Pugnaire F.I. Valladares F. Functional Plant Ecology
Подождите немного. Документ загружается.

canopy such that light is equally limiting for leaves at all levels, giving a strict proportionality
between the integrated PFD intercepted and Amax
area
(Field 1983, Farquhar 1989). Depend-
ing on crown architecture and the resulting light gradients an optimal versus a uniform
N distribution among leaves can theoretically have a substantial impact on canopy photo-
synthesis. The increase ranges from 1% to 4% in open-canopied shrubs and trees with
relatively low total canopy N concentrations (Field 1983, Leuning et al. 1991) to as much
as 20%–40% in dense-canopied stands of with high-canopy N contents (Hirose and Werger
1987a, Hirose 1988, Evans 1993). Evans (1993) compared the consequences of nitrogen
redistribution within a leaf due to acclimation (investment in chlorophyll–protein complexes
versus carboxylation capacity) with that of nitrogen redistribution within a canopy. Leaf
photosynthetic acclimation alone (optimal partitioning between photosynthetic components
within a leaf at a constant N content) was shown to potentially increase canopy photosyn-
thesis by about 4%. On the other hand, redistribution of N among leaves potentially increased
canopy photosynthesis by 20% for the same canopy. Thus, in terms of maximizing photo-
synthesis in heterogeneous light environments within canopies, nitrogen redistribution among
leaves is more important than nitrogen redistribution within leaves.
In the field, these theoretical returns from optimal resource distributions may never be
attained. Measurements within a variety of canopies have found gradients in Amax
area
tending toward but not equal to an optimal gradient (Hirose and Werger 1987b, Hirose
et al. 1988). Leaves have a minimum LMA and N content and a higher Amax
area
in the most
shaded locations than predicted by a strict proportionality with light (Niinemets et al. 1998b,
Meir et al. 2002). On the other hand, stresses, including high temperature, excessive light, and
low water potentials may constrain full acclimation at the top of the canopy (Niinemets and
Valladares 2004).
The patterns of leaf development within a canopy also have important consequences for
the nature of the plastic responses to the resulting light heterogeneity. In species with a single,
early-season leaf flush, light gradients are created simultaneously with and by leaf develop-
ment. Gradients in Amax
area
in these species occur primarily in response to differences in
partitioning of N between more rapidly growing leaves that develop high LMA in the sunnier
crown positions and more slowly growing leaves with low LMA in the shadier crown
positions (Ninnemets et al. 2004). In species that flush leaves continuously, lower leaves
may either be those that developed in high light but were then overtopped, or leaves that
developed in the shade (Pearcy and Seemann 1990). The former maintain the high LMAs
from their earlier high-light existence and have limited capacity for acclimation as compared
with those developing in the shade. Senescence therefore plays a greater role in establishing
canopy gradients in these continually flushing canopies. Still another model is the evergreen
canopies with leaf life spans of several years. In some Mediterranean species, leaves continue
to accumulate mass for several years due to thickening and lignification of cell walls
(Valladares and Pearcy 1999, Niinemets et al. 2006). Although N per unit mass decreases
because of the mass dilution effect, N per unit area remains constant. Thus there is apparently
little or no reallocation of N from lower to higher leaves as predicted by optimal N allocation.
Wall thickening and lignification may be important in water stress resistance but a conse-
quence is a decrease in Amax
area
due to greater wall diffusion limitations.
RESPONSES TO DIRECTIONAL LIGHT GRADIENTS
Plants near the edges of gaps or in competing plant populations often receive much more of
their light on one side because of shading by nearby plants on the other. Phototropic
reorientation of leaves and stems, which increases light capture from the prevailing direction,
can often be observed in these plants (Ackerly and Bazzaz 1995). Additionally, preferential
branch growth may occur to fill available space on the open side leading possibly to
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 230 30.4.2007 7:57pm Compositor Name: DeShanthi
230 Functional Plant Ecology
unbalanced crowns (Young and Hubbell 1991). Since branches are often thought to exhibit
semiautonomy, the simplest explanation could be differential carbon gain on the two sides.
Indeed a model of growth in crowded populations based on this principle adequately
predicted the general patterns of branch growth and thinning (Takenaka 1994). However,
hormonal signals may also be involved and branches are certainly limited in their extension
perhaps by hydraulic constraints (Mencuccini 2002). Gilbert et al. (1995, 2001) found that
reflected far-red light acting through phytochrome inhibited branch growth on the side
nearest to a neighboring plant. Novoplansky (1990) showed that Portulaca oleracea prefer-
entially branched in a direction away from a reflected or transmitted far-red light source.
Additionally, for early successional herbaceous plants, reflected far-red radiation can be a
signal of potential competitors, leading to internode elongation and accelerated height growth
at the expense of tillering or branch growth (Ballare et al. 1987, Ballare 1994, Ballare and
Casal 2000). This response occurs before any direct shading and therefore allows the plant to
effectively anticipate a potential competitive interaction.
