Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.


110 4
Heterogeneous
Systems
4.6 Equilibrium Phase Transformations
According to Sec. 2.3, heat transferred during an isobaric process between
two homogeneous states of the same phase is proportional to the change of
temperature. Under those circumstances, the constant of proportionality is
the specific heat at constant pressure
cp.
By contrast, heat transfer during an
isobaric process between two heterogeneous states of the same two phases
involves no change of temperature (Fig. 4.3). Instead, heat transfer results
in a conversion of mass from one phase to the other (which is associated
with a change of internal energy) and in work being performed when the
system's volume changes. Conversely, an isobaric transformation of phase can
occur only if it is accompanied by a transfer of heat to support the change of
internal energy and the work performed by the system during its change of
volume.
4.6.1 Latent Heat
The preceding effects are collected in the change of enthalpy, which equals
the heat transfer into the system during an isobaric process (2.15). Analogous
to the specific heat capacity at constant pressure, the specific
latent heat
of
transformation is defined as the heat absorbed by the system during an isobaric
phase transformation
1 = t~qp
= dh,
(4.27.1)
which, by the first law, equals the change in enthalpy of phase transformation.
In (4.27.1), the mass in I refers only to the substance undergoing the transforma-
tion of phase (e.g., to the water component in a mixture with dry air). The spe-
cific latent heats of vaporization, fusion (solid ~ liquid), and sublimation (solid
vapor) are denoted Iv, 1 I, and
ls,
respectively, and are related as
l~ = If + I v.
(4.27.2)
Like specific heat capacity, latent heat is a property of the system. Thus, l
