Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.


120 5
Transformations of Moist
Air
from which the dimensionless quantities 3' and K (2.21) follow directly. Like
virtual temperature,
co, Cp, y,
and K differ only slightly from the constant
values for dry air.
5.1.2 Saturation Properties
Consider now the gas phase of the system in the presence of a condensed
phase of water. If the vapor is in chemical equilibrium with the condensed
phase, it is said to be
saturated.
Corresponding to this condition and for a
given pressure and temperature are particular values of the foregoing moisture
variables, which are referred to as
saturation values.
According to the Gibbs-
Dalton law, the
saturation vapor pressure
with respect to water
ew
is identical
to the equilibrium vapor pressure Pw of a single-component system of vapor
and water (Sec. 4.6). Likewise, the saturation vapor pressure with respect to
ice
e i
is identical to
Pi
for a single-component system. 2
The saturation vapor pressure
ec,
where c denotes either of the condensed
phases, is a function of temperature alone and described by the Clausius-
Clapeyron relations (4.39) and (4.40). The
saturation specific humidity qc
and
the
saturation mixing ratio r c,
which follows from
ec
through (5.7), also describe
the abundance of vapor at equilibrium with a condensed phase. Like the
saturation vapor pressure, these quantities are state variables. But, because
they refer to the mixture and not just the vapor,
qc
and
r c
also depend on
pressure, in accord with Gibbs' phase rule (4.24) for a two-component system
involving two phases. However, the strong temperature dependence of
ec(T)
in the Clausius-Clapyeron equation is the dominant influence on
qc(P, T)
and
rc( p, T).
Therefore, a decrease of temperature following from adiabatic
expansion sharply reduces the saturation values
qc
and
r c.
Just the reverse
results from an increase of temperature following from adiabatic compression.
Contrary to saturation values, which change with the thermodynamic state
of the system, the abundance of vapor actually present changes only through
a transformation of phase. If no condensed phase is present (e.g., under un-
saturated conditions), the abundance of vapor is preserved. A decrease of
temperature then results in a decrease of
rc,
but no change of r. On the other
hand, if the system is saturated, r =
rc( p,
T). A change of state in which the
system remains saturated must then result in a change of both
rc(p, T)
and r,
that is, the vapor and condensate must adjust to preserve chemical equilibrium
between those phases.
2
Strictly, the water component of the two-component system does not behave exactly as it would
in isolation. Discrepancies in that idealized behavior stem from (1) near saturation, departures
of the vapor from the behavior of an ideal gas, (2) the condensed phase being acted on by the
total pressure and not just that of the vapor, and (3) some of the air passing into solution with
the water. However, these effects introduce discrepancies that are smaller than 1%, so they can
be ignored for most applications; see Iribarne and Godson (1981) for a detailed treatment.

5.2 Implications for the Distribution of
Water Vapor
121
Two other quantities are used to describe the abundance of water vapor.
The
relative humidity
is defined as
RH-- Nv
e r
= ___ , (5.13)
ec rc
where
N~c
denotes the saturation molar abundance of vapor, and equilibrium
with respect to water is usually implied. The
dew point Td
is defined as that
temperature to which the system must be cooled "isobarically" to achieve
saturation. If saturation occurs below 0~ that temperature is the
frost point
Tf.
The
dew point spread
is given by the difference
(T- Td).
For a given
temperature T, a high dew point implies a small dew point spread. Each cor-
responds to a large abundance of vapor, which requires only a small depres-
sion of temperature to achieve saturation.
Neither relative humidity nor dew point spread are direct measures of va-
por concentration but, rather, describe how far the system is from saturation.
Because saturation values increase sharply with temperature, inferring mois-
ture content from the aforementioned quantities is misleading. For instance,
r w at 1000 mb and 30~ is nearly 30 g kg -1, but only 4 g
kg -1 at
the same pres-
sure and 0~ Therefore, a relative humidity of 50% implies an abundance of
vapor of 2 g kg -~ in the latter but of 15 g kg -~ in the former~more than
seven times as large.
