa
in addition to the long-term trend, it is not directly convertible
to irradiance because the IMF has a different origin from
magnetic activity associated with sunspots (Lean et al., 2002).
Solar variability and cosmogenic isotopes
The Earth is continually being bombarded by galactic cosmic
radiation (GCR): high-energy charged particles originating
mostly from outside the solar system. The charge on cosmic
ray particles subjects them to deflection by solar and terrestrial
magnetic fields. The partial shielding of Earth by the coupled
solar and geomagnetic fields is modulated by variability in
those fields. This is clear from cosmic ray neutron monitor meas-
urements taken since the 1950s. Figure S40 shows the neutron
monitor counting rate (GCR flux) from Climax, Colorado, USA,
which varies inversely with sunspot number.
Cosmic rays interact with Earth materials to produce cos-
mogenic isotopes, both in the atmosphere and in geologic
materials. Two atmospherically produced cosmogenic isotopes,
14
C and
10
Be, are among the most useful proxy records for solar
modulation of cosmic rays. Carbon-14 is produced from cos-
mic ray neutron reactions with nitrogen. It is rapidly oxidized
to
14
CO
2
, and mixed throughout the atmosphere within about
one year. Living trees incorporate
14
C in their tissues during
photosynthesis. Measurements of
14
C in German oak tree rings
provide a continuous record of atmospheric
14
C concentration
back to almost 12,000 years. One complication in using atmo-
spheric
14
C as a proxy for solar variability is its sensitivity to
cycling of carbon in the ocean (Stuiver, 1994). In some ocean
basins, surface water is transported to the deep ocean via ther-
mohaline circulation (THC), where it may reside for more than
1,000 years before returning to the surface. Rapid changes in
THC can affect atmospheric
14
CO
2
concentration independently
of its production. By increasing THC, more
14
CO
2
-equilibrated
water is sequestered in the ocean, drawing down atmospheric
14
C. Atmosphere-ocean carbon cycling effects in tree ring
records may be evaluated by comparison with records of other,
non-cycling cosmogenic isotopes, such as
10
Be.
Beryllium-10 is produced from oxygen and nitrogen in the
atmosphere, rapidly sorbed to suspended aerosols, and flushed
out in precipitation. The highest quality
10
Be records are found
in polar ice cores. While ocean circulation changes cannot
directly affect
10
Be flux, the ocean can influence polar climate,
changing ice-core accumulation rates. Beryllium-10 concentra-
tion in the ice is directly convertible to
10
Be flux if ice-core
accumulation rates are known. Holocene ice-core accumula-
tion rates are believed to have been constant, allowing
10
Be
variations to reflect changes in production. Figure S41 shows
14
C and
10
Be production rates for the past 11,000 years.
Ca II emission of sun-like stars
Conversion of the cosmogenic production rates to irradiance
remains a major challenge. One approach is to construct an
estimate of irradiance for some point in the record, which is
then used to scale back from modern irradiance. The most
widely cited estimates of irradiance beyond the observed
record come from observations of Sun-like stars at wavelengths
associated with brightness variations, such as singly ionized
calcium (Ca II) (Radick, 2003). Non-cycling stars are observed
to be dimmer than those with 8–15 year periods in Ca II
emission. Such observations suggest that the Sun was around
0.25% dimmer during the Maunder Minimum, when sunspot
cycles ceased (Lean et al., 1995, 1998; Figure S42).
A critical assumption remains unverified, that non-cycling
Sun-like stars are in a period of Maunder-type inactivity.
None of the non-cycling Sun-like stars observed over the past
two decades have resumed cycling. Given this uncertainty, true
irradiance change during the Maunder Minimum could be
either larger or equal to that during an 11-year cycle.
Correlations between solar variabili ty and climate
In a landmark paper, Eddy (1976) presented evidence suggest-
ing solar influence on climate for periods longer than the
11-year cycle. He noted that the Maunder Minimum corre-
sponded to the coldest years of the Little Ice Age, a period
of European history known for its climatic extremes. Eddy
described the striking correspondence between minima in sun-
spot observations and maxima in the tree-ring record of cos-
mogenic
14
C(Figure S38), suggesting that cold years during
the Maunder Minimum were linked in some way to reduced
solar activity.
In the modern observational record, there have been many
attempts to correlate the last three solar cycles with climate-
related variables. While some correlations appear convincing,
the observational records are typically too short for statistical
Figure S40 The geomagnetic aa index, neutron monitor count rate, and sunspot numbers demonstrate the influence of solar
magnetic variability on the geomagnetic field. Cosmic ray flux is lower during solar-geomagnetic maxima (data from National Geophysical
Data Center, USA).
