Baca A.G., Ashby C.I.H. Fabrication of GaAs Devices
Подождите немного. Документ загружается.

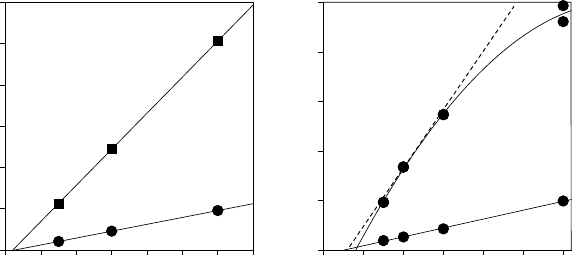
Wet oxidation for optoelectronic and MIS GaAs devices
oxidation rates are achievable with 98% Al compositions while
remaining within a virtually linear growth region.
The transition from reaction-limited (linear) to diffusion-limited
(parabolic) behaviour is often difficult to detect for relatively short
oxidation depths. A comparison of the oxidation behaviour of 94%
Al and 98% Al layers at 400 and 440
◦
C with 80
◦
C water and
an N
2
flow rate of 3.0 slm in a 4
diameter three-zone furnace
(FIGURE 10.6) reveals that the slower-oxidising material remains
in the linear regime at both temperatures, while a shift from linear
towards parabolic is seen for the faster-reaction 98% Al mater-
ial at the higher temperature [2]. However, for shorter reaction
times (shallower oxidation depths), the parabolic dependence is
well approximated by a straight line and the assumption of linear
time dependence would be adequate for selecting oxidation times
for depths less than 15 μm. The specific times and temperatures
at which the linear-to-parabolic transition will be observed will
depend on the water vapour supply, which is controlled by the
bubbler temperature and gas flow rate, and can vary depending on
specific operating conditions.
From these studies, it is clear that faster oxidation rates, whether
due to higher Al mole fraction or higher oxidation temperatures,
will shift the balance from linear towards parabolic time depend-
ences. It appears in general that for oxidation rates of AlGaAs
below 0.2 μm/min, the process appears reaction-rate limited while
initial rates above 1.3 μm/min appear diffusion limited at longer
times. The transition region lies between these rates. The slower
reaction rate obtained with higher Ga mole fractions enables the
loss of As, forming the porous oxide, to keep pace with the advan-
cing dense oxidation front. This allows such materials to remain
oxidised depth (mm)
time (min)
0 10203040506070
0
2
4
6
8
10
12
400°C
98%
94%
0 5 10 15 20 25 30
0
5
10
15
20
25
440°C
time (min)
98%
94%
(a) (b)
FIGURE 10.6 Change between linear and parabolic oxidation versus
composition and temperature from Ashby et al. [2].
312
Wet oxidation for optoelectronic and MIS GaAs devices
in the linear regime at higher temperatures and for longer times
than their higher-Al analogues.
It is clear in FIGURE 10.6 that some time may pass between
insertion into the furnace at t = 0 and the onset of oxidation.
In these cases, t = 0 was defined as the time at which the wafer-
loaded boat was returned to the central zone of a three-zone tube
furnace through which water vapour was flowing entrained in N
2
.
The lag in the apparent onset of oxidation may be attributed to
the much slower rates while the wafer and boat were reheating
to the furnace reaction temperature. Thermal equilibration times
will vary between different reactors and may be significant or
negligible. This will be discussed further in Section 10.4.
10.3.2 Layer thickness effects
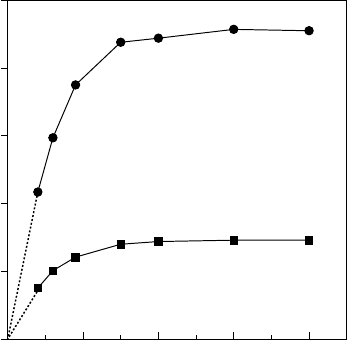
Above a certain layer thickness (about 100 nm), the oxidation rate
for a particular AlGaAs composition is not appreciably influenced
by the layer thickness. However, for thicknesses below 80 nm, a
very strong dependence on layer thickness appears, as illustrated in
FIGURES 10.7 and 10.8. This effect is probably related to the ease
of diffusive transport of either reactants or oxidation products from
the advancing oxidation front. A similar retardation of etching
reactions is seen when trying to selectively etch a thin layer, as in
selective channel recessing, discussed in Section 4.5.