DIFFERENTIAL GROWTH OF CLONAL PLANTS
Clonal plants can exhibit varying degrees of physiological integration, which may be important
in their exploitation of patchy environments (Slade and Hutchings 1987, de Kroon and
Hutchings 1995, de Kroon et al. 2005). A type of foraging behavior manifested as a greater
concentration of ramets and leaves in favorable, high-light patches as compared with unfavor-
able, shaded patches can result from morphological plasticity. In particular, shorter petioles
coupled with increased bud activation may be elicited when a clone reaches a favorable patch,
whereas in unfavorable patches, longer internodes and petioles may help ramets and leaves
escape (de Kroon and Hutchings 1995). Resource sharing via physiological integration of ramets
in favorable versus unfavorable microenvironments may modify the responses of ramets. For
example, Dong (1995) found that integration in the stoloniferous understory herb Laminastrum
galeobdolon significantly evened out the morphological differences across high- and low-light
patches. In contrast, there was no such dampening in the morphologically similar fenland species,
Hydrocotyle vulgaris. Since a major source of heterogeneity in the understory is spatially
unpredictable sunflecks, the evening out of responses across ramets may be beneficial in terms
of whole-clone performance. Patches of light are spatially more predictable in the fenland, and
therefore independence in morphological adaptation of ramets may be beneficial.
There is also evidence that clonal integration may enhance utilization of heterogeneous
resources via spatial division of labor (Stuefer et al. 1994, 1996). In experiments where
heterogeneous light and soil water environments were created with strictly negative covari-
ance (high light always associated with low water and vice versa), interconnected ramets of
Trifolium repens exhibited specialization for uptake of the locally most abundant resource,
with resource sharing across ramets (Stuefer et al. 1996). Moreover, total clonal growth was
significantly enhanced relative to that of clones subjected to homogeneous environments
that were either well-watered but shaded or in high light but water-limited. Inherently,
negative relationships between patchiness in resources such as light and water may be fairly
common in natural environments. However, even in the absence of patchiness of one
resource, spatial integration may prove beneficial. For example, in experiments with uni-
form soil moisture, integration enhanced the growth of ramets of the herbs Potentilla
replans and Potentilla anserina in high light that were interconnected to ramets in low
light, apparently because water uptake by the low-light ramets supported in part the high
transpiration rates of the high-light ramets (Stuefer et al. 1994). Severing the connections
reduced the growth of the low-light ramets in both species because of the restriction in
carbon supply and also reduced the growth of the high-light ramets because of a reduction
in water supply.
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 231 30.4.2007 7:57pm Compositor Name: DeShanthi
Responses of Plants to Heterogeneous Light Environments 231
TEMPORAL HETEROGENEITY IN LIGHT ENVIRONMENTS
T
IMESCALES OF TEMPORAL CHANGES AND RESPONSES
Temporal changes in light of interest in ecophysiology involve timescales differing by several
orders of magnitude. At one end of the spectrum, the formation and the closure of canopy
gaps due to tree falls takes place over several to many years. Annual changes occur because of
day length and solar elevation angle changes as well as seasonal variation in cloudiness.
Diurnal changes occur because of the earth’s rotation and the resulting path of the sun across
the sky. Finally, at the other end of the spectrum, changes on the order of seconds to minutes
occur in sunflecks under plant canopies and because of intermittent cloudiness.
The responses to these temporal changes can be conveniently divided into two categories,
one involving the acclimation and developmental plasticity occurring in response to long-term
changes in the light environment and the other involving regulatory responses to short-
term changes that occur during the normal diurnal course of solar radiation or during more
rapidly changing irradiance such as that due to sunflecks in an understory. On the order of a
day appears to be the time dividing temporal changes for which acclimation and develop-
mental plasticity are of paramount importance and those for which regulatory mechanisms
are prominent. Acclimation to a change in light requires on the order of 4–5 days for leaves of
fast-responding species such as peas (Chow and Anderson 1987a,b) and up to 45 days in some
slow-responding species, such as the tropical tree Bischofia javanica (Kamaluddin and Grace
1992). These types of responses involve changes in enzyme concentrations within leaves that
function to alter photosynthetic capacity. Both acclimation and developmental plasticity are
sensitive to the level of other resources, especially nitrogen supply (Osmond 1983, Ferrar and
Osmond 1986), and set in particular the maximum photosynthetic capacity that a leaf can
achieve. Mature leaves have limited physiological plasticity so further change affecting whole-
plant performance involves development of new leaves that have different structural properties
and even changes in branching architecture. These types of changes obviously require even
longer periods (weeks to months) with the maximal change in whole-plant physiological
properties not occurring until all leaves have turned over. Indeed as discussed earlier, in the
context of spatial heterogeneity, growth also generates a temporally heterogeneous light
environment as newer branches and leaves overtop and shade lower branches and older leaves.
