Smith G.T. Cutting Tool Technology: Industrial Handbook
Подождите немного. Документ загружается.

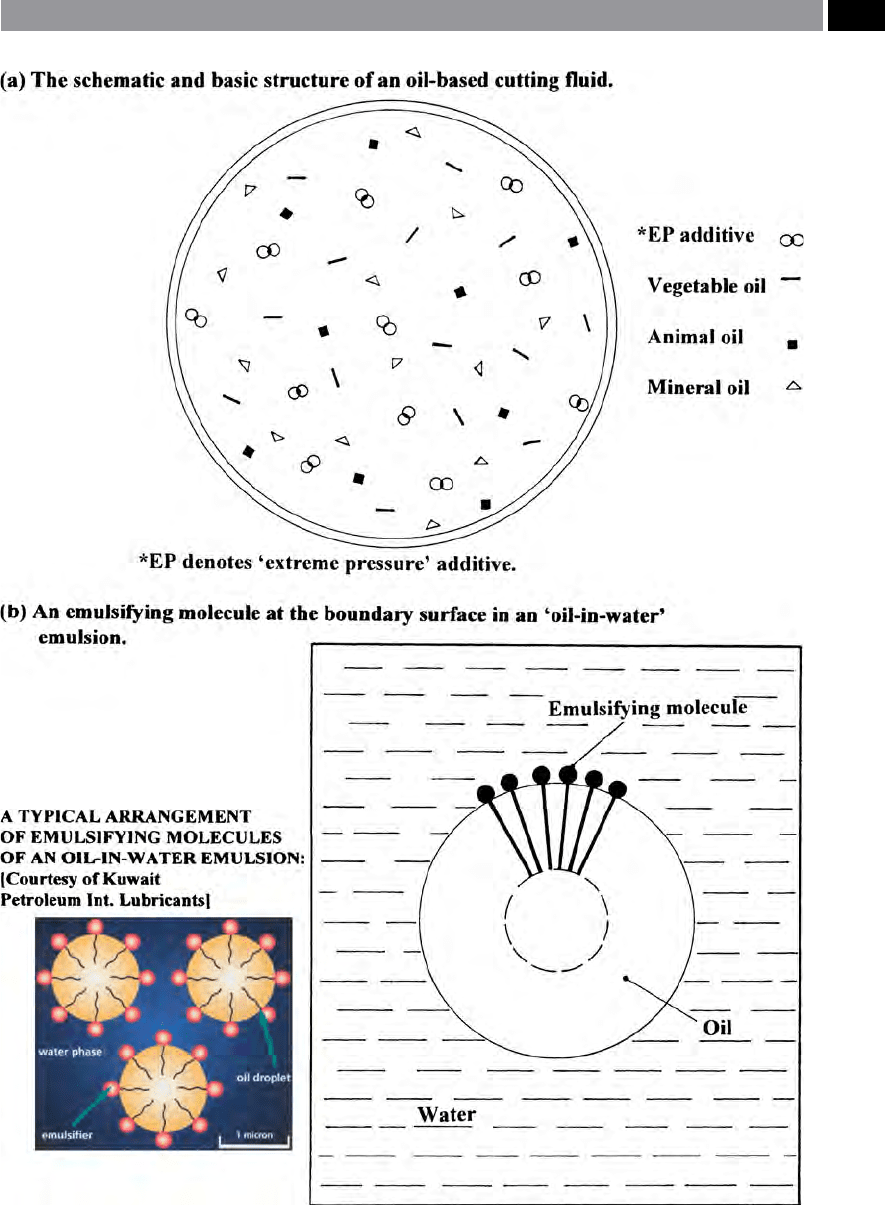
Figure 198. The basic structure of an oil-based cutting uid and an ‘oil-in-water’ emulsifying molecule. [Courtesy of
Cimcool]
.