The rate of diffusion-controlled reactions can be limited by the
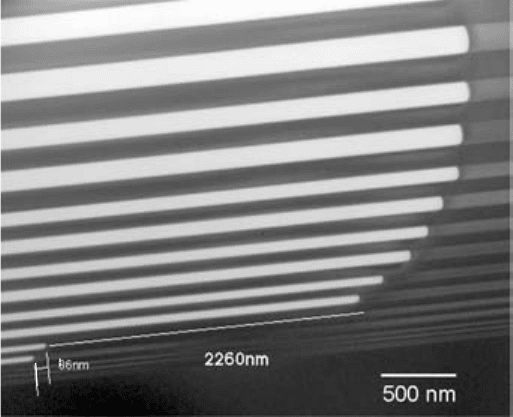
diffusion of either reactants or products. A study of the time
FIGURE 10.7 TEM of oxidation front region showing layer thickness effect.
313
Wet oxidation for optoelectronic and MIS GaAs devices
AlGaAs layer thickness (nm)
0 50 100 150 200
oxidation rate (mm/min)
0.0
0.1
0.2
0.3
0.4
0.5
425ºC
Al
0.94
Ga
0.04
As
Al
0.98
Ga
0.02
As
FIGURE 10.8 Effect of layer thickness on oxidation rate.
dependence as a function of layer thickness could distinguish
between the two in this case, since a transition from linear to
parabolic behaviour would signal the formation of a progressively
thicker interfacial front if the formation and removal of products
failed to keep pace in the thinner channels.
10.3.3 Proximity enhancement effect
The close proximity of a rapidly oxidising layer can accelerate the
oxidation of a lower Al-content layer relative to that obtained for an
isolated layer. A possible cause of the enhancement is the injection
of defects from adjacent oxidised regions. The enhancement can
be modelled as the combination of the regular lateral oxidation of
an isolated layer and a contribution from a species diffusing from
nearby oxidised zones [5]. The activation energy for this species
is consistent with the diffusion of column III vacancies, which are
known to enhance oxidation and whose diffusion is enhanced by
strain, which is present in the layers adjacent to the oxidised layer.
For example, lattice expansion is observed in thin (55 nm) GaAs
layers in oxidised superlattices so adjacent layers are strained.
From a practical perspective, this enhancement must be con-
sidered when profile control is important. Compositional steps
are used to fabricate tapered-oxide current apertures for optimum
VCSEL performance (Section 10.5) so the observation of profiles
that show appreciably deeper oxidation than expected from com-
positional effects alone is important. The enhancement resulting
from adjacent regions with faster oxidation rates actually simplifies
314
Wet oxidation for optoelectronic and MIS GaAs devices
(c)
(a)
(b)
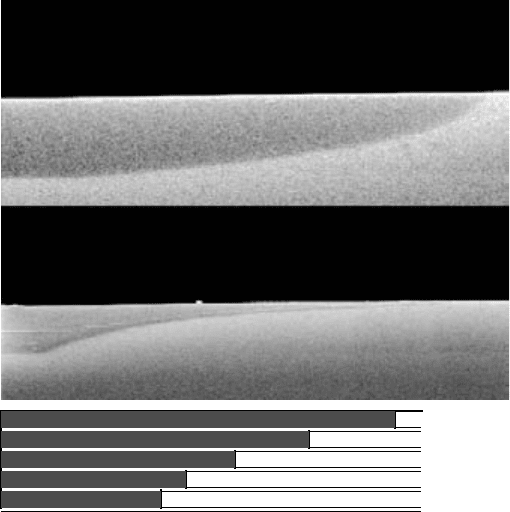
FIGURE 10.9 Enhancement effects from adjacent oxidised layers:
(a) continuous compositional grade from 98 to 90% Al in AlGaAs, (b) step
grade of 0.002% Al from 98 to 90% Al with 5 nm GaAs barriers between
AlGaAs layers and (c) schematic of oxidised step-graded structure.
the grown-in structure for a sharp taper. A sharp, smooth taper can
be obtained with only three layers and only two compositions, as
shown in FIGURE 10.14.