5.2 Implications for the Distribution of Water Vapor
Saturation values describe the maximum abundance of vapor that can be sup-
ported by air for a given temperature and pressure. At that abundance, dif-
fusion of mass from vapor to condensate is balanced by diffusion of mass in
the opposite sense. If the system is heterogeneous and has a vapor abundance
below the saturation value (e.g., an unsaturated air parcel in contact with a
warm ocean surface), vapor will be absorbed until the difference of chemical
potential between the phases of water has been eliminated. For this transfor-
mation to occur, the water component must absorb heat equal to the latent
heat of vaporization. Conversely, if the system is heterogeneous and has a va-
por abundance slightly above the saturation value (e.g., a supersaturated air
parcel containing an aerosol of droplets), vapor will condense to relieve the
imbalance of chemical potential. For this transformation to occur, the water
component must reject heat equal to the latent heat of vaporization.
At temperatures and pressures representative of the atmosphere, the satu-
ration vapor pressure seldom exceeds 60 mb and the saturation mixing ratio
seldom exceeds 30 g kg -1 or 0.030. It is for this reason that water vapor exists
122
5
Transformations of Moist
Air
only in trace abundance in the atmosphere. One should note that the foregoing
moisture properties refer only to vapormnot to the total water content of the
system. Condensation results in a reduction of q and r, but a commensurate
increase of condensate according to (4.6.2). Unless condensate precipitates
out of the system, the total water content of an air parcel is preserved.
According to the Clausius-Clapeyron equation, saturation vapor pressure
depends exponentially on temperature. Thus, air can support substantially
more vapor in solution at high temperature than at low temperature. For this
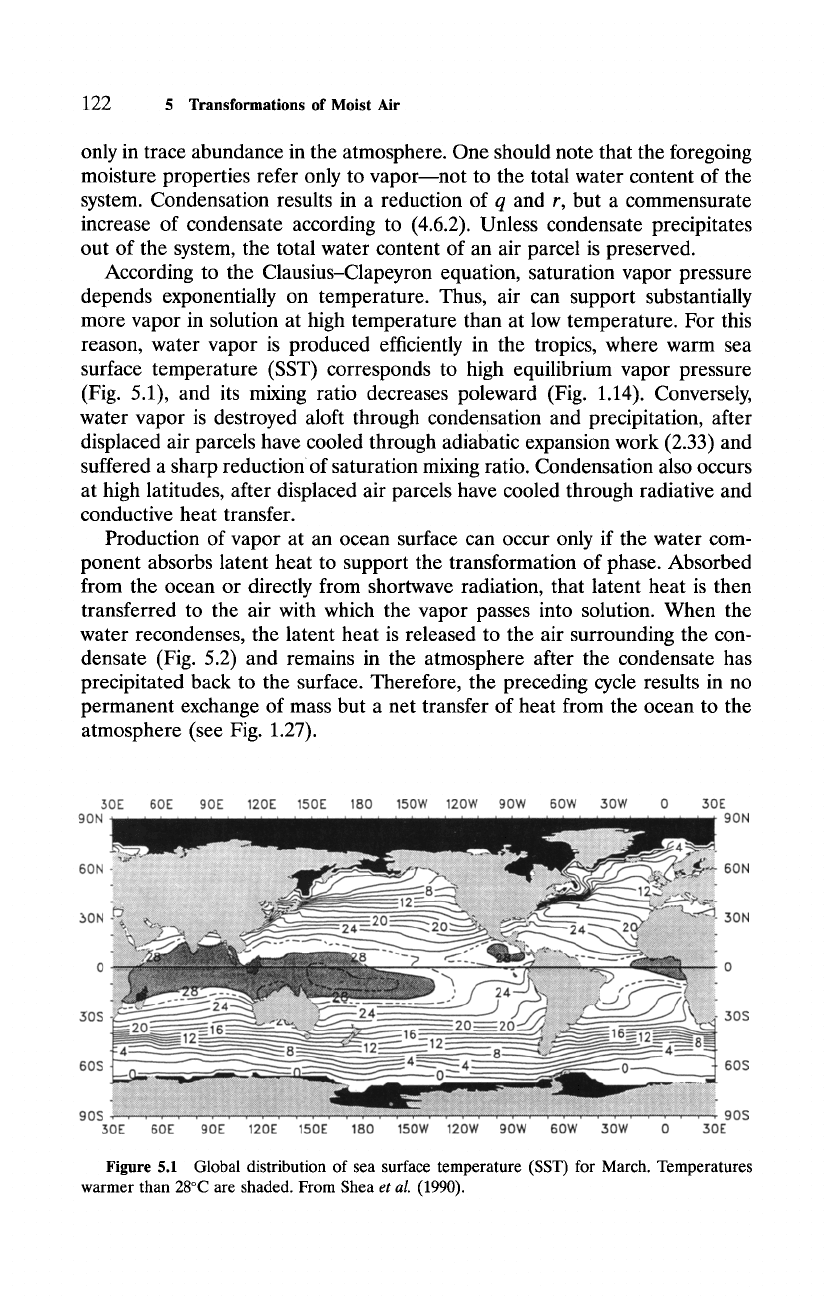
reason, water vapor is produced efficiently in the tropics, where warm sea
surface temperature (SST) corresponds to high equilibrium vapor pressure
(Fig. 5.1), and its mixing ratio decreases poleward (Fig. 1.14). Conversely,
water vapor is destroyed aloft through condensation and precipitation, after
displaced air parcels have cooled through adiabatic expansion work (2.33) and
suffered a sharp reduction of saturation mixing ratio. Condensation also occurs
at high latitudes, after displaced air parcels have cooled through radiative and
conductive heat transfer.