depends on the thermodynamic state, which may be expressed
l - l(T)
for a
heterogeneous system of two phases. The dependence on temperature of I can
be established by considering a transformation from one phase to another and
how that transformation varies with temperature. Consider a homogeneous
state wherein the system is entirely in phase a and another homogeneous state
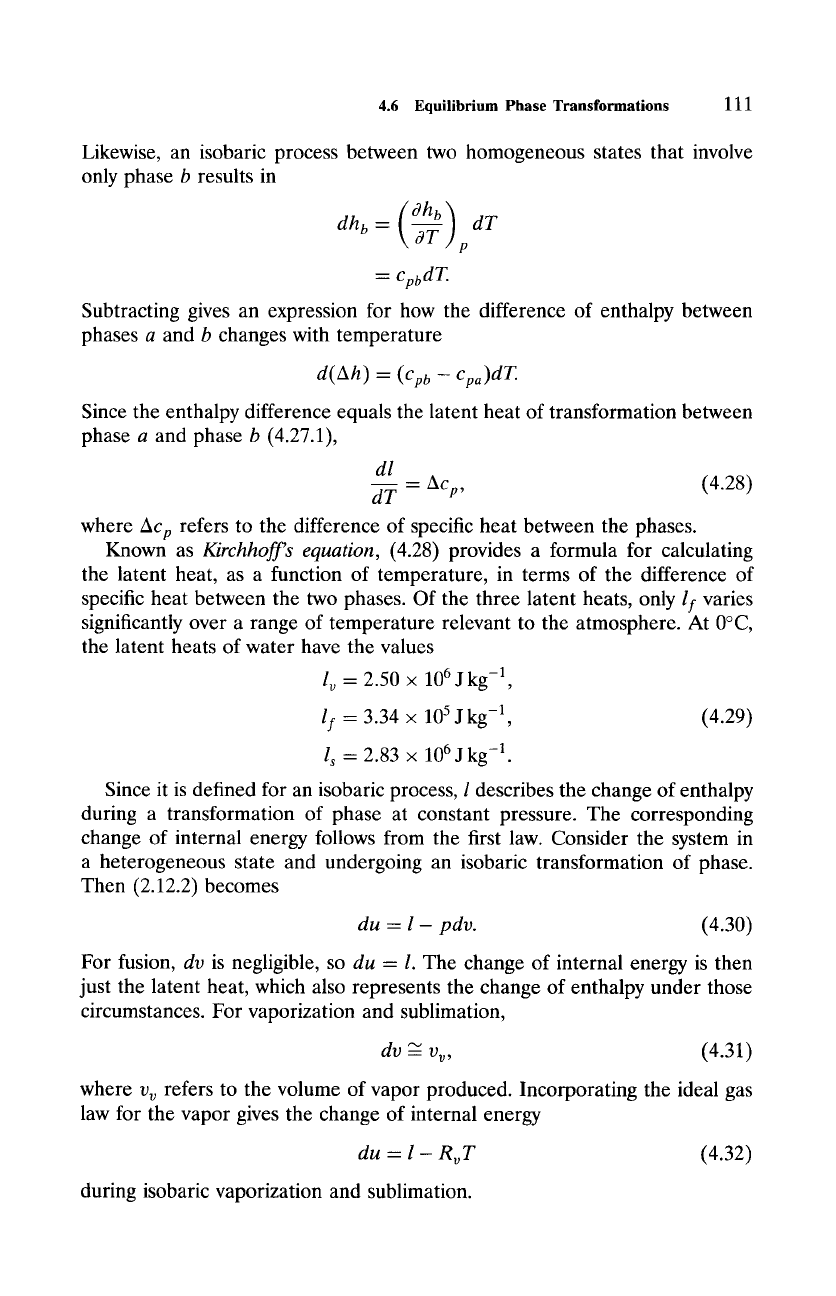
wherein it is entirely in phase b. By (2.23.2), an isobaric process between two
homogeneous states that involve only phase a results in a change of enthalpy
(dha] dT
dha -- ---~-] p
= cpadT.
4.6
Equilibrium Phase Transformations
111
Likewise, an isobaric process between two homogeneous states that involve
only phase b results in
dr
dhb -
~T /] p
-~ CpbdT.
Subtracting gives an expression for how the difference of enthalpy between
phases a and b changes with temperature
d(~Xh) - (Cpb -- Cpa)dT.
Since the enthalpy difference equals the latent heat of transformation between
phase a and phase b (4.27.1),
dl
dT = Acp,
(4.28)
where
Acp
refers to the difference of specific heat between the phases.
Known as
Kirchhoff's equation,
(4.28) provides a formula for calculating
the latent heat, as a function of temperature, in terms of the difference of
specific heat between the two phases. Of the three latent heats, only
If
varies
significantly over a range of temperature relevant to the atmosphere. At 0~
the latent heats of water have the values
l~ - 2.50 • 106 J kg-1,
If-
3.34 • 105 J kg -1, (4.29)
l s -2.83
x 106 J kg -1.
Since it is defined for an isobaric process, l describes the change of enthalpy
during a transformation of phase at constant pressure. The corresponding
change of internal energy follows from the first law. Consider the system in
a heterogeneous state and undergoing an isobaric transformation of phase.