Enhancement is most pronounced when a continuous gradation
of Al percentage is employed in the structure (FIGURE 10.9(a)).
Where the compositional grading was expected to produce a
convex oxidation front replicating the Al-composition profile (as in
FIGURE 10.9(b)), a concave oxidation profile is obtained due to
enhancement from adjacent higher-Al-content regions.
The enhancement can be mitigated by insertion of thin (5 nm)
layers of GaAs between layers of a step-graded structure that
covers the same composition range (FIGURES 10.9(b) and
10.9(c)). The GaAs layers servetoimpedetheoxidation-enhancing
diffusion from the oxidised layers and the final oxidation profile
is closer to that expected based on composition alone.
10.3.4 Wet oxidation of other materials
While most wet oxidation work has focused on AlGaAs materials,
other III–V semiconductors with high Al content also have the
potential to undergo rapid wet oxidation.
315
Wet oxidation for optoelectronic and MIS GaAs devices
As mole fraction
0.0 0.2 0.4 0.6 0.8 1.0
0
20
40
60
80
100
120
oxidised depth (mm)
25 min at 380°C
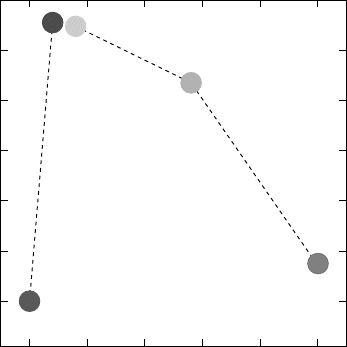
FIGURE 10.10 Wet oxidation of AlAsSb as a function of As mole fraction.
10.3.4.1 Sb-containing materials
Another III–V material family that might be amenable to wet
oxidation is the AlAsSb family of compounds. Among the
thermodynamically favoured products are As, which can mostly
escape as in the AlGaAs case, and Sb, which will remain behind.
Oxidation of these materials proceeds at temperatures lower than
those required for AlGaAs (325–380
◦
C.)
In the absence of As, wet oxidation produces a non-porous film
and the oxidation proceeds at a slow, diffusion-controlled rate. The
introduction of a small amount of As is sufficient to accelerate the
reaction by increasing the porosity (FIGURE 10.10). The fall-off
towards higher As mole fractions reflects the slower oxidation rates
for AlAs at these relatively low temperatures.
The oxidation of AlAsSb lattice matched to InP has been stud-
ied [6]. The structure shown in FIGURE 10.11 consisted of a
109 nm cap of In
0.53
Ga
0.47
As, 288 nm layer of AlAs
0.56
Sb
0.44
,
109 nm layer of In
0.53
Ga
0.47
As and an InP substrate. While surface
oxidation of AlSb, GaSb and InSb produces a layer of Sb which
moves ahead of the advancing oxidation front, in lateral oxidation
this Sb layer segregates to one of the oxide/semiconductor lateral
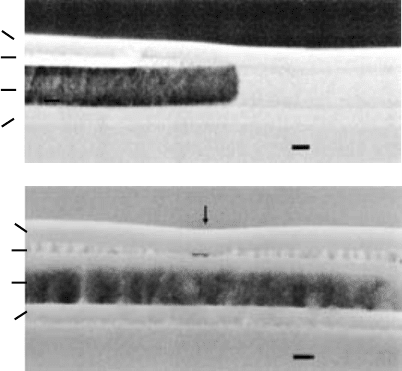
interfaces, typically the top one as shown in FIGURE 10.11, where
deformation can accommodate the volumetric changes caused by
oxidation. There is significant deformation of the surface layer
as the front advances, as can be seen in FIGURE 10.11(a), and
a dip where the fronts meet if the layer is completely oxidised
(FIGURE 10.11(b)).