Production of vapor at an ocean surface can occur only if the water com-
ponent absorbs latent heat to support the transformation of phase. Absorbed
from the ocean or directly from shortwave radiation, that latent heat is then
transferred to the air with which the vapor passes into solution. When the
water recondenses, the latent heat is released to the air surrounding the con-
densate (Fig. 5.2) and remains in the atmosphere after the condensate has
precipitated back to the surface. Therefore, the preceding cycle results in no
permanent exchange of mass but a net transfer of heat from the ocean to the
atmosphere (see Fig. 1.27).
30E 60E 90E 120E 150E 180 150W 120W 90W 60W 30W 0 30E
90N 90N
60N
50N
30S
60S
60N
50N
30S
60S
90S 90S
30E 60E 90E 120E 150E 180 150W 120W 90W 60W 30W 0 30E
Figure
5.1 Global distribution of sea surface temperature (SST) for March. Temperatures
warmer than 28~ are shaded. From Shea
et al.
(1990).
5.3 State Variables of the Two-Component System
123
9 v9
\
\ 9 , .... ~: '~"::~. ~ ~. ~:~ .... ~:~ "ff '"::: " ~: "~$~i~i~,~'~ ,~:::~:
.... ~,::~,:!!.. ~ ~ .:~ .: .,.~ ..,~:~~iii~?ii?~i~i~ii!i~iil~,i~iiii~'~iiii!!~:~:i~ :: ....
" i
liiiii!!!4i !i!!
and Vapor
", 9 9 9 - '
" \ ~ o 9 00 ~ , 9 9 00 /
\
,['~ "l, r
"-.'. "" ".. ". ". ". ". 0-" //
Figure 5.2 Schematic illustration of a saturated air parcel undergoing condensation, in which
latent heat q is released to the gas phase and some of the condensate precipitates out of the
parcel.
5.3 State Variables of the Two-Component System
Under unsaturated conditions, the two-component system involves only a sin-
gle phase. By Gibbs' phase rule (4.24), the system then possesses three ther-
modynamic degrees of freedom. Thus, three intensive properties are required
to specify the system's state if no condensate is present. Usually, the state of
moist air is specified by pressure, temperature, and a humidity variable like
mixing ratio, for example, 0 =
O(p, T, r).
Under saturated conditions, two
phases are present and one of the independent state variables is eliminated by
the constraint of chemical equilibrium [e.g., r =
rc( p,
T)]. This leaves two de-
grees of freedommthe same number as for a homogeneous single-component
system. Thus, under saturated conditions, a state variable can be specified as
0 = O(p, T).
5.3.1 Unsaturated Behavior
Under unsaturated conditions, thermodynamic processes for moist air occur
much as they would for dry air because thermal properties are modified only
slightly by the trace abundance of vapor. A change of temperature leads to
124 5
Transformations of Moist
Air
changes of internal energy and enthalpy given by (2.18), but with slightly mod-
ified specific heats (5.12). The saturation mixing ratio r c, which measures the
capacity of air to support vapor in solution, varies with the system's pressure
and temperature. By contrast, the mixing ratio r of vapor actually present
remains constant so long as the system is unsaturated.