Although there are enzymes that show marked diurnal variation in concentration
(Huffaker and Peterson 1976), photosynthetic enzymes involved in carbon metabolism and
electron transport, and the light-harvesting chlorophyll–protein complexes in leaves, are
typically already present in quite high concentrations. Affecting a significant relative change
in concentrations due to either synthesis of degradation may require longer times than a day
and would be energetically costly. Therefore, changes in photosynthetic enzyme concentra-
tions may be evolutionarily precluded as too slow and too costly to be a mechanism
for responding to diurnal changes in solar radiation or to sunflecks. Instead, responses to
these faster changes involves modulation of enzyme activity by small regulatory molecules
such as thioredoxin (Buchanan 1980), regulatory enzymes such as Rubisco activase (Portis
1992) binding of substrates to regulatory sites, chloroplast stromal pH, or energization of the
thylakoid membranes (Foyer et al. 1990, Harbinson et al. 1990a,b). In addition, the regulatory
mechanisms governing stomatal opening and closure are also important. These regula-
tory mechanisms appear to serve to match the capacity of the component steps to each other
and to the supply of light energy and to the supply of CO
2
. When the PFD is low, down-
regulation of several enzymes in the PCRC occurs so that the capacity for carbon metabolism
matches the supply of light. When light is in excess, regulatory mechanisms involving
the xanthophyll cycle in the pigment beds cause the excess energy to be dissipated as heat,
thereby affording protection from photoinhibition (Demmig-Adams and Adams 1992b).
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 232 30.4.2007 7:57pm Compositor Name: DeShanthi
232 Functional Plant Ecology
Stomatal conductance also adjusts to maintain a balance between the supply of CO
2
and the
capacity to use it, as well as to affect the compromise between water loss and carbon gain
(Cowan and Farquhar 1977). The time constants for these regulatory changes typically range
from 0.5 to 15 min to as long as an hour or so, depending on the particular process. Little is
known about the quantitative energetic costs of these regulatory mechanisms but they are
undoubtedly much less than those involved in acclimation and developmental plasticity.
RESPONSES TO SUNFLECKS
Temporal Nature of Sunfleck Light Regimes
Light environments in canopies and understories present a particularly complex case of
temporal heterogeneity. Sunflecks in these environments occur because of the juxtaposition
of the solar track for a particular day with a gap in the canopy. Since the sun moves along the
track at 158 h
1
, sunflecks move across the forest floor at a rate determined by the height to
the canopy gap. Penumbral effects in tall vegetation cause the PFD in a sunfleck to increase
and decrease gradually as the solar disk moves across the gap. However, movement due to
wind causes the edges of a canopy gap to continually change and even for gaps to open and
close. Thus, what may be one gradually changing long sunfleck on a completely still day is
more often broken up into many brief sunflecks in a cluster. Since the solar track shifts with
time of the year, a sunfleck occurring at a particular time and place may do so for only a few
days to weeks and then only when cloud cover does not intervene. Moreover, seasonal
changes in solar elevation also influences the occurrence of sunflecks because most gaps are
at high angles.
The complexity of sunfleck light regimes makes the task of defining a sunfleck difficult
(Chazdon 1988, Smith 1989). Since the PFD transients are rapid, sampling rates must be high
to adequately describe the record. Sampling theorem states that the sampling frequency must
be double the rate of the most frequent event of interest if the objective is to reconstruct the
record accurately. In understories, this dictates sampling of PFDs at intervals of 1–10 s,
whereas in canopies, where leaf flutter creates highly dynamic light environments, sampling
frequencies of 0.1 s may be in order (Pearcy et al. 1990, Roden and Pearcy 1993b). Once an
adequate record is obtained, one approach is to define a sunfleck as a transient excursion of
the PFD above a threshold level that is just above the background diffuse PFD. Alternatively,
the threshold can be some value that is physiologically meaningful. This approach has been
criticized since the threshold is arbitrary and different thresholds have to be used in different
canopies and with different species (Baldocchi and Collineau 1994). Wavelet analysis provides
an objective method for detecting sunfleck events in a data record and does not require a
threshold (Baldocchi and Collineau 1994). Once sunflecks are detected, then they can be
quantified as to their duration, PFD, and so on. However, wavelet analysis depends on
selection of the appropriate wavelet transform function. Finding one that works well is
difficult, given the highly variable and complex nature of sunflecks. So in the end it is not
yet clear whether application of wavelet analysis provides a significant advantage over the
threshold approach. Spectral analysis in which periodicity in the light environment repre-
sented by characteristic frequencies is detected in a record using fast Fourier transform
techniques has been used in crop canopies (Desjardins 1967, Desjardins et al. 1973). Its
usefulness is limited since usually sunflecks are so variable and irregular that no characteristic
frequencies can be detected. The exceptions are canopies such as aspen or poplar where leaves
flutter (Roden and Pearcy 1993a).