Cutting Fluids 393
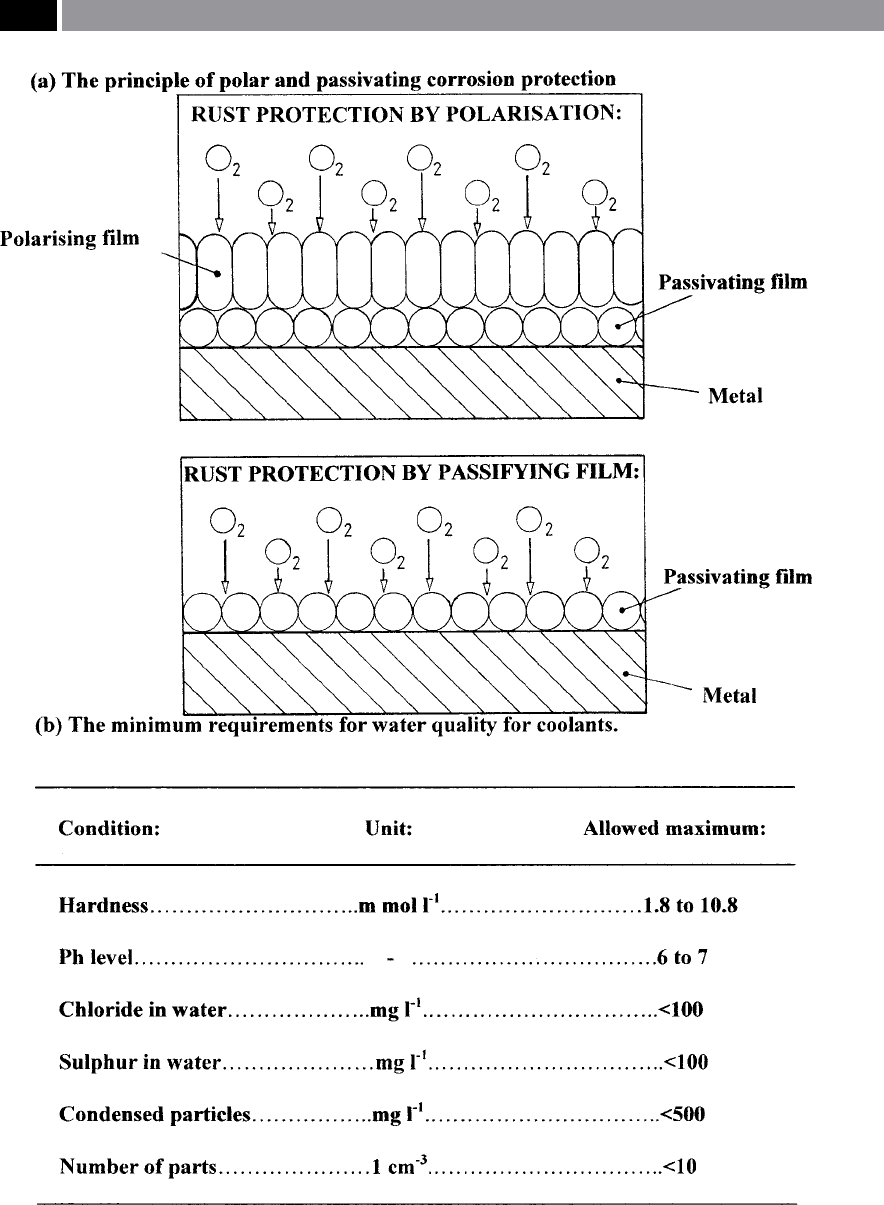
Figure 199. The principle of polar and passivating corrosion protection and the minimum requirements for water
quality. [Courtesy of Cimcool]
.
394 Chapter 8

the oil
8
, keeps the machine tool cleaner and will delay
the formation of a layer of ‘tramp-oil’ on the surface –
which might otherwise encourage unwanted bacterial
growth. e
denition o
f Semi-synthetic cutting uids
9
can cause some diculty, but generally the oil content
is much l
ower than with the mineral-soluble oils, rang-
ing from approximately 10 to 40%.
Additives for: corrosion inhibition; bacterial con-
t
rol; lubricity
10
; EP; are employed in the same manner
as for mineral-soluble oils, also, there is oen an addi-
t
ion of a blue, or pink dye, as these translucent micro-
emulsions can appear to look somewhat ‘watery’ oth-
e
rwise. Although translucent micro-emulsions
a
re
initially formed, Semi-synthetics d
o not go cloudy in
use. ey contain excess emulsiers to ensure that ne
micro-emulsion of oil particles are formed in water. As
previously mentioned, these ‘spare’ emulsiers enable
the micro-emulsion to absorb tramp oil. Hence, as
these ‘spare’ emulsiers are consumed by suspending
the ‘tramp-oil’ , both the amount of oil in the emulsion
and the oil particle size increases. is
increase i
n oil
particle size causes m
ore incident light to be reected
and results in the visual ‘clouding eect’ w
ithin the lu-
bricant. In particular, this ‘cloudiness’ of the lubricant
is not necessarily an indication that there is anything
wrong with the uid,
i
t is merely an suggestion of the
oil absorbed by the cutting uid.
All cutting uids, whether ‘aqueous-’ , or ‘oil-based’ ,
may contain some: mineral oils; synthetic products; or
a combination of both. e choice of raw material and
composition depends on certain parameters and their
actual composition (i.e its formulation) will depend
8 ‘Emulsication of tramp-oil’ when using Semi-synthetic oils,
will only occur, until all of the ‘spare’ emulsiers are used up!
erefore, aer this time, the excess ‘tramp-oil’ will oat on
the cutting uid’s surface.
NB Some Semi-synthetic formulations will emulsify only
small quantities of ‘tramp-oil’ , while others can emulsify
much larger concentrations.
9 P
erhaps the easiest and best uid denition is this: ‘A semi-
synthetic cutting uid forms a translucent emulsion and con-
tains mineral oil’.
10
‘Lubricity’ , o
r ‘Oiliness’ as it is oen known, is dicult to de-
ne with any precision. One reasonable denition is that Lu-
bricity is: ‘[e signicant] dierences in friction greater than
can be accounted for on the basis of viscosity, when comparing
dierent lubricants under identical test conditions.’ [Source:
American Society of Automotive Engineers]
upon many factors, which is closely-guarded secret by
any lubricant
m
anufacturer.