The same is approximately true of the potential temperature. Under adia-
batic conditions, pressure and virtual temperature change in such proportion
to preserve the virtual potential temperature
To (5.14)
where Kd denotes the value for dry air. Like virtual temperature, Ov is nearly
identical to its counterpart for dry air, 0. Therefore, virtual properties of moist
air like T v and 0~ will hereafter be referred to by their counterparts for dry
air, but the former will be understood to apply in a strict sense.
5.3.2 Saturated Behavior
Under saturated conditions (e.g., in the presence of an aerosol of droplets),
the aforementioned relationships no longer hold. Because the vapor must then
be at equilibrium with a condensed phase, e = ec. A change of thermodynamic
state that alters ec then also alters e, which must result in a transformation of
mass from one phase of the water component to another. Accompanying that
transformation of mass is an exchange of latent heat between the condensed
and gas phases of the heterogeneous system (Fig. 5.2), one that alters the
potential temperature of the gas phase through (2.36).
State variables describing the two-component heterogeneous system must
account for these changes. For a closed system, (4.7) gives the change of total
enthalpy
~H dT + dp + (ho - hc)dmo. (5.15)
dH = ~
pm Tm
Because
H = mdh d + mvh v + mch c,
- ma \
dT ,I
+ m~ \ dT +
mc
--~ pm pm pm
\OT
pm
Similarly,
= mdCpd -1- mvCpv + mcCpc.
= m d + m v W m c
-~P Tm ~ Tm \--~P,]Tm -~P
Tm
(5.16)
For the gas phase,
5.3 State
Variables of the Two-Component System
125
dp ,J Tm k Op Tm
by (2.18). For the condensed phase, the corresponding change of enthalpy can
be expressed
0 c)
Tm - vc(1- TOtp)'
where
ffp ~ --
V p
defines the isobaric expansion coefficient (e.g., Denbigh, 1971). Because C~p is
small for condensed phases,
- PJ m
The contribution to (5.15) from this pressure term can be shown to be neg-
ligible compared to the corresponding contribution from temperature (5.16).
Incorporating the above into (5.15) and identifying the specific heat of the
heterogeneous system as
mdCpd -Jr- mvCpv n t- mcCpc
(5.17)
Cp --
m
and the latent heat as the difference of enthalpy between the phases of water
l= h~- hc, (5.18)
obtains the change of enthalpy for the system:
dh = cpdT + l dmv
m
-~ cpdT + ldr. (5.19)
The last expression holds exactly if the specific enthalpy refers to a unit mass
of dry air, derivation of which is left as an exercise. In (5.19), the term
6q - -ldr
represents the heat transferred to the gas phase (which is chiefly dry air) from
the water component when the latter undergoes a transformation of phase.
If l is treated as constant (Sec. 4.6.1), (5.19) may be integrated to yield an
expression for the absolute enthalpy of the two-component heterogeneous
system:
h = cpT + lr + ho, (5.20)
where h0 denotes the enthalpy at a suitably-defined reference state.
126
5
Transformations of Moist
Air
Expressions for the internal energy and entropy of the system follow in
similar fashion:
u = cvT + lr +
u0, (5.21)
s lr s o
Cp
=
lnT- Kdlnp + ~ -t-
~Cp
lr s o
= In 0 + + --. (5.22)
Cp T Cp
Like (5.19), the expressions for absolute enthalpy, internal energy, and entropy
of the two-component system are exact if referenced to a unit mass of dry air.
5.4 Thermodynamic Behavior Accompanying Vertical Motion
The thermodynamic state of a moist air parcel changes through vertical mo-
tion. Vertical displacement alters the environmental pressure, which varies
hydrostatically according to (1.17). To preserve mechanical equilibrium, the
parcel expands or contracts, which results in work being performed. Compen-
sating that work is a change of internal energy, which alters the temperature
and hence the saturation vapor pressure of the two-component system.