Then (2.12.2) becomes
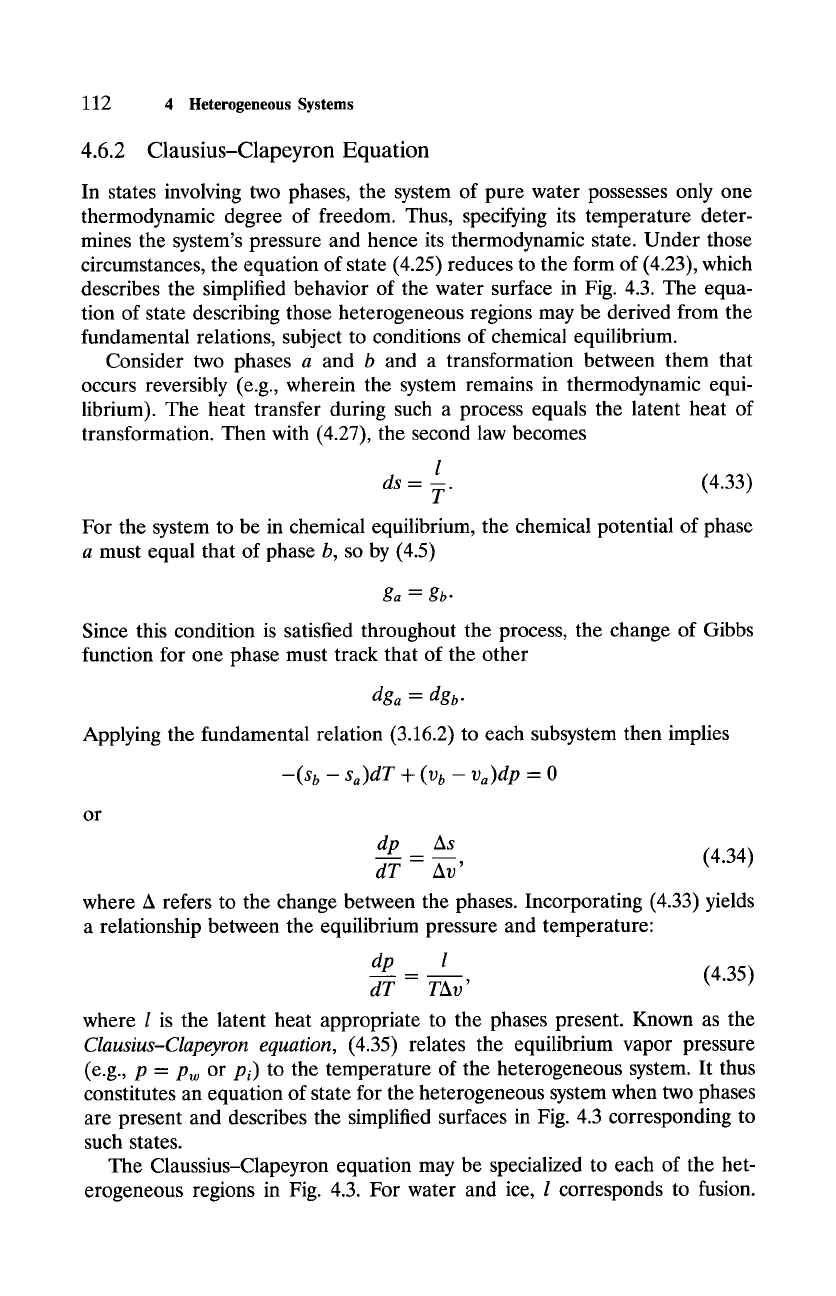
du = l- pdv.
(4.30)
For fusion,
dv
is negligible, so
du = I.
The change of internal energy is then
just the latent heat, which also represents the change of enthalpy under those
circumstances. For vaporization and sublimation,
dv "~ v v,
(4.31)
where vv refers to the volume of vapor produced. Incorporating the ideal gas
law for the vapor gives the change of internal energy
du - l- R~T
(4.32)
during isobaric vaporization and sublimation.
112 4
Heterogeneous Systems
4.6.2 Clausius-Clapeyron Equation
In states involving two phases, the system of pure water possesses only one
thermodynamic degree of freedom. Thus, specifying its temperature deter-
mines the system's pressure and hence its thermodynamic state. Under those
circumstances, the equation of state (4.25) reduces to the form of (4.23), which
describes the simplified behavior of the water surface in Fig. 4.3. The equa-
tion of state describing those heterogeneous regions may be derived from the
fundamental relations, subject to conditions of chemical equilibrium.
Consider two phases a and b and a transformation between them that
occurs reversibly (e.g., wherein the system remains in thermodynamic equi-
librium). The heat transfer during such a process equals the latent heat of
transformation. Then with (4.27), the second law becomes
l
ds = --. (4.33)
T
For the system to be in chemical equilibrium, the chemical potential of phase
a must equal that of phase b, so by (4.5)
ga =
gb"
Since this condition is satisfied throughout the process, the change of Gibbs
function for one phase must track that of the other
dg a = dg b.