8.4.2 Aqueous-Based Cutting Fluids
A large proportion of cutting uids used for machin-
ing operations are still of the aqueous-based types (Fig.
197), as they combine the excellent heat-absorbing ca-
p
acity of water, with the lubricating power of chemical
substances. Such cutting uids oer excellent cooling,
lubricating and wetting properties. Machine tools re
-
qu
ire protection from the lubricant ingress and should
be compatible with lubricating and hydraulic systems
on the machine, making it possible to apply water-
mixed cutting uids to the manufacturing environ-
ment. e aqueous-based lubricants can be utilised
across quite a diverse range of workpiece materials,
ranging from steels, to non-ferrous metals.
An aqueous cutting uid can consist of naturally
occurring oils such as: mineral oil; synthetic mater-
ial; or a combination of both, but generally they are
present in the form of an emulsion, or solution – as
previously discussed. Other forms of cutting uids,
such as: suspensions; gels; pastes; are rarely used in
the production process. Hence, the commonest form
in which aqueous cutting uids are used is as an emul
-
s
ion. Much of this cutting uid terminology has al-
r
eady been discussed, but is worth restating, to ensure
that its signicance is suciently comprehended. An
emulsion is a disperse system formed by
m
ixing two
uids which are not soluble in each other. In the emul
-
s
ion, one of the uids forms the internal phase, which
is dispersed in the form of droplets suspended in the
external phase, or ‘medium’ – as its is oen known.
Such corresponding cutting uids are of two types:
‘emulsive’ , or ‘emulsiable’ – of which the former type
is normally the most commonly used. e ‘emulsive’
cutting uid consists of an oil-in-water emulsion, in
which the oil forms the internal phase. While its coun
-
t
erpart, the ‘emulsive’ type is the ‘emulsiable’ solu-
t
ion, consisting of a water-in-oil emulsion, but here,
the water is the internal phase – lately this cutting uid
has become less important.
An aqueous ‘emulsive’ cutting uid always contains
a stock oil, usually having a: mineral oil; synthetic
hydrocarbon; synthetic ester; or fatty oil, etc.; together
with certain additives to the formulation. e most
important additives tend to be: ‘emulsiers’; corro
-
s
ion inhibitors; stabilisers and solubilisers; anti-foam
Cutting Fluids 395

agents; micro biocides; as well as complex formers (i.e.
see Fig. 197). Consideration will now be given to each
of these ‘additives’ in turn:
‘Emulsifiers’
e ‘emulsiers’ are necessary to help form a stable
emulsion and as such, are very important for the tech-
n
ical characteristics of the cutting uid. ‘Emulsiers’
make it possible for the oil droplets to form and re-
m
ain suspended in water, preventing them from merg-
i
ng and oating upwards to form a surface layer in the
uid’s tank. ‘Emulsiers’ r
educe the surface tension and
form a lubricating lm a
t the boundary surface. ese
‘emulsier’ molecules are bipolar in characteristic and
as a result ‘line-up’ like the bristles on a brush, with
one end toward the oil and the other end facing the
water, as shown in Fig. 198b. In this way, the ‘emulsi
-
er’ forms a lm which is one molecule thick at the
boundary surface.
Corrosion Inhibitors
e main task of a corrosion inhibitor in any aqueous
cutting uid is to prevent the water in the uid from
corroding the exposed portions of the machine tool,
such as its: slideways; spindle nose; ballscrews; etc. e
mechanism by which dierent corrosion inhibitors
operate, will vary widely and one commonly used ver
-
s
ion of ‘inhibitor’ , consists of an additive
w
hich forms
a protective lm on the exposed metal’s surface
11
.
11 ‘Galvanic corrosion’ , for two metals in contact in the ‘electro-
chemical series’ the further apart they are in this ‘series’ , the
greater their electro-potential and the faster the rate of corro-
sion. For example, in this ‘series’ gold (i.e. being a ‘noble metal’)
is at one extreme, thus having a potential dierence of +1.70
v – being cathodic, while at the other end of the galvanic table,
calcium (i.e. being a ‘base metal’) has a potential dierence
of –2.87 v – being anodic. Hence, the anodic metal will cor-
rode, while the cathode remains unchanged, hence in gold’s
case, the term ‘noble’ metal is used.us, water-miscible u-
ids can penetrate between bolt threads, setscrews and xtures
and as water is an electrolyte – a liquid that can conduct an
electrical current, the presence of water produces a galvanic
electrical current ow between these mating parts. So, say on
a lightweight workpiece xture – perhaps made from alumin-
ium (–1.67 v) with this being located onto a machine tool’s
table – normally produced from cast iron (–0.44 v). us, the
potential dierence here being 1.23 v, which is not too acute,
as both these metals are in fact, relatively close-together in the
‘electro-chemical series’.
ese corrosion inhibitors consist of long, narrow
molecules which are negatively-charged and
a
s such,
are attached to the metal in contact (Fig. 199a – top
schematic diagram, shows:
rust protection b
y polari-
sation, w
hereas the lower schematic diagram depicts;
rust protection b
y a passifying lm). ese ‘lms’ that
are subsequently formed, are no thicker than just a
few molecules and as such, are invisible. Nevertheless,
such ‘lms’ can eectively prevent the electro-chemi
-
c
al process of corrosion, such as passivation by means
of nitride, but the latter type is now being eectively
phased-out.