316
Wet oxidation for optoelectronic and MIS GaAs devices
InGaAs
InGaAs
InSbO
x
Sb
InGaAs
InGaAs
InSbO
x
Sb
(a)
(b)
100 nm
100 nm
FIGURE 10.11 Sb segregation at interfaces in InAsSb wet oxidation from
Blum et al. [6].
10.3.4.2 P-based materials
Wet oxidation of P-based materials has also been the subject
of study. These materials differ appreciably in their principal
chemistry from As-based ones. While post-oxidation materials
analysis shows that most of the As is lost from the oxide, most
of the P is retained. Thermodynamic calculations suggest that the
principal products of this oxidation are very different for AlAs
and AlP. While the formation of As, Al
2
O
3
and AlO(OH) are most
favoured for AlAs, the change in Gibbs free energy is twice as great
for the formation of AlPO
4
than for P and Al
2
O
3
or AlO(OH).
Rates for phosphide materials are slower, and temperatures in
excess of 500
◦
C are employed.
The oxidation of In
0.5
Al
0.5
P has been compared with that of
Al
0.5
Ga
0.5
As. While InAlP oxidises more slowly than AlGaAs, it
has a lower activation energy. With oxidation at 500
◦
C, In is seen
to build up at the oxide/semiconductor interface. At 550
◦
C, the In
is distributed uniformly throughout the film and a parabolic time
dependence prevails. At 650
◦
C, In is depleted at the interface [7].
An interesting and potentially useful property of GaP is the
ability of a very thin layer (two monolayers) to serve as a diffusion
barrier to the movement of As away from the oxidised layer and
into adjacent semiconductor layers. The importance of this will be
discussed in Section 10.6.
10.3.5 Miscellaneous observations
The observed change in layer volume upon oxidation of AlAs and
AlGaAs is not as expected if calculated based upon theoretical
317
Wet oxidation for optoelectronic and MIS GaAs devices
molar volumes of the starting semiconductor and a dense, crys-
talline oxide. AlAs shrinks by about 13% while Al
0.98
Ga
0.02
As
shrinks only 6.7% upon oxidation. Analysis of oxidised films
has revealed the presence of appreciable quantities of hydrogen,
suggesting the presence of a considerable amount of hydrous
species such as AlO(OH) in the oxidised layer. This is consist-
ent with thermodynamic predictions. Since the relative amounts of
AlO(OH) and Al
2
O
3
are not well defined and may change depend-
ing on the specific process conditions, a priori prediction of film
volume is not strictly possible at this time. Similar behaviour is
expected for AlGaAs materials.
A serious mechanical problem can exist when oxidation is taken
to completion, i.e. when the oxidation fronts from opposing mesa
walls coalesce. The oxide/semiconductor interface is weak, and
the upper layers of the structure will delaminate from the oxide
unless a portion of unoxidised structure is present to hold the device
together. This is naturally the case for oxide-aperture VCSELs, but
use of wet oxidation for GOI (GaAs-on-insulator) devices will face
serious problems unless provision is made for holding the device
together. This problem is most pronounced for AlAs and is less
problematic with AlGaAs alloys.
Changes in composition mean changes in dimension, producing
stresses, especially in overlying layers. These can deform if they
are not too physically constrained by a thick semiconductor over-
layer. As seen in FIGURE 10.2, the oxidation-front profile tends to
be slightly deeper at the top interface than at the bottom interface.
This may be related to stress, although the origin of the effect is
not definitively established and the effect is rather small.
It is generally not possible to oxidise a certain distance into
a layer, pull the wafer from the furnace and then reinsert the
wafer to oxidise further for a controlled final depth. Wet oxida-
tion under these conditions is not a simple time-additive process.
There appears to be a change in the chemical or structural nature of
the oxide-semiconductor reaction front that discourages restarting
the wet oxidation process, perhaps by inhibiting the free access
of water to AlAs or AlGaAs for the generation of the H that is
essential for the rapid removal of As from the oxidising layer.
When inserting a wafer into a furnace with wet gas flow, there
may be a delay in the onset of rapid wet oxidation (FIGURE 10.6).