Applying the fundamental relation (3.16.2) to each subsystem then implies
--(Sb -- sa)dT + (Vb -- va)dP = 0
or
dp As
= (4.34)
dT Av '
where A refers to the change between the phases. Incorporating (4.33) yields
a relationship between the equilibrium pressure and temperature:
dp l
= (4.35)
dT TAr'
where l is the latent heat appropriate to the phases present. Known as the
Clausius-Clapeyron equation, (4.35) relates the equilibrium vapor pressure
(e.g., p = Pw
or
Pi)
to
the temperature of the heterogeneous system. It thus
constitutes an equation of state for the heterogeneous system when two phases
are present and describes the simplified surfaces in Fig. 4.3 corresponding to
such states.
The Claussius-Clapeyron equation may be specialized to each of the het-
erogeneous regions in Fig. 4.3. For water and ice, l corresponds to fusion.

4.6 Equilibrium Phase Transformations
113
Under those circumstances, (4.35) is expressed most conveniently in inverted
form
dT TAr
dp 1 '
which describes the influence on melting temperature exerted by a change
of pressure. Because the change of volume during fusion is negligible, the
equation of state in the region of water and ice reduces to
(d_pT) ~'0. (4.36)
fusion
Changing the pressure has only a negligible effect on the temperature at which
water is at equilibrium with ice. Consequently, the surface of water and ice in
Fig. 4.3 is vertical.
For vapor and a condensed phase, 1 corresponds to the latent heat of vapor-
ization or sublimation. Under those circumstances, the change of volume is
approximately equal to that of the vapor produced (4.31). Thus,
Av Z RvT
P
which transforms (4.35) into
din p
)
For l ~ const, (4.37) gives
l
= (4.37)
vaporization
RvT 2"
sublimation
Using the value of l~
respect to water:
2.354 x 103
10810
Pw "~
9.4041 - T ' (4.39)
where Pw is in millibars and T = 0~ is used as a reference state. Similarly,
the value of
ls
in (4.29) yields the equilibrium vapor pressure with respect to
ice
2.667
x 103
log10
Pi "
10.55 - T ' (4.40)
which differs from (4.39) only modestly.
Equations (4.39) and (4.40) describe the simplified surfaces in Fig. 4.3
that correspond to vapor being in chemical equilibrium with a condensed
phase and to the system pressure equaling the equilibrium vapor pressure
Pw
or
Pi.
If the system's pressure is below the equilibrium vapor pressure
for the temperature of the system, water will evaporate or sublimate until the
in (4.29) yields the equilibrium vapor pressure with

114 4
Heterogeneous Systems
system's pressure reaches the equilibrium vapor pressure. A pressure above the
equilibrium vapor pressure will result in the reverse transformations. Owing
to the exponential dependence in (4.39) and (4.40), Pw and
Pi
vary sharply
with temperature. In the presence of a condensed phase, substantially more
water can exist in vapor phase at high temperature than at low temperature.
We will see in Chapter 5 that the principles governing a single-component
heterogeneous system carry over to a two-component system of dry air and
water. The equilibrium vapor pressure is then the maximum amount of vapor
that can be supported by air at a given temperature.
The exponential dependence of
Pw
on T has an important implication for
exchanges of water between the earth's surface and the atmosphere. Accord-
ing to (4.39), warm tropical oceans with a high sea surface temperature can
transfer substantially more water into the atmosphere than can colder extra-
tropical oceans. For this reason, tropical oceans serve as the primary source
of water vapor for the atmosphere, which is subsequently redistributed over
the globe by the circulation (refer to Fig. 1.15). Much of the water vapor ab-
sorbed by the tropical atmosphere is precipitated back to the earth's surface
in organized convection inside the Inter Tropical Convergence Zone (ITCZ)
(Fig. 1.25). However, latent heat that is released during condensation remains
in the overlying atmosphere. Thus, cyclic transfer of moisture between ocean
surfaces and the tropical troposphere results in a net transfer of heat to the
atmosphere. Eventually converted into work, that heat generates kinetic en-
ergy, which, along with radiative transfer from the earth's surface (Fig. 1.27),
maintains the general circulation against frictional dissipation.