Stabilisers and Solubilisers
Stabilisers considerably extend the life of the concen-
trate, w
hile solubilisers act to increase the oil’s solubil-
ity. V
arious alcohols and glycols can be used as stabi-
lisers, or solubilisers.
Anti-Foaming Agents
Anti-foaming agents are oen known by the alter-
native names of: ‘anti-froth-’; or ‘defrothing-agents’;
being utilised to prevent the formation of foam. Sili-
c
ones, while being subject to certain restrictions have
proved in the past to be very popular anti-foaming
agents. A typical restriction to that of using silicones
additives in machining operations, might be because
aerward it may prove dicult to either: paint; coat;
or adhesively-bond to the machined parts. In the
past when both the coolant pressures and ow rates
were low, foaming did not present too great a prob
-
l
em, but nowadays, the pressures and ow rates are
much greater and severe coolant agigtation can re-
s
ult, creating potential foaming conditions. Foaming
is at its most prevalent when a newly-charged clean
and fresh cutting uid is employed and as this coolant
is contaminated with: ‘tramp-oil’; metal nes; abra
-
s
ive grains; from the subsequent machining process,
these contaminants will tend to suppress foaming
tendencies.
NB Galvanic corrosion occurs between contact of dissimilar
metals – in the presence of an electrolyte. is electrolytic con-
tact might at the least cause either: surface staining; mild corro-
sion; or pitting, with its severity depending upon how long the
two metallic surfaces are in contact in the presence of water.
396 Chapter 8

Today, anti-foaming agents tend to be ‘branch-
chained’ higher alcohols – being insoluble in wa-
t
er, or as mentioned above, silicones. Both alcohols
and silicones evidently disrupt the foam-producing
surface lm with that of an alternative gas-perme
-
a
ble surface lm, causing the surface-active liquid
surrounding each bubble to drain away, causing the
foam layer to collapse. If severe foaming occurs, anti-
foaming agents are not the answer, as eventually these
‘anti-foams’ get carried away, or ltered-out of the
coolant on the resultant machined: chips and swarf;
workpieces; or on coolant lters. e problem to
foaming may
not b
e due to the lack of ‘anti-foams’ ,
b
ut may be the result of air leaks that are sucking air
into the coolant stream. ese air leaks oen arise
around the pipe unions, or at pipe-connectors to
either the valves and pumps in the coolant delivery
system.
Microbiocides
Microbiocides are oen added to the aqueous-based
cutting uid as they help prevent the dramatic and un-
c
ontrolled growth of microbes in the coolant. Micro-
biocides uses are normally limited, owing to the po-
t
ential skin-care consideration – more will be said
concerning this very important topic later in the chap-
t
er, when ‘health-issues’ will be discussed.
8.4.3 Water Quality
e main constituent of any aqueous-based cutting
uid is obviously water and by nature, it is impure. e
impurity depends on the source: rain-; river-; spring-;
ground-water; etc. e water may also contain: dust
particles; oxygen; nitrogen; calcium and magnesium
salts; oen with smaller quantities of: ammonia; bo
-
r
on; ourine; iron; nitrate; strontium; aluminium;
arsenic; barium; phosphate; copper and zinc. Addi-
t
ionally, the water has in its presence micro-organ-
i
sms, such as: algae; bacteria; fungi and viruses (i.e.
see Fig. 203); although in dierent orders of magni-
tu
de. So, depending on its composition, water can
aect the aqueous-based cutting uid in many ways
and since the composition varies throughout the
year, these seasonal variations will have an eect on
its use. By far the greatest eect on the properties of
the cutting uid is caused by the hardness of the water.
Water’s hardness depends on the concentration of ele
-
ments
12
such as: calcium, magnesium and other heavy
metals like iron and manganese. Hard water may cause
a soapy deposit, which will eventually block lters, or
destabilise the emulsion and may have a detrimental
eect on the uid’s corrosion protection. Equally, so
water can be a problem, but for a dierent reason, in
this case it can promote foaming under ‘abusive’ ma
-
c
hining conditions.
e degree of alkalinity of the water can be ex-
p
ressed as a pH-value (
i.e. see the pH-scale shown in
Fig. 202b) and this is an important measurement, as
it aects its usage and can react to human skin
13
caus-
ing ‘serious complaints’ – more will be said concerning
these health issues later in the chapter. Alkalinity in
the main, aects the growth of microbes (i.e. see Fig.