This is more of a problem for depth control of shallow oxidations,
since the apparent incubation time can be a substantial fraction
of the total time in the furnace. For such processes, it may be
especially important to load into dry gas, thermally equilibrate
to the final reaction temperature and then introduce the wet gas
flow. Since the heat capacities of dry nitrogen and water-saturated
nitrogen are different, it may be necessary to usedifferent flowrates
318
Wet oxidation for optoelectronic and MIS GaAs devices
of dry and wet nitrogen to maintain a constant furnace temperature
when switching gases. This will require calibration for a specific
reactor.
10.4 PRACTICAL WET OXIDATION
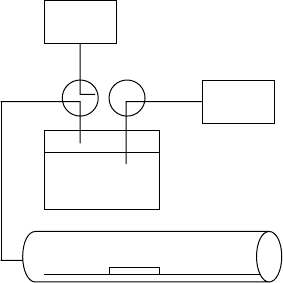
The apparatus for performing wet oxidation can be very simple,
consisting of a water bubbler, an inert gas source and a tube fur-
nace. It is best to use a quartz tube to avoid introducing contaminant
species, such as Na, that can be a problem with ordinary glass
at the elevated temperatures required for oxidation (350–525
◦
C).
A general system schematic is shown in FIGURE 10.12. For
Al
0.98
Ga
0.02
As layers thicker than 90 nm, oxidation at 400–440
◦
C
using 80–90
◦
C water and an N
2
flow rate of 3 slm in a 4
diameter
three-zone tube furnace works well. It is best to work at a temper-
ature at least two degrees below the boiling point of water, which
will depend on the elevation of the laboratory above sea level.
dry N
2
dry N
2
water
bubbler
boat
3-zone tube furnace
FIGURE 10.12 Diagram of wet
oxidation apparatus.
Water vapour is entrained in inert gas (N
2
or Ar) that is bubbled
through liquid water. The water temperature will determine the
equilibrium vapour pressure of water in the flowing gas and there-
fore controls the water concentration experienced at the wafer.
Both nitrogen and Ar work well as carrier gases as long as they
are oxygen-free. Even rather small amounts of oxygen in the gas
will stop the wet oxidation process (Section 10.2.1). The bubbler
should be of sufficient size that the water temperature within it
does not change more than a fraction of a degree during the reac-
tion. Heating the tubing between the bubbler and the inlet to the
furnace avoids condensation in transit to the reaction zone.
These reactions are highly temperature dependent, so it is vitally
important to maintain a uniform temperature in the reaction zone
and to avoid temperature-lag effects as much as possible while
loading wafers. It is helpful to use a three-zone furnace for thermal
stability in the oxidation region. Generally, the wafer will be
situated in the central zone. This allows the flowing gas to pass
through a hot zone before reaching the wafer. For AlGaAs, oxid-
ation temperatures are generally between 400 and 475
◦
C. For
P-containing materials, higher temperatures are used (>500
◦
C),
while Sb-containing materials are oxidised at lower temperatures
(325–380
◦
C).
Since reaction initiation can be sensitive to the surface condition,
it is most advantageousto perform the oxidation as soon as possible
after exposing the layer edge with a mesa etch. Minimal formation
of native oxide or its removal (Section 3.2.2) just prior to oxidation
is generally desirable if a sample has been etched a long time
before oxidising. This is especially important for Sb-containing
319
Wet oxidation for optoelectronic and MIS GaAs devices
materials, which can form a number of surface oxides that vary in
their reactive chemistry.
While reactant-gas access to the AlGaAs layers is easily
providedby mesa etching, an alternative approach involves etching
trenches into the wafer in the regions where one wishes oxidation
to occur. This has the advantage of providing an essentially
planar surface for subsequent metallisations and may prove highly
advantageous for reliable fabrication of arrays of devices, such as
VCSELs.