Suggested Reading
The Principles of Chemical Equilibrium
(1971) by Denbigh includes a thorough
development of phase equilibria in heterogeneous systems.
A detailed treatment of water substance and accompanying thermodynamic
properties is presented in
Atmospheric Thermodynamics
(1981) by Iribarne
and Godson.
Problems
4.1. The Gibbs-Dalton law implies that the partial pressure of vapor at equi-
librium with a condensed phase of water is the same in a mixture with
dry air as the equilibrium vapor pressure if the water component were
in isolation. Since it corresponds to the abundance of vapor at which no
mass is transformed from one phase to another, this vapor pressure de-
scribes the state at which air is
saturated.
For a lapse rate of 6.5 K km -a,
which is representative of thermal structure in the troposphere (Fig. 1.2),
calculate (a) the equilibrium vapor pressure as a function of altitude and

Problems
115
4.2.
4.3.
4.4.
4.5.
4.6.
4.7.
4.8.
4.9.
(b) the corresponding mixing ratio of water vapor as a function of alti-
tude.
Derive the fundamental relations (4.17) for a mixture involving multiple
phases.
Derive alternate expressions for chemical equilibrium under (a)
isothermal-isochoric conditions and (b) reversible-adiabatic and isobaric
conditions.
Consider a mixture of dry air and water. Describe the state space of
this generally heterogeneous system and the geometry its graphical rep-
resentation assumes, noting the number of thermodynamic degrees of
freedom in regions where one, two, and three phases are present.
Use (4.39) to estimate the latent heat of vaporization for water.
A more accurate version of (4.39) is given by
2937.4
log10
Pw - T
4.9283 log10 T + 23.5471 (mb).
Estimate the boiling temperature for water at the altitude of (a) Denver,
5000 ft, (b) the continental divide, 14,000 ft; and (c) the summit of Mt.
Everest, 29,000 ft.
Present-day Venus contains little water, most of which is thought to
have been absorbed by its atmosphere and eventually destroyed dur-
ing the planet's evolution (Sec. 8.7). (a) For present conditions, wherein
Venus has a surface pressure of 9
• 10 6
Pa, at what surface tempera-
ture does water vapor become the atmosphere's primary constituent?
Compare this to Venus' present surface temperature of 750 K. (b) Were
these conditions to prevail to altitudes where energetic UV radiation is
present, what would be the implication for the abundance of water on
the planet? (c) Contrast these circumstances with present-day conditions
on Earth.
Clouds seldom form in the stratosphere because air there is very dry.
However, polar stratospheric clouds (PSCs) are known to form over the
Antarctic and, less frequently, over the Arctic. The thicker of these clouds
(Type II PSCs), which are still quite tenuous compared to tropospheric
clouds, are known to be composed of ice. (a) For a mixing ratio at
80 mb of 3 ppmv, which is representative of water vapor in the lower
stratosphere (refer to Fig. 17.8), calculate the temperature at which ice
cloud forms. (b) Referring to Fig. 1.7 and to the discussion in Sec. 17.3.2,
where are such clouds likely to form?
The average precipitation rate inside the ITCZ is of order 10 mm day -1
(Fig. 9.38). (a) In watts per square meter, calculate the average column
heating rate inside the ITCZ. (b) Compare this value with the longwave

116 4
Heterogeneous Systems
radiative flux emitted to (and largely absorbed by) the atmosphere if the
surface behaves as a blackbody at a temperature of 300 K (1.29).