203b) and the degree of corrosion protection aorded
12 ‘Water hardness levels’ , are calculated based upon the quantity
of ‘grains’ of hardness minerals the water contains. By way
of example, one grain of calcium carbonate, constitutes 17.1
parts million
–1
(ppm) per 3.785 litres (i.e. equivalent to a U.S.
gallon). ‘Salts’ such as sodium chloride and sodium sulphate
are found in hard water, where they contribute to corrosion, or
rust – if not ‘inhibited’. Moreover, the greater the cutting uid’s
solution salt content, the more coolant concentrate is required
to prevent subsequent corrosion. Further, coolant degradation
occurs with time and usage. For example, a new charge of
relatively so water admixed with coolant concentrate, will
initially have say, a 3-grain hardness, but aer one month’s use
its hardness will have increased to between 12–14 grains and,
aer two months this hardness will have increased still further,
to between 24–27 grains. is problem is exacerbated if the
water content evaporates, needing periodic cutting uid analy-
sis to maintain optimum coolant performance.One method of
signicantly reducing water of its hardness minerals, is to run
it through a water soener, which removes the calcium and
magnesium ions, replacing them with sodium ions, although
residue build-up will be signicantly reduced, corrosion may
now be a problem, so for this reason soened water is not
recommended when using water-miscible coolants. Other-
wise, boil the water – ensuring that no soener, or anti-cor-
rosion agents were present prior to using the condensed water
product (i.e from the boiling process). Deionized water is the
best source of pure water, as a deionizer removes all dissolved
minerals, creating distilled water.
13 ‘Human skin’ , varies from one body-region to another, but
generally, it has a pH-level slightly biased toward the acidic
region of the scale, at approximately 6.8 pH (e.g. a value of 7.0
pH is considered as ‘neutral’).