Measurements now completed in a wide variety of forest understories show that on clear
days, from 10% to 80% of the daily PFD at any given location may be due to sunflecks
(Pearcy 1983, Chazdon 1988, Pfitsch and Pearcy 1989b, Singsaas et al. 2000, Leakey et al.
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 233 30.4.2007 7:57pm Compositor Name: DeShanthi
Responses of Plants to Heterogeneous Light Environments 233
2003b). Thus sunflecks contribute a large fraction of the light available for photosynthesis
and in turn could be expected to have a large effect on carbon balance and growth of
understory plants. The characteristics of sunflecks depend on attributes such as canopy
height, flexibility, and leaf size as well as weather conditions such as wind and cloudiness.
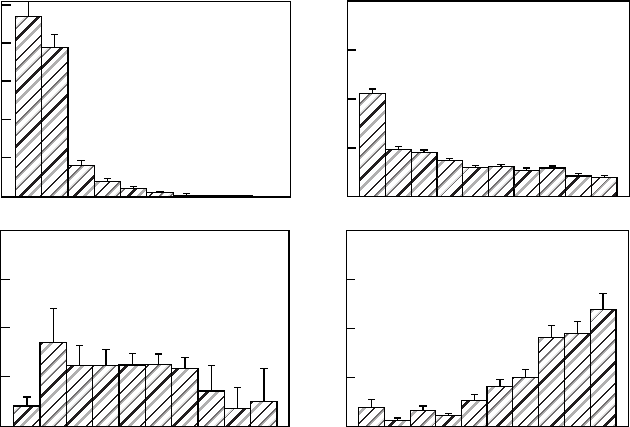
Figure 7.5 shows histograms of sunfleck characteristics for a tropical forest on Barro
Colorado Island (BCI), Panama, and for sensors mounted within a poplar canopy. Most
sunflecks in forest understories are brief and have low-peak PFDs and contribute only a small
proportion of daily PFD as compared with long-duration sunflecks. By contrast, short-
duration sunflecks are a much more important contributor of PFD in aspen canopies. In
the tropical forest understory on BCI, sunflecks tended to be shorter in the dry as compared
with the wet season because of the more windy conditions in the former. In the wet season,
most sunflecks occurred in the mornings because clouds typically built up and rain fell in the
afternoons. Most days in the dry season were clear in both the mornings and the afternoons.
Overall, the additional cloudiness in the wet season as compared with the dry season reduced
the PFD contributed by sunflecks by about 50%. This is consistent with the reductions in
PFD above the canopy in the wet versus the dry season.
Dynamic Responses of Photosynthesis to Sunflecks
Photosynthetic responses to sunfleck light regimes are complex because several components
with markedly different time constants are involved. The photosynthetic response to an individ-
ual sunfleck, or its artificial counterpart—a lightfleck, is quite rapid but the carbon gain achieved
by this response is determined by factors that change over a much longer timescale. If the leaf
has been in the shade for a long time, photosynthetic induction (Figure 7.6) must occur in
order for the full photosynthetic potential to be achieved (Walker 1981, Pearcy 1988).
0.2 1
Cottonwood canopy California
2 4 8 16 32 64 128>
50
40
30
20
10
Percent of sunflecks
0
1
Tropical forest understory Panama
2 4 8 16 32 64 128 256>
40
30
20
10
Percent of sunflecks
0
0.2 1 2 4 8 16 32 64 128> 256
40
30
20
10
Percent of sunfleck PFD
0
1 2 4 8 16 32 64 128 256>
40
30
20
10
Percent of sunfleck PFD
0
Sunfleck duration (s)
FIGURE 7.5 Histograms showing variation in sunfleck characteristics between a Populus fremontii
canopy in California (left side) and a tropical forest understory on Barro Colorado Island, Panama
(right side).