5.4.1 Condensation and the Release of Latent Heat
From (4.38), the change of saturation vapor pressure between a reference
temperature T O and a temperature T can be expressed
ln(ecC0 ) =
Rvl
(1T T01)" (5.23)
Then (5.7) implies that the saturation mixing ratio varies with pressure and
temperature as
rc
exp [-~ (1_ ~)]
rco ( ot
(5.24)
According to (5.24), the saturation mixing ratio increases with decreasing
pressure. However,
r c
decreases sharply with decreasing temperature, which
likewise accompanies upward motion. Therefore, even though an ascending
parcel's pressure decreases exponentially with altitude, the temperature de-
pendence in (5.24) prevails, so its saturation mixing ratio decreases monoton-
ically with altitude.
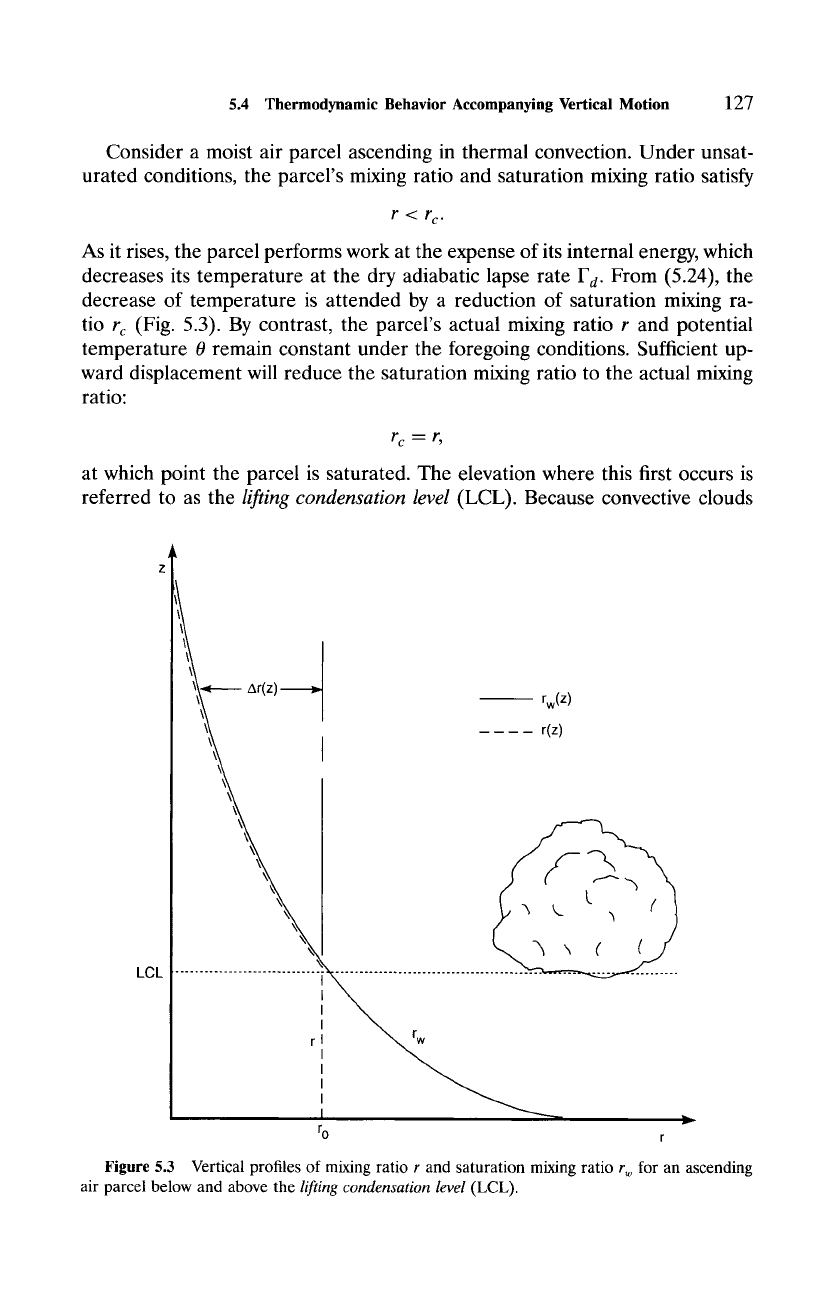
5.4 Thermodynamic Behavior Accompanying Vertical Motion
127
Consider a moist air parcel ascending in thermal convection. Under unsat-
urated conditions, the parcel's mixing ratio and saturation mixing ratio satisfy
r< r c.