NB Skin also has a protective layer of natural oils, that act
to retard moisture evaporation, acting as a form of ‘defensive
shield’ against some forms of biological attack.
Cutting Fluids 397

by the emulsion. If alkaline levels increase this results
in improved protection, particularly when machining
ferrous workpieces. In view of the importance of water
composition for the eectiveness of a water-mixed
cutting uid, it is essential to know the quality of the
water source available and to take account of this fac
-
t
or when selecting a concentrate. Cutting uid manu-
f
acturers undertake water analysis, as do local water
companies. In Fig. 199b, the minimum requirements
for water quality for aqueous-based cutting uids is
shown.
8.5 Cutting Fluid
Classification – According
to Composition
Generally speaking, cutting uids are purchased under
the following classications, according to
t
heir com-
position:
•
Synthetic uids – are those cutting uids which
contain very little, or no natural oil. e various
components such as the actual cutting uid are
nely distributed in water, as such, they form a
watery transparent solution – shown in a schematic
representation in Fig. 200a. e applications of
synthetic cutting uids range from light-to-heavy
cutting, together with usage in grinding applica
-
t
ions. In order to ensure the necessary lubricating
power desirable for heavy cutting operations, some
of these products contain synthetic lubricants (Fig.
200b). e major properties of synthetic cutting
uids can be summarised as follows:
–
A very clean and transparent uid,
–
Excellent corrosion protection,
–
A long life cutting uid,
–
Outstanding cooling capabilities,
–
Easy to mix,
–
Does not burn, or smoke.
•
Semi-synthetic uids – can contain up to 41% oil
and when mixed with water they have a translucent
property (Fig. 200c). Extreme pressure (EP) addi-
t
ives and synthetic lubricant can be added, in order
to widen the range of potential workpiece materials