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 234 30.4.2007 7:57pm Compositor Name: DeShanthi
234 Functional Plant Ecology
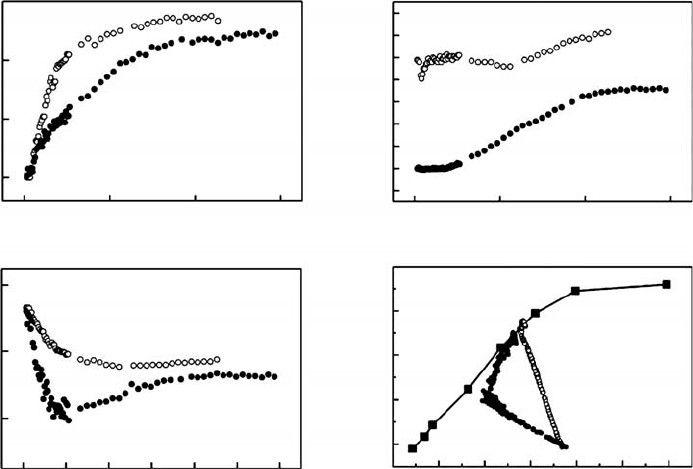
Induction is important in determining the maximum assimilation rate that can be achieved
during a sunfleck. A sunfleck received after a long period of shade results in only relatively
low maximum photosynthetic rates during the sunfleck, whereas one received after a period
of sunfleck activity results in much higher photosynthetic rates (Pearcy et al. 1985, Chazdon
and Pearcy 1986). Thus, in effect, a sunfleck or a series of sunflecks primes the leaf so that it is
better able to use subsequent sunflecks.
The actual transient response to a sunfleck measured with a fast-responding gas exchange
system reveals further complex kinetics. Postillumination CO
2
fixation occurs as the primary
CO
2
acceptor molecule, ribulose bisphosphate (RuBP), and the high-energy precursors to it
that build up during the sunfleck are used for continued CO
2
fixation after the sunfleck. These
pools of RuBP and its high-energy precursors essentially act as a capacitance that is charged
as electron transport initially runs much faster than CO
2
fixation. Then the charge is used to
support postlightfleck CO
2
fixation. In an uninduced leaf with a low capacity for utilization
of RuBP, this capacitance is drained slowly and postillumination CO
2
fixation may continue
at a decelerating rate for 20–60 s. In contrast, postillumination CO
2
fixation lasts for only a
few seconds in an induced leaf. For brief lightflecks (less than 10 s), postillumination CO
2
fixation can enhance carbon gain by 50%–150% but in longer lightflecks it makes a dimin-
ishing contribution. A further kinetic complexity is introduced in relatively long lightflecks
where a postillumination burst of CO
2
is released due to photorespiration. This burst occurs
6
0.16
0.12
0.08
0.04
0.00
4
2
0
0
2
4
6
8
0 600 1200
Time (s)
(a) (b)
(c)
(d)
A (µmol m
−2
s
−1
)
A (µmol m
−2
s
−1
)
g
s
(mol m
−2
s
−1
)
1800 0 600 1200
Time (s)
1800
0 100 200 300 400 500 600
400
200
300
0 600 1200
Time (s)
c
i
(µbar bar
−1
)
c
i
(µbar bar
−1
)
1800
FIGURE 7.6 The time courses of (a) assimilation, (b) stomatal conductance, (c) c
i
and (d) the relation-
ship between assimilation and c
i
during photosynthetic induction for a P. marginata leaf measured in the
morning (open symbols) and the afternoon (closed symbols). PFD was increased from darkness to
saturating levels at time zero. In addition, shown in (d) is the steady-state assimilation versus c
i
curve
(closed squares) measured after full induction. The open circles are from an induction response
measured on a wet-season morning, whereas the closed circles are from one measured on the same
leaf in the afternoon when the initial g
s
was lower. (Adapted from Allen, M.T. and Pearcy, R.W.,
Oecologia, 122, 470, 2000a. With permission.)
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 235 30.4.2007 7:57pm Compositor Name: DeShanthi
Responses of Plants to Heterogeneous Light Environments 235
because some time is required to build up the photorespiratory metabolites and the shuttle of
these metabolites from the chloroplasts to the glyoxysomes and mitochondria.
Mechanistically, the induction requirement, which strongly influences the capacity to use
sunflecks, is primarily a consequence of three main factors: (1) the light activation of Rubisco,
(2) the increase in g
s
, and (3) the light activation of enzymes in the RuBP regeneration
path. Up-regulation of the light-activated enzymes in RuBP regeneration (fructose-1,6-
bisphosphatase, seduheptulose 1,7 bisphosphatase and ribulose-5-P kinase) occurs within
1–2 min following a light increase (Woodrow and Walker 1980, Sassenrath-Cole et al. 1994,
Pearcy et al. 1996), thus allowing a sufficient capacity for RuBP regeneration so that RuBP
concentrations are not rate-limiting for Rubisco. These enzymes are regulated by the redox
state of thioredoxin, which in turn depends on photosynthetic electron flow (Buchanan 1980).