Samples are inserted into the reaction zone by sliding in a quartz
“boat” holding the wafers. Usually the boat is pulled quickly from
the furnace, loaded quickly atthe mouth of the furnace and returned
to the central zone of the furnace as rapidly as possible. Temper-
ature equilibration is fairly rapid but not instantaneous; even with
rapid handling it can take a few minutes to achieve thermal sta-
bility. This can manifest itself as a non-zero intercept in depth
versus time plots (FIGURE 10.6). If an initial delay is observed
under a specific set of reaction conditions, it may be desirable to
employ a bypass flow design for the most uniform and repeatable
process. Dry nitrogen or Ar is flowed through the tube furnace
until the boat and wafers thermally equilibrate. As discussed in
Section 10.3.5, the best dry-gas flow rate to use during equilibra-
tion may differ from the flow rate of the water-saturated gas. When
the desired reaction temperature is attained, the water-saturated gas
is switched to pass through the reaction zone. Likewise, switching
back to dry gas before unloading will remove reaction time uncer-
tainties. This is less of a problem during unloading than during
loading since cooling after unloading is rapid compared to the ini-
tial time needed to thermally equilibrate a boat that has been pulled
briefly from the furnace for loading.
Flow rates and tube dimensions typically used in wet oxida-
tion result in the development of a parabolic flow-velocity profile
across the diameter of the tube. The virtually zero flow rate at
the tube wall and the maximum flow at the tube centre produce
a non-uniform reaction rate across a wafer due to variation in the
effective water concentration. For a particular water temperature,
wafer temperature and tube radius, the rate increases as the sample
becomes more distant from the first wall, then plateaus across the
distance where a certain flow rate is exceeded, and drops again
as the flow rate drops while approaching the second wall. This
can give uniform oxidation across the central portion of the boat,
but samples outside the plateau zone will oxidise irregularly. For
example, a wafer of mesa-etched VCSELs that is 1.5
wide can
be uniformly oxidised in a 4
tube furnace.
This problem can be solved by disrupting or “spoiling” the para-
bolic flow profile by inserting a baffle system just upstream from
320
Wet oxidation for optoelectronic and MIS GaAs devices
the position of the boat. This baffle can be a simple honeycomb
structure mounted on the upstream end of the boat. The disruption
of the flow profile produces a uniform flow rate across the entire
width and length of the boat where the samples are located. This is
especially important for trench oxidation; mesa structures produce
some spoiling of the flow without an additional baffle.
10.5 APPLICATIONS IN OPTOELECTRONIC DEVICES
Wet oxidation is a true enabling technology for high efficiency
VCSELs, so this will be the primary focus of this section. There
are both structural considerations and electronic consequences due
to defect generation that should be considered by the VCSEL
designer. These are discussed below.
10.5.1 Structural issues for oxide VCSELs
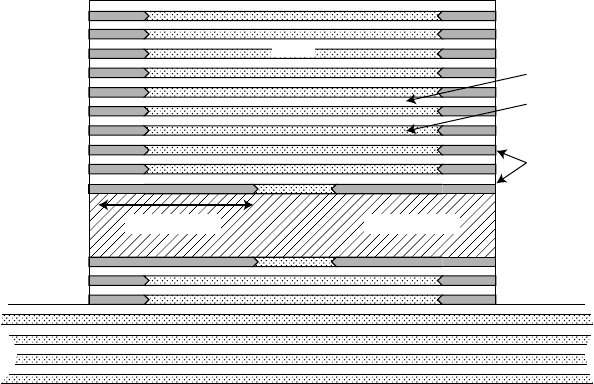
Wet oxidation of AlGaAs can be extremely useful for locally alter-
ing the refractive index of a heterostructure since the oxide has a
much lower refractive index of n = 1.6. The insulating character of
the oxide also makes current channelling within a device possible.
High-efficiency vertical-cavity surface-emitting lasers (VCSELs)
with wall-plug efficiencies in excess of 50% have been made pos-
sible by wet oxidation processing. They employ oxide apertures
made by incorporating a higher Al-content layer next to the optical
cavity than those used in the distributed Bragg reflector (DBR)
mirror stacks, as illustrated in FIGURE 10.13. Note that while
GaAs
AlGaAs
oxide
layers
mesa
optical cavityoxide extent
FIGURE 10.13 Oxide aperture VCSEL.
321