SUN-CLIMATE CONNECTIONS 931
significance (Lean and Rind, 1999). The reviews of Lockwood
(2002), Solanki and Fligge (2002), and Haigh (2003) provide
excellent summaries of recent Sun-climate correlations using
observational records. One example is a study of irradiance
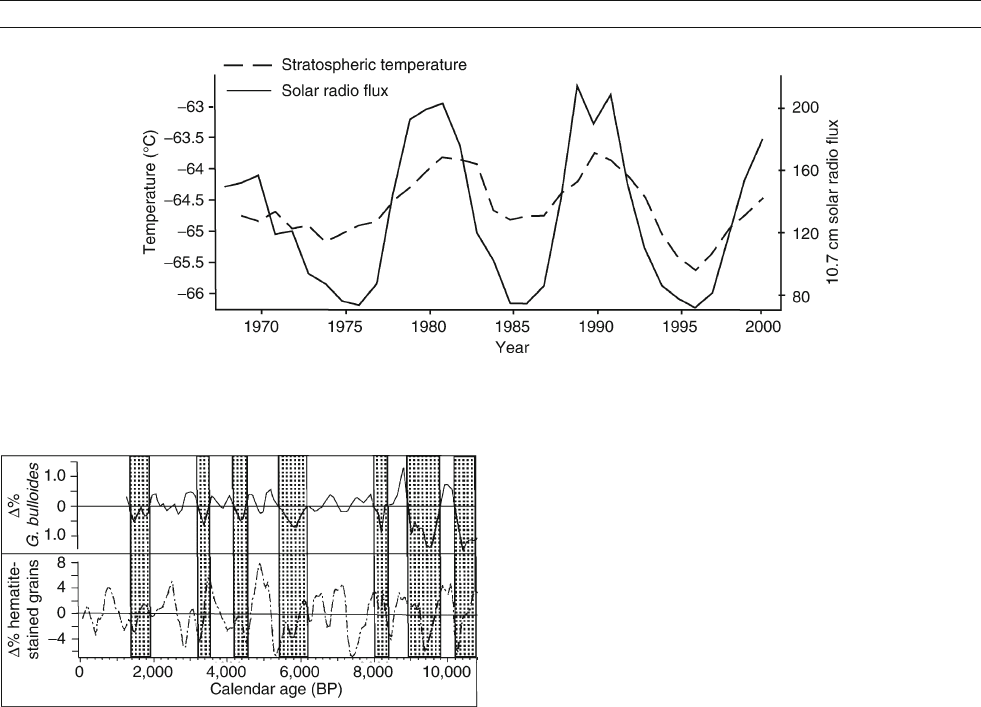
correlated with stratospheric temperature by Labitzke and
Matthes (2003; Figure S43).
Some correlations imply a physical mechanism other than
direct radiative forcing. For example, Marsh and Svensmark
(2000) correlated cosmic ray intensity with North Atlantic
regional cloud cover. Cloud cover is a potentially powerful
climate feedback, critically important in defining climate sensi-
tivity to external forcing in models. The authors suggest a
direct link between atmospheric ionization by cosmic rays
and cloud nucleation processes. Sun and Bradley (2002) argue
that these correlations do not hold using updated data sets.
Furthermore, large increases in cosmogenic isotope production
such as the Laschamp event around 40 ka appear unassociated
with climate. Carslaw (2002) has reviewed the mechanisms
of cloud response to cosmic rays in detail.
Paleoclimate and solar variability
Ocean sedimentcores with highaccumulation rates (>20 cm ka
1
)
can provide paleoclimate records with up to multi-centennial
resolution, currently limited by the accuracy of accelerator mass
spectrometer radiocarbon dating. Bond et al. (2001) presented
Holocene records of North Atlantic ice rafted detritus (IRD),
together with
10
Be flux and
14
C production rate (Figure S41).
IRD consists of mineral grains once frozen in drifting glacier
or sea ice. Its occurrence in ocean sediment is evidence of
enhanced ice production or survivability, i.e., a cold period.
Though climate change could possibly influence ice core
10
Be and ocean circulation may influence atmospheric
14
C, it is
unlikely the agreement between nuclides with different produc-
tion and transport chemistries could be produced independent
of solar variability. The complex patterns of the co-varying
IRD and cosmogenic cycles make a strong argument for solar
influence on North Atlantic climate.
The Asian monsoon is a climate response to intense summer
heating of the Tibetan Plateau, leading to rising of air and low
pressure. This low pressure reverses the direction of prevailing
winds during summer to bring Indian Ocean moisture onshore.
A proxy record for upwelling of deep ocean water from a
sediment core off the coast of Oman indicates that during the
centennial to millennial coolings in the North Atlantic, winds
associated with the summer monsoon were weaker (Gupta et al.,
2003; Figure S44).