As it rises, the parcel performs work at the expense of its internal energy, which
decreases its temperature at the dry adiabatic lapse rate F d. From (5.24), the
decrease of temperature is attended by a reduction of saturation mixing ra-
tio
r c
(Fig. 5.3). By contrast, the parcel's actual mixing ratio r and potential
temperature 0 remain constant under the foregoing conditions. Sufficient up-
ward displacement will reduce the saturation mixing ratio to the actual mixing
ratio:
rc m r,
at which point the parcel is saturated. The elevation where this first occurs is
referred to as the
lifting condensation level
(LCL). Because convective clouds
LCL
i
\\= Ar(z) -------~ ()
\~ r w z
\~
l r(z)
......................
r
r o
r
Figure
5.3 Vertical profiles of mixing ratio r and saturation mixing ratio r w for an ascending
air parcel below and above the
lifting condensation level
(LCL).
128
5
Transformations of Moist
Air
form through the process just described, the LCL defines the base of cumulus
clouds that are fueled by air originating at the surface.
Below the LCL, the parcel's thermodynamic behavior can be regarded as
adiabatic because the characteristic timescale for vertical displacement (e.g.,
from minutes in cumulus convection to 1 day in sloping convection) is small
compared to the characteristic timescale for heat transfer. Therefore, the par-
cel evolves in state space along a dry adiabat, which is described by Poisson's
equations (2.30) and characterized by a constant value of 0.
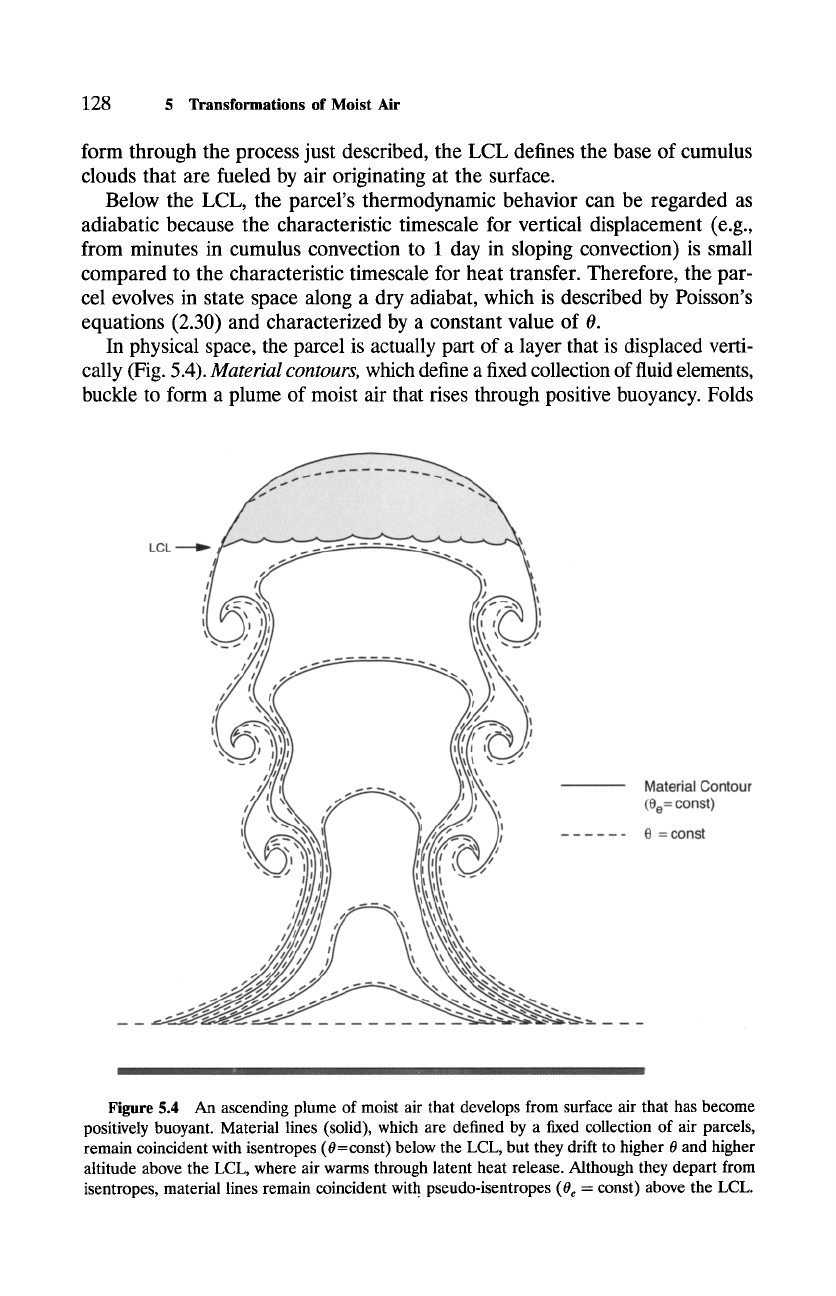
In physical space, the parcel is actually part of a layer that is displaced verti-
cally (Fig. 5.4).