and applications. e properties of semi-synthetic
cutting uids can be summarised in the following
manner:
–
Very clean in appearance,
–
Excellent corrosion protection,
–
Long life of cutting uid,
–
Outstanding cooling capabilities,
–
Good wetting properties,
–
Easy to mix,
–
Does not burn, or smoke.
•
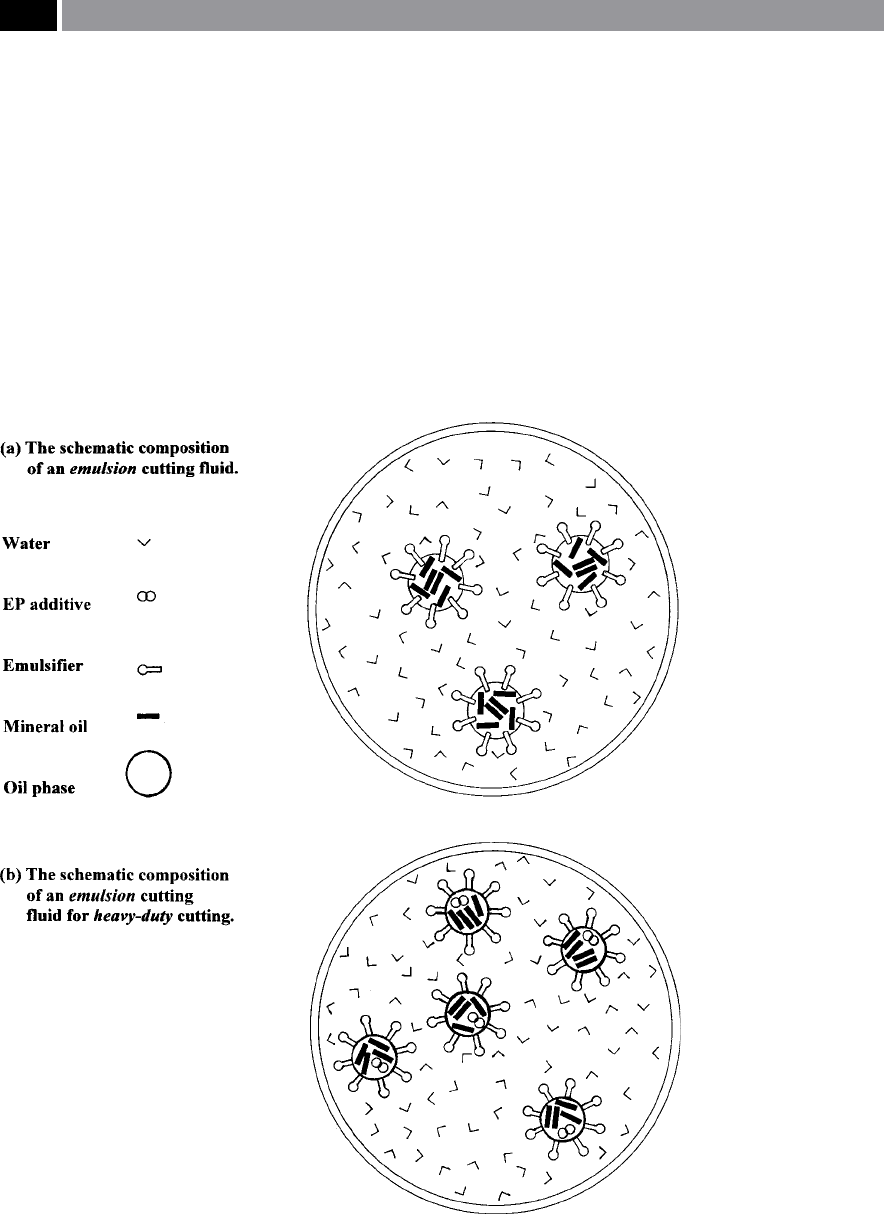
Emulsion uids – contain a high proportion of oil
and when the concentrate is mixed with water it
has a ‘milky appearance’ (Fig. 201a). Cutting uid
products intended for very heavy cutting operations
additionally contain EP additives (Fig. 201b). e
properties of an emulsion cutting uid, are sum
-
m
arised below:
–
Clean,
–
Oer good corrosion resistance,
–
Long life of emulsion,
–
Outstanding cooling capabilities,
–
Easy to mix,
–
Do not burn, or smoke.
Finally, for all of these various cutting uid types and
compositions, the dierences in the range of applica
-
t
ion of: synthetic; semi-synthetic; emulsion uids; de-
p
ends upon the respective machining requirements.
I
n
general, the heavier t
he cutting operation, the higher the
cutting forces p
roduced and the greater the oil content
required. is observation, means that synthetics a
re
normally used for lighter cutting o
perations, whereas,
emulsions a
re usually utilised for heavy-cutting appli-
cations, while the semi-synthetics t
end to be employed
as a general-purpose (
i.e. alternative) cutting uid.
8.6 Computer-Aided
Product Development
e latest cutting uids are very complex products and
a considerable amount of research and development (R
and D) is required to perfect them. e quantity of raw
materials that have diering characteristics and the
number of interactions between them, means that the
possible combinations are potentially enormous. Even
when most of the possible combinations are obviously
unnecessary and hence could be disregarded, this still
leaves the possibility of many thousands of coolant ad
-
d