Light activation of Rubisco itself is a slower process requiring 5–10 min for completion
(Seemann et al. 1988, Woodrow and Mott 1988). Rubisco activity is regulated by covalent
binding of Mg
þþ
and CO
2
and by an enzyme, Rubisco activase, that removes bound sugar-
phosphates from Rubisco, allowing catalytic activity (Campbell and Ogren 1992, Portis
2003). In addition, removal of a tight-binding inhibitor, carboxyarabinatol-1-phosphate,
seems to be important in some species (Sage et al. 1993). Rubisco activase itself is light
activated due to an ATP requirement (Campbell and Ogren 1992, 1995). Antisense plants
with reduced levels of Rubisco activase have reduced rates of Rubisco activation and
induction (Mott et al. 1997). Modeling studies suggest that there is an optimal balance
between investment in Rubisco and Rubisco activase that may involve tradoffs between
maintaining high steady-state photosynthetic rates and high rates of Rubisco activation,
improving photosynthesis during sunflecks (Mott and Woodrow 2000).
In terms of assimilation rates, the key to understanding the limitations during induction is
Rubisco, since the observed rates of CO
2
uptake are largely a mirror of its kinetics (Woodrow
and Berry 1988). The relative limitations imposed by RuBP regneration, Rubisco activation,
or g
s
at any given time during induction determine the in vivo rate of Rubisco and hence the
time course of CO
2
assimilation. This can be conveniently visualized by plotting assimilation
versus c
i
during induction (Figure 7.6d). Each point in the trajectory occurs on an imaginary
assimilation versus c
i
curve at a particular time during induction. As induction proceeds, the
slope of this curve increases and gradually approaches the steady-state assimilation versus c
i
curve, indicating an increasing carboxylation capacity of Rubisco. If the initial g
s
is low then
there is a significant decline in c
i
, limiting assimilation, and a generally more sigmoidal
increase in assimilation (Tinoco-Ojanguren and Pearcy 1993a, Allen and Pearcy 2000a). On
the other hand, when the initial g
s
is high, c
i
remains high during induction, resulting in a
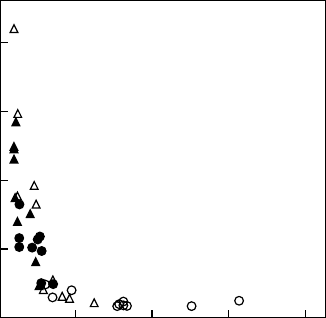
faster increase in assimilation. Differences in the time required for induction are strongly
dependent on the initial g
s
(Figure 7.7). Lower initial g
s
and slower induction in the afternoon
as compared with the morning has been observed in tropical forest understory shrubs and
redwood forest herbs (Pfitsch and Pearcy 1989a, Allen and Pearcy 2000a).
RuBP regeneration limitations are only important as long as the Rubisco activation and
stomatal limitations are small. Thus, after a leaf has been in the shade for a long period so
that g
s
and the Rubisco activation state are low, the enzymes in RuBP regeneration impose
almost no limitation early in induction. However, RuBP regeneration limitations can be quite
significant in leaves that have been shaded for 5–10 min, since the RuBP regenerating
enzymes are deactivated much more rapidly than Rubisco or the decline in g
s
(Sassenrath-
Cole and Pearcy 1994). This can lead to a rather prominent fast induction phase during which
the photosynthetic rate increases over the first 1–2 min before a transition occurs to a slower
increase that is due to stomatal opening and Rubisco activation (Kirschbaum and Pearcy
1988b, Tinoco-Ojanguren and Pearcy 1993b).
The relative roles of stomatal and biochemical limitations during induction have been the
subject of some controversy. Early experiments were consistent with a greater role for
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 236 30.4.2007 7:57pm Compositor Name: DeShanthi
236 Functional Plant Ecology
biochemical as compared with stomatal limitations (Usuda and Edwards 1984, Chazdon and
Pearcy 1986a). This may well be true in some circumstances (Barradas and Jones 1996) but
separation of the limitations using classical gas exchange approaches in which c
i
is calculated
is subject to a number of sources of error. When stomatal conductances are low, as they can
be early in induction, it is necessary to consider the cuticular pathway for water loss, which
when ignored can lead to a considerable overestimation in c
i
(Kirschbaum and Pearcy 1988a).
Moreover, considerable heterogeneity (patchiness) of stomatal conductance may be present
during induction or even before it is in the shade, also leading to an overestimation of c
i
(Cardon et al. 1994, Bro et al. 1996, Eckstein et al. 1996, Kuppers et al. 1999). However,
computation of the errors due to patchiness in the estimate of c
i
suggests that errors in
partitioning the limitations between stomata and biochemistry are typically no more than
10%–15% (M.T. Allen and R.W. Pearcy unpublished results).