4.10. The Gibbs-Dalton law implies that equilibrium properties of water vapor
in solution with dry air are identical to those of pure water. In light of
this result, explain why the vertical profile of atmospheric water vapor is
distinguished from other trace gases in Fig. 1.20. In particular, use the
global-mean temperature (Fig. 1.2) to demonstrate why the mixing ratio
of water vapor decreases sharply with height in the troposphere.

Chapter 5
Transformations of Moist Air
The atmosphere is a mixture of dry air and water in varying proportions.
Although its abundance varies widely, water vapor seldom represents more
than a few percent of air by mass. We shall consider a two-component system
comprised of these species, with water appearing in possibly one condensed
phase. According to the Gibbs-Dalton law (which is accurate at pressures be-
low the critical point), an individual component of a mixture of gases behaves
the same as if the other components were absent. Consequently, the abun-
dance of vapor at equilibrium with a condensed phase in a mixture of water
and dry air is the same as if the water component were in isolation. ~ For this
reason, concepts established in Chapter 4 for a single-component system of
pure water carry over to a two-component system of dry air and water.
5.1 Description of Moist Air
5.1.1 Properties of the Gas Phase
For the moment, we focus on the gas phase of this system, irrespective of
whether condensate happens to be present. The vapor in solution with dry air
is represented by its partial pressure e, which obeys the equation of state
In (5.1.1),
evv = R~T.
(5.1.1)
V
vo
= (5.a.e)
my
is the specific volume of vapor (not to be confused with the partial volume),
R*
R v =
Mo
1
= -rid, (5.1.3)
and ev =
Mv/Md ~-
0.622 is the ratio of molar weights defined by (1.15.2).
aThis and other implications of the Gibbs-Dalton law can be found in Keenan (1970).
117

118 s Transformations of Moist Air
The
absolute humidity Pv - 1/vv
measures the absolute concentration of
vapor. The relative concentration of vapor is measured by the
specific humidity:
q_ p~ _ _ m__~v , (5.2)
p m
which equals the ratio of the masses of vapor and mixture. Closely related is
the mass
mixing ratio r,
which is referenced to to the mass of dry air (1.14).
Since
m - m d n t- my,
(5.3)
the mixing ratio is approximately equal to the specific humidity
q
r--
1-q
=q
(5.4)
because vapor exists only in trace abundance (Table 1.1). Hence, to a good
approximation, q and r can be used interchangeably. Both are conserved for an
individual air parcel outside regions of condensation. By contrast, measures
of absolute concentration like e and p~ change for an individual air parcel
through changes of i.ts pressure, even if the mass of vapor remains fixed.
Despite the advantages of relative concentration, chemical equilibrium of
the water component is controlled by the absolute concentration of vapor. For
this reason, it is convenient to express the mixing ratio r in terms of the vapor
pressure e. The dry air component of the mixture obeys the equation of state
PdVd -- RdT ,
(5.5)
where
Pd
and
Vd
denote the partial pressure and specific volume of dry air.