itive permutations and their respective interactions
to investigate, which would be a ‘Herculean task’ to
398 Chapter 8
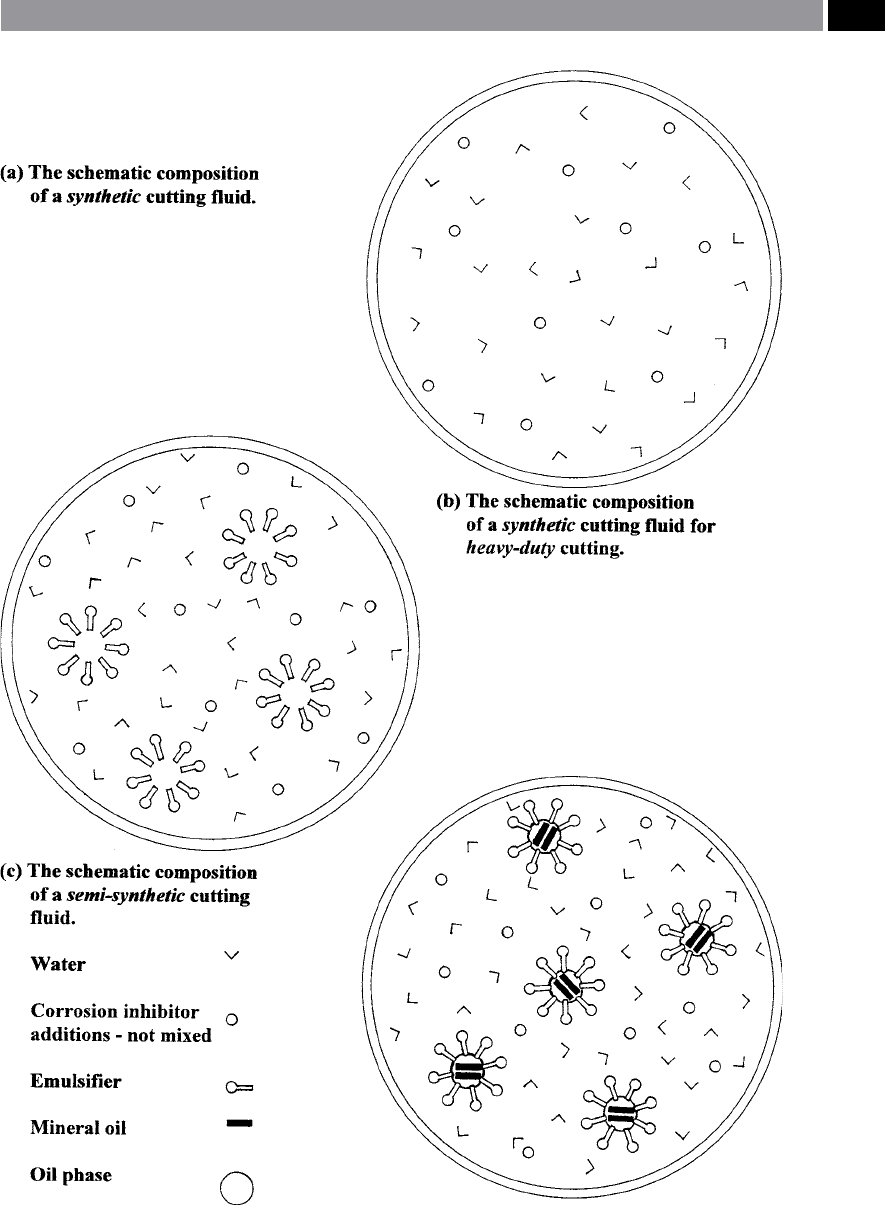
Figure 200. Schematic representation of synthetic variaties of cutting uids. [Courtesy of Cimcool].
Cutting Fluids 399
decipher and then to optimise! To press the point still
further, this situation of determining the optimum
combination is analogous to that of: ‘looking for a
needle in a haystack’ , where the conventional empiri
-
c
al methods become no better that in eect, searching
at random! Luckily a solution is at hand, by the evalu-
a
tion using computer technology, when utilised with
specially-developed programs. Computer-aided prod-
u
ct development will as a result, eciently provide a
solution backed-up by statistical techniques, enabling
many thousands of combinations to be assessed, re
-
d
ucing the nal choices to just a few cutting uid com-
b
inations. In this way it
i
s possible to rapidly and ac-
c
urately optimise the solution, as depicted in Fig. 204,
where a Computer-aided Design (CAD) application is
used to select – in this case – a corrosion inhibitor for
a potentially-new cutting uid. Such computer-based
techniques have brought about a means of develop
-
i
ng cutting uid products, using the CAD to not only
‘screen-out’ formulations which do not t the present
machining requirements, but can also uncover previ
-
o
usly unsuspected properties – resulting form syner-
Figure 201. Schematic representation of emulsion varieties of cutting uids. [Courtesy of Cimcool].
400 Chapter 8
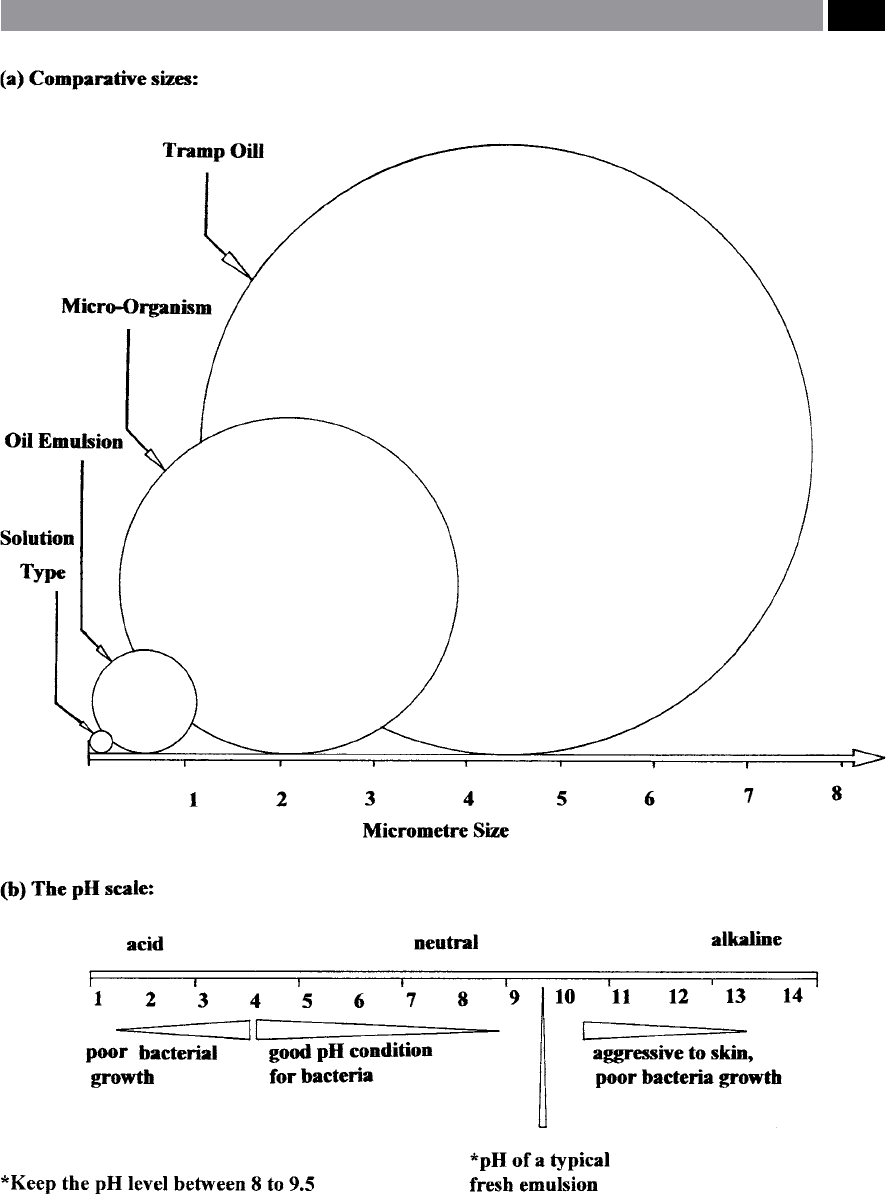
Figure 202. A cutting uid emulsion’s diametral size (0.2 to 1.5 µm) in comparison with micro-organisms and ‘tramp oil’, together
with the ‘pH scale’. [Courtesy of Kuwait Petroleum International Lubricants]
.
Cutting Fluids 401
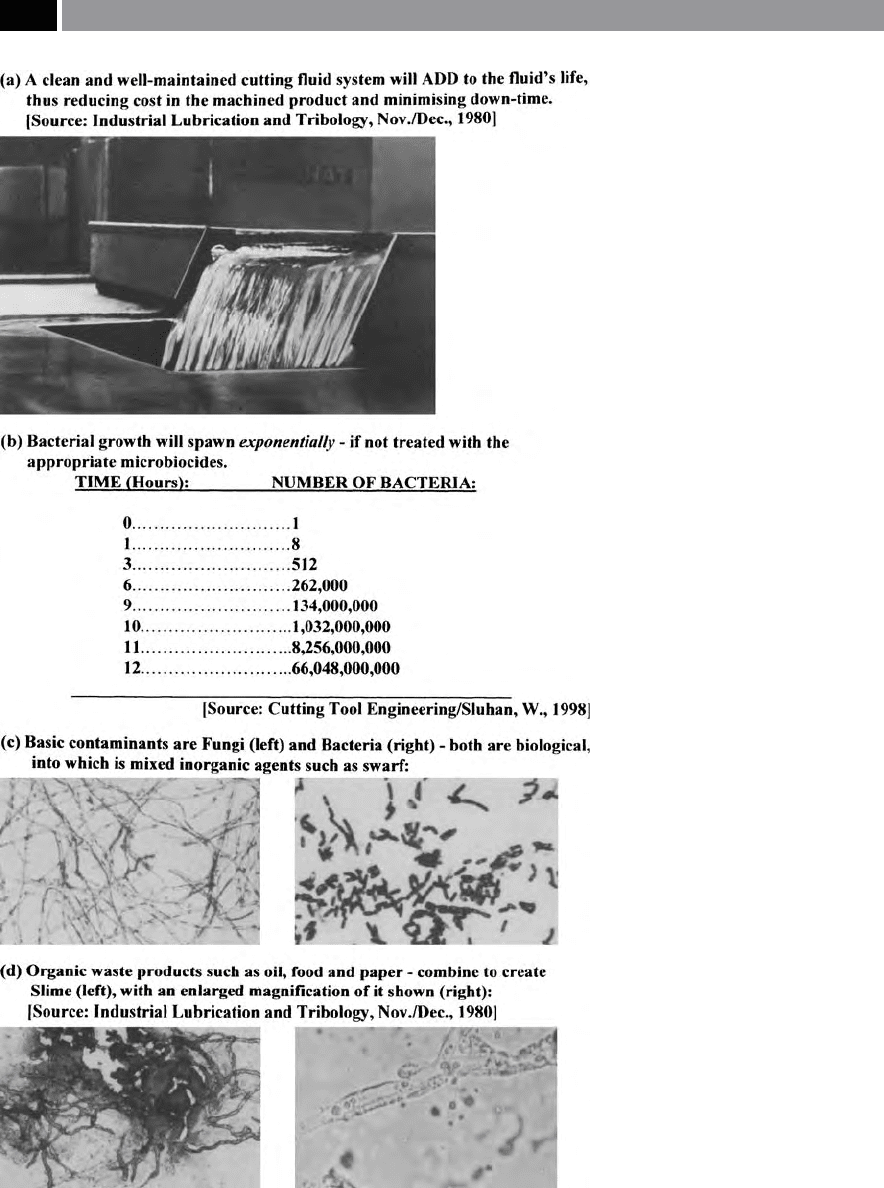
Figure 203. Bacterial contamination: aqueous cutting uid.
402 Chapter 8