Since shade-tolerant plants in a forest understory receive 10%–70% of the available PFD
in the form of sunflecks, these plants might be expected to have mechanisms that enhance
sunfleck utilization by minimizing induction limitations. However, Naumberg and Ellsworth
(2000) summarized the induction times from the literature and found that induction dynamics
are not closely related to species shade tolerance, though individual studies with ecologically
or phyllogenetically narrower groups of species often seem to be consistent with it (Poorter
and Oberbauer 1993, Kuppers et al. 1996, Ogren and Sundlin 1996, Valladares et al. 1997,
Rijkers et al. 2000, Hull 2002). The only clear trend from the literature survey of Naumberg
and Ellsworth was that conifers tend to take longer to induce than angiosperms. Species
differences in rates of Rubisco activation may play a minor role in differences in induction
(Ogren and Sundlin 1996) but mostly they may be due to differences in stomatal behavior.
The capacity for lightfleck utilization was much greater in a shade-tolerant species, Piper
aequale, than in a pioneer species, P. auritum, when both were grown in the shade (Tinoco-
Ojanguren and Pearcy 1992). These differences appeared to be related to stomatal behavior
since stomatal conductance responded strongly to lightflecks in P. aequale, whereas almost no
response occurred in P. auritum.
0
500
1000
1500
2000
Time to 50% of Amax (s)
Initial g
s
(mmol m
−2
s
−1
)
0.00 0.04 0.08 0.12 0.16
FIGURE 7.7 Relationship between initial stomatal conductance of Psychotria marginata leaves before
the beginning of induction and time required to reach 50% of the final steady-state Amax. Circles
represent measurements made in the morning, whereas triangles show afternoon measurements. Open
symbols represent wet-season values, and closed symbols are values from the dry season. (Adapted from
Allen, M.T. and Pearcy, R.W., Oecologia, 122, 470, 2000a. With permission.)
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 237 30.4.2007 7:57pm Compositor Name: DeShanthi
Responses of Plants to Heterogeneous Light Environments 237
Differences in the utilization of transient light have also been found for species native to
high-light habitats. Knapp and Smith (1987, 1990a,b) have identified species differences in the
tracking response of stomatal conductance and hence photosynthesis to sun-shade transitions
typical of those created by intermittent cloud cover. In some species, rapid stomatal opening
and closure occurred in response to sun-shade transitions that limited the increase in photo-
synthesis during the light increase but overall enhanced water use efficiency. Other species
exhibited reduced stomatal opening and closing responses and consequently were able to use
the light increases more efficiently but at an overall lower water use efficiency. Although
herbs, which tended to exhibit rather large water potential changes as compared with the
woody species, were initially identified as having the more conservative response (Knapp and
Smith 1989), this may not always be so (Knapp 1992). The high g
s
of poplar species even in
the shade may enhance the capability of these species to use the highly dynamic light
environments found in their crowns due to leaf flutter (Roden and Pearcy 1993a,b). These
species typically occur in habitats with abundant soil moisture so the high conductances and
resulting low water use efficiency may cost relatively little as compared with the enhanced
carbon gain. A similar lack of stomatal control of induction and consequently a fast induction
response was reported for Nothofagus cunninghamii (Tausz et al. 2005). An alternative
hypothesis to a sun shade-driven difference in induction times may be that species differences
reflect different priorities in the trade-offs between carbon gain and water use (Naumburg and
Ellsworth 2000).
The light environment during growth could also be expected to influence induction times
and lightfleck utilization, but so far no clear picture has emerged. The higher photosynthetic
capacity of high light-acclimated plants tends to increase carbon gain in sunflecks (Lei and
Lechowicz 1997), but trade-offs between maximizing photosynthesis in low light versus
maximizing it in sunflecks make this an unsuccessful strategy. Moreover, induction limita-
tions would cause the realized carbon gain from the higher photosynthetic capacity to be
lower. Rijkers et al. (2000) contrasted gap and understory plants of three species and found no
difference in the rate of induction during a sequence of lightflecks. Despite higher photosyn-
thetic capacities in the gap plants, the actual assimilation rate achieved during the lightflecks
was either the same or was higher in the understory than in the gap plants. The understory
plants did, however, maintain a higher induction state in each lightfleck in the sequence.
Faster induction in shade as compared with sun leaves has been found (Kuppers and
Schneider 1993, Kuppers et al. 1996) but other factors complicate the picture. For example
sun-grown P. auritum leaves exhibit a large fast induction component, whereas shade-grown
leaves do not (Tinoco-Ojanguren and Pearcy 1993b). Shade leaves have also been found to
have higher lightfleck utilization efficiencies than sun-acclimated plants (Chazdon and Pearcy
1986b, Kuppers et al. 1996, Tang et al. 1994, Ogren Sundlin 1996). Pons and Pearcy (1992)
found no difference in the capacity to use brief lightflecks (0.25 s) between sun- and shade-
grown soybeans, but longer lightflecks were used less efficiently by the sun- as compared with
the shade-grown plants. Induction should have little influence on utilization of brief light-
flecks but would impact utilization of longer lightflecks.