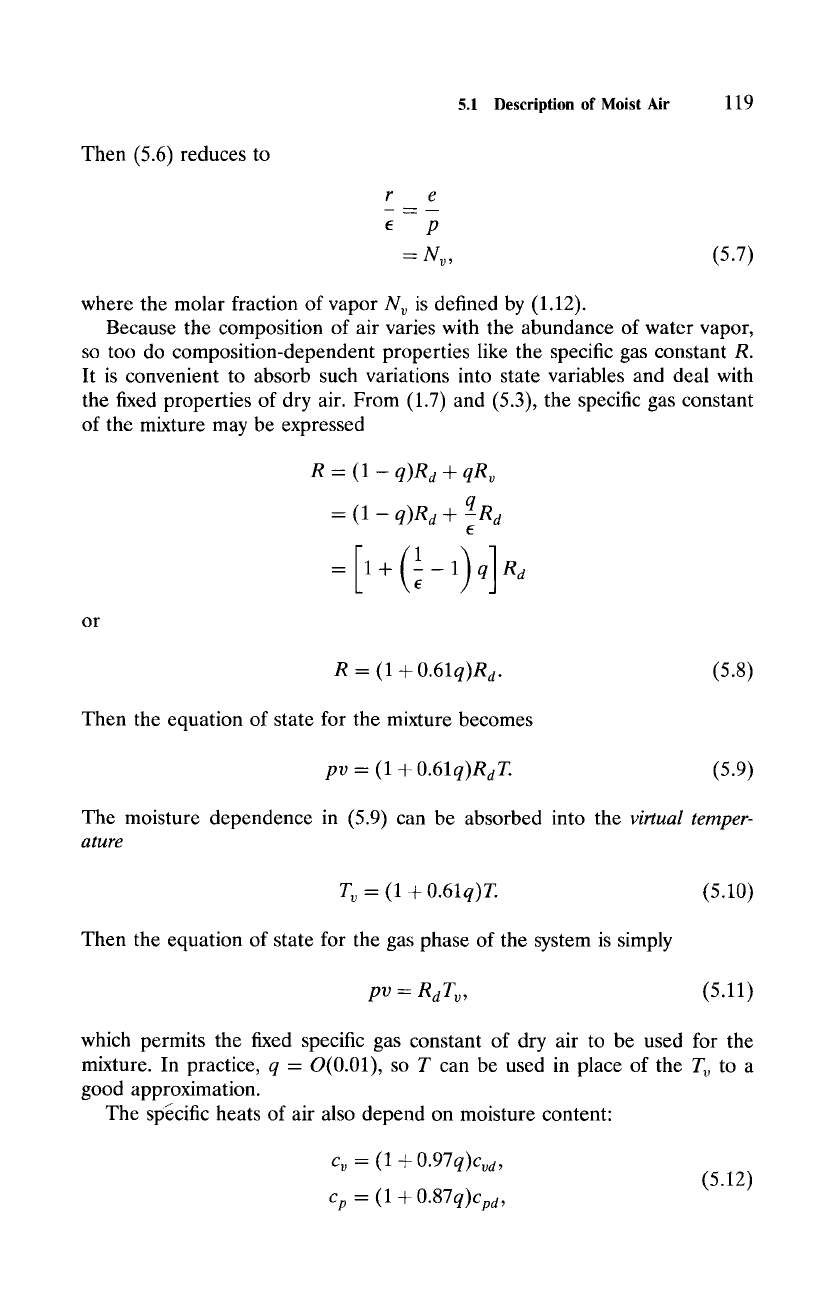
Dividing (5.1) by (5.5) obtains
where
e- %
is understood. Now
my
1
r
and, since vapor exists only in trace abundance,
p--Pd+e
~-- Pd"
5.1 Description of Moist Air
119
Then (5.6) reduces to
e
P
--Nv,
(5.7)
where the molar fraction of vapor Nv is defined by (1.12).
Because the composition of air varies with the abundance of water vapor,
so too do composition-dependent properties like the specific gas constant R.
It is convenient to absorb such variations into state variables and deal with
the fixed properties of dry air. From (1.7) and (5.3), the specific gas constant
of the mixture may be expressed
R - (1 -
q)R d + qR v
= (l -- q)Rd + q Rd
E
or
R -- (1 +
0.61q)R d.
(5.8)
Then the equation of state for the mixture becomes
pv -
(1 +
0.61q)RdT.
(5.9)
The moisture dependence in (5.9) can be absorbed into the
virtual temper-
ature
T~ - (1 + 0.61q)T. (5.10)
Then the equation of state for the gas phase of the system is simply
pv- RdT,,,
(5.11)
which permits the fixed specific gas constant of dry air to be used for the
mixture. In practice, q = O(0.01), so T can be used in place of the T~ to a
good approximation.
The specific heats of air also depend on moisture content:
c v --
(1 +
0.97q)cvd ,
(5.12)
Cp - (1 + 0.87q)Cpd,