Material contours,
which define a fixed collection of fluid elements,
buckle to form a plume of moist air that rises through positive buoyancy. Folds
LCL
Material Contour
(ee= const)
e = const
Figure 5.4 An ascending plume of moist air that develops from surface air that has become
positively buoyant. Material lines (solid), which are defined by a fixed collection of air parcels,
remain coincident with isentropes (0=const) below the LCL, but they drift to higher 0 and higher
altitude above the LCL, where air warms through latent heat release. Although they depart from
isentropes, material lines remain coincident with pseudo-isentropes (0e = const) above the LCL.

5.4
Thermodynamic Behavior Accompanying Vertical Motion
129
in the wall of the plume reflect turbulent entrainment with surrounding air,
which dilutes the plume's buoyancy with that of the environment. Associated
mixing introduces diabatic effects that are ignored in the present analysis, but
play an essential role in dissipating cumulus convection (Chapter 9). Under
unsaturated adiabatic conditions, 0 and r are both conserved for individual
fluid elements. Therefore, a material line that coincides initially with a cer-
tain isentrope 0 = 00 and mixing ratio contour r = r 0 remains coincident with
those isopleths~as long as they reside beneath the LCL.
Above the LCL, the foregoing behavior breaks down. Continued ascent and
expansion work reduces the saturation mixing ratio
r c
below the mixing ratio
r of vapor present. To restore chemical equilibrium, some of the vapor must
condense~just enough to maintain saturation:
r--r c.
Thus, above the LCL, r and
r c
both decrease with height (Fig. 5.3), the de-
crease of vapor being reflected in an increase of condensate (4.6.2).
In this fashion, ascent above the LCL wrings vapor out of solution with
dry air and produces condensate (e.g., cloud droplets). The production of
condensate is attended by a release of latent heat to the gas phase of the
system. Internal to the parcel, that heating alters the potential temperature
of the gas phase through (2.36) and adds positive buoyancy, which in turn
promotes continued ascent. The change of water vapor mixing ratio that results
from a displacement Az above the LCL is given by
Ar(z) :
- ro,
where z, p, and T are understood to refer to the displaced parcel. Because
r c
decreases monotonically with altitude, the greater the displacement above the
LCL, the less vapor that remains in the gas phase of the parcel and the greater
the abundance of condensate and the liberation of latent heat. Cloud droplets
produced in this fashion grow (through mechanisms described in Chapter 9)
until they can no longer be supported by the updraft, at which point they
precipitate out of the parcel.
Since 0 and r are no longer conserved, the material contour coincident initial-
ly with the isopleths 0 = 00 and r = r0 deviates from those isopleths (Fig. 5.4).
The release of latent heat increases 0 (2.36), so the material contour advances to
isentropes of greater potential temperature. For reasons developed in Chap-
ter 7, these lie at a higher altitude than the original isentrope. Similarly, con-
densation reduces r, so the material line moves to isopleths of smaller mixing
ratio, which likewise lie at higher altitude.
Saturated air that is descending undergoes just the reverse behavior. Adia-
batic compression then increases the internal energy and temperature of the
gas phase, which increases the saturation mixing ratio
r c
over the actual mix-
ing ratio r. Condensate can then evaporate to restore chemical equilibrium:
r = r c.
Evaporation of condensate must be attended by absorption of latent