Figure S42 Two components of solar irradiance variability, background and the 11-year cycle, are summed to provide an estimate of total solar
irradiance (TSI) since 1610 (data from Lean et al., 1998).
Figure S41 Percentage of hematite-stained quartz grains increases during Holocene cosmogenic isotope maxima, indicating North Atlantic
cooling during solar minima. The most recent cold phase corresponds to the Little Ice Age in Europe (data from Bond et al., 2001).
932 SUN-CLIMATE CONNECTIONS
Carbon isotope indicators of humidity from lake sediments
(Hong et al., 2003) show drier conditions during North Atlantic
coolings. Furthermore, Neff et al. (2001) found that rainfall
intensity was weaker during solar minima based on a record
of oxygen isotopes in Oman cave calcite. These three compo-
nents of monsoon climate: winds, humidity, and rainfall, all
point to a weaker monsoon during periods of reduced solar
magnetic activity. Whether it is a direct response, as would
be expected only for a large irradiance change, or a response
to the solar-influenced climate of the North Atlantic is not yet
known.
Amplification of solar influence
The direct radiative temperature response to solar variability is
extremely small, around 0.06
C for a typical solar cycle irradi-
ance change of 0.1%. A measurable climate response therefore
requires amplifying mechanisms. One of the simplest ampli-
fiers yet proposed may be the climate system responding with
stochastic resonance (Benzi et al., 1982). According to this
model, a small signal (solar forcing) by itself would not pro-
duce climate change, but once added to a background level of
climate system “noise,” the signal crosses a response threshold
and is detectable. One candidate for climate noise may be the
El Niño, which has a recurrence interval (2–7 years) well sui-
ted to amplifying 11-year solar variability. Modeling has shown
that El Niño noise may amplify the solar cycle into decadal
variability of climate-related atmospheric anomalies, such as
the Pacific North American pattern (Ruzmaikin, 1999).
Another possible amplifier is stratospheric ozone. Irradiance
changes in ultraviolet (UV) wavelengths are up to ten times lar-
ger over the course of the solar cycle relative to total irradiance
change (Lean, 2000). The resulting stratospheric heating due to
UV absorption can change ozone concentrations, either ampli-
fying or damping the initial radiative forcing depending on
the altitude of ozone change. Aerosols from volcanic eruptions
may have affected observational data for the last two solar
cycles, so the actual ozone response to the solar cycle is still
unclear (Haigh, 2003).
Shindell et al. (2001) simulated climate of the Maunder
Minimum assuming a TSI reduction of 0.25%. Ozone concen-
trations were allowed to vary in response to the larger UV
reduction of spectrally resolved irradiance. In the model, the
irradiance decrease resulted in Maunder Minimum cooling via
two interacting mechanisms. First, stratospheric ozone changed
such that the initial negative radiative forcing was amplified.
Second, there was a dynamical response resulting in a surface
atmospheric pressure pattern resembling the negative phase of
the Arctic Oscillation/North Atlantic Oscillation (AO/NAO).
The modeled negative AO/NAO was associated with a cooling
of nearly 1–2
C during winter in Northern Europe, consistent
with estimates of the coldest Little Ice Age temperatures. Glob-
ally, modeled cooling was only around 0.35
C, emphasizing
the role of this amplification mechanism in producing regional
climate responses to solar variability.
IRD data of Bond et al. (2001) indicate that for the Little Ice
Age, the most recent of the Holocene millennial cycles, the
North Atlantic did not cool in the typical dipole pattern of the
AO/NAO. Instead, cooling appeared basin-wide. Basin-wide
cooling during the Holocene could be achieved via a reduction
in thermohaline circulation. Thermohaline circulation transports
heat to the North Atlantic region by surface ocean flow from
the tropics. It is sensitive to transport of fresher (lighter) water
into regions of density-driven convection. If solar influence
results in an atmospheric response capable of affecting North
Atlantic THC, the regional climate effect could be substantially
amplified. A persistent negative phase of the AO/NAO may
Figure S43 Stratospheric (70 hPa) temperatures at a point 30
N / 136
W are correlated with solar radio emission, a proxy for irradiance.
The consequences for surface climate depend on uncertain mechanisms of stratosphere-troposphere interaction (adapted from Labitzke
and Matthes, 2003).
Figure S44 Weak monsoon wind strength results in less nutrient-rich
water upwelling off the coast of Oman. This reduces abundance of
G. bulliodes, a hard-shelled plankton whose skeletons may be found in
sediment cores. Periods of weak monsoon winds (shaded) correspond
to North Atlantic coolings (adapted from Gupta et al., 2003).
SUN-CLIMATE CONNECTIONS 933