Other environmental factors linked to shade and sun environments can also impact
sunfleck utilization. High humidity characteristic of the understory caused slower decreases
in stomatal conductance on shading and hence more carryover of induction from one light-
fleck to the next (Tinoco-Ojanguren and Pearcy 1993a). Bright sunflecks can cause significant
increases in leaf temperatures especially in large leaves with low stomatal conductance and
these high temperatures can significantly inhibit utilization of sunflecks (Leakey et al. 2003a,
2005). Photoprotective systems in shade leaves appear to be fast responding (Logan et al.
1997), but still some photoinhibion cannot be avoided. This photoinhibition would be
expected to primarily affect carbon gain in the low light following the lightfleck (Pearcy
1994, Zhu et al. 2004). Induction states have been found to be higher in the morning than the
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 238 30.4.2007 7:57pm Compositor Name: DeShanthi
238 Functional Plant Ecology
afternoon and in the wet versus the early dry season in a tropical forest (Allen and Pearcy
2000a,b). These effects are not readily explained by diurnal or seasonal environmental
differences so their origin and significance remain elusive.
Models for Assessing the Consequences of Sunfleck Light Regimes
Since sunfleck light regimes are so temporally complex, a modeling strategy is needed to
evaluate their overall consequences for carbon gain. Photosynthesis models such as either the
simple Johnson–Thornley equation (Johnson and Thornley 1984) for the light dependence of
photosynthesis or the more mechanistic Farquhar–von Cammerer (FvC) model are both
steady-state models and thus do not provide an accurate simulation under dynamic light
conditions. One approach is to measure assimilation during a sunfleck regime and then
compare it to a steady-state model simulation as done by Pfitsch and Pearcy (1989a) and
Schulte et al. (2003). In a steady-state model it is assumed that photosynthesis responds
instantaneously to a change in PFD and therefore the limitations due to induction or the post-
CO
2
assimilation are not present. If agreement between model and measurement is achieved it
means that induction and postlightfleck CO
2
assimilation are of either little consequence for
carbon gain or that the two cancel out. Using this type of approach Pfitsch and Pearcy
(1989a) found that the steady-state Johnson–Thornley equation predicted about 20% more
carbon gain than measured, indicating that induction limitations predominated and signifi-
cantly lowered carbon gain in the understory. Essentially similar results were reported by
Schulte et al. (2003).
To probe the consequences of sunflecks for carbon gain, more complex dynamic photo-
synthesis models are needed and several have now been developed. The models of Pearcy et al.
(1997) and Kirschbaum et al. (1998) are both based on a combined dynamic stomatal model
(Kirschbaum et al. 1988) and a dynamic assimilation model. The dynamic assimilation model
is a modification of the FvC model (Gross et al. 1991), made dynamic by adding differential
equations for activation or deactivation of Rubisco and RuBP regeneration and inclusion of
metabolite pools. One difficulty with these models is that there are a large number of
parameters, some of which require fairly elaborate gas exchange measurements for determin-
ation. Thus a more empirical model that simulates the essential dynamic features of the
response to sunfleck light regimes (Stegemann et al. 1999) is an attractive approach.
Despite the considerable effort to parameterize them, mechanistic dynamic models do
give good agreement between measured and modeled responses. Pearcy et al. (1997) found
that assimilation of Alocasia macrorrhiza measured under a 3 h long sequence of lightflecks
and simulated with their dynamic model agreed to within 3%. In contrast, a steady-state
version of the model predicted 50% more carbon gain. The Pearcy et al. model can be run
with input of PFD obtained with quantum sensors connected to data loggers that sample light
at intervals as short as 0.1 s to make predictions of carbon gain and to visualize the time
course of different limitations (Figure 7.8). The effects of induction on assimilation during
sunflecks are clearly visible by comparing Figure 7.8a and Figure 8b. In addition, evident are
the different time dependencies of g
s
(Figure 7.8d), Rubisco (Figure 7.8e), and metabolite
pool sizes (Figure 7.8f ). Comparison of the dynamic and steady-state model predictions for
carbon gain with light measurements in different microsites and days showed that the
dynamic model gave a 3%–25% lower carbon gain (Table 7.1). The lowest values were for
microsites and days with lower sunfleck activity but, because of the complex nature of the
responses and the sunfleck regimes, no single characteristic of the sunfleck light regime such
as numbers of sunflecks or their average duration was predictive of the limitations imposed.
With defined lightfleck regimes, the dynamic limitation to carbon gain was much greater
when there were just three 5 min lightflecks as compared with where there were one hundred
and sixty nine 5 s lightflecks imposed on a low background PFD. This is consistent with the
Francisco Pugnaire/Functional Plant Ecology 7488_C007 Final Proof page 239 30.4.2007 7:57pm Compositor Name: DeShanthi
Responses of Plants to Heterogeneous Light Environments 239
