Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


Thus, we see another example of how this domain has been recruited in evolution because of its ability to couple
nucleoside triphosphate hydrolysis to conformational changes.
The nitrogenase component is an α
2
β
2
tetramer (240 kd), in which the α and β subunits are homologous to each other
and structurally quite similar (Figure 24.4). Electrons enter at the P clusters, which are located at the α-β interface.
These clusters are each composed of eight iron atoms and seven sulfide ions. In the reduced form, each cluster takes the
form of two 4Fe-3S partial cubes linked by a central sulfide ion. Each cluster is linked to the protein through six
cysteinate residues. Electrons flow from the P cluster to the FeMo cofactor, a very unusual redox center. Because
molybdenum is present in this cluster, the nitrogenase component is also called the molybdenum-iron protein (MoFe
protein). The FeMo cofactor consists of two M-3Fe-3S clusters, in which molybdenum occupies the M site in one cluster
and iron occupies it in the other. The two clusters are joined by three sulfide ions. The FeMo cofactor is also coordinated
to a homocitrate moiety and to the α subunit through one histidine residue and one cysteinate residue. This cofactor is
distinct from the molybdenum-containing cofactor found in sulfite oxidase and apparently all other molybdenum-
containing enzymes except nitrogenase.
The FeMo cofactor is the site of nitrogen fixation. Note that each of the six central iron atoms is linked to only three
atoms, leaving open a binding opportunity for N
2.
It seems likely that N
2
binds in the central cavity of this cofactor
(Figure 24.5). The formation of multiple Fe-N interactions in this complex weakens the N N bond and thereby lowers
the activation barrier for reduction.
24.1.2. Ammonium Ion Is Assimilated into an Amino Acid Through Glutamate and
Glutamine
The next step in the assimilation of nitrogen into biomolecules is the entry of NH
4
+
into amino acids. Glutamate and
glutamine play pivotal roles in this regard. The α-amino group of most amino acids comes from the α-amino group of
glutamate by transamination (Section 23.3.1). Glutamine, the other major nitrogen donor, contributes its side-chain
nitrogen atom in the biosynthesis of a wide range of important compounds, including the amino acids tryptophan and
histidine.
Glutamate is synthesized from NH
4
+
and α-ketoglutarate, a citric acid cycle intermediate, by the action of glutamate
dehydrogenase. We have already encountered this enzyme in the degradation of amino acids (Section 23.3.1). Recall that
NAD
+
is the oxidant in catabolism, whereas NADPH is the reductant in biosyntheses. Glutamate dehydrogenase is
unusual in that it does not discriminate between NADH and NADPH, at least in some species.
The reaction proceeds in two steps. First, a Schiff base forms between ammonia and α-ketoglutarate.The formation of a
Schiff base between an amine and a carbonyl compound is a key reaction that takes place at many stages of amino acid
biosynthesis and degradation.
Schiff bases can be easily protonated. With glutamate dehydrogenase, the protonated Schiff base is reduced by the
transfer of a hydride ion from NADPH to form glutamate.
This reaction is crucial because it establishes the stereochemistry of the α-carbon atom (S absolute configuration) in
glutamate. The enzyme binds the α-ketoglutarate substrate in such a way that hydride transferred from NAD(P)H is
added to form the l isomer of glutamate (Figure 24.6). As we shall see, this stereochemistry is established for other
amino acids by transamination reactions that rely on pyridoxal phosphate.
A second ammonium ion is incorporated into glutamate to form glutamine by the action of glutamine synthetase. This
amidation is driven by the hydrolysis of ATP. ATP participates directly in the reaction by phosphorylating the side chain
of glutamate to form an acyl-phosphate intermediate, which then reacts with ammonia to form glutamine.
A high-affinity ammonia-binding site is formed only after the formation of the acyl-phosphate intermediate. A specific
site for ammonia binding is required to prevent attack by water from hydrolyzing the intermediate and wasting a
molecule of ATP. The regulation of glutamine synthetase plays a critical role in controlling nitrogen metabolism
(Section 24.3.2).
Glutamate dehydrogenase and glutamine synthetase are present in all organisms. Most prokaryotes also contain an
evolutionarily unrelated enzyme, glutamate synthase, which catalyzes the reductive amination of α-ketoglutarate with
the use of glutamine as the nitrogen donor.
The side-chain amide of glutamine is hydrolyzed to generate ammonia within the enzyme, a recurring theme throughout
nitrogen metabolism. When NH
4
+
is limiting, most of the glutamate is made by the sequential action of glutamine
synthetase and glutamate synthase. The sum of these reactions is
Note that this stoichiometry differs from that of the glutamate dehydrogenase reaction in that ATP is hydrolyzed. Why
do prokaryotes sometimes use this more expensive pathway? The answer is that the value of K
M
of glutamate
dehydrogenase for NH
4
+
is high ( 1 mM), and so this enzyme is not saturated when NH
4
+
is limiting. In contrast,
glutamine synthetase has very high affinity for NH
4
+
. Thus, ATP hydrolysis is required to capture ammonia when it is
scarce.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.1. Nitrogen Fixation: Microorganisms Use ATP and a Powerful Reductant to Reduce Atmospheric Nitrogen to Ammonia
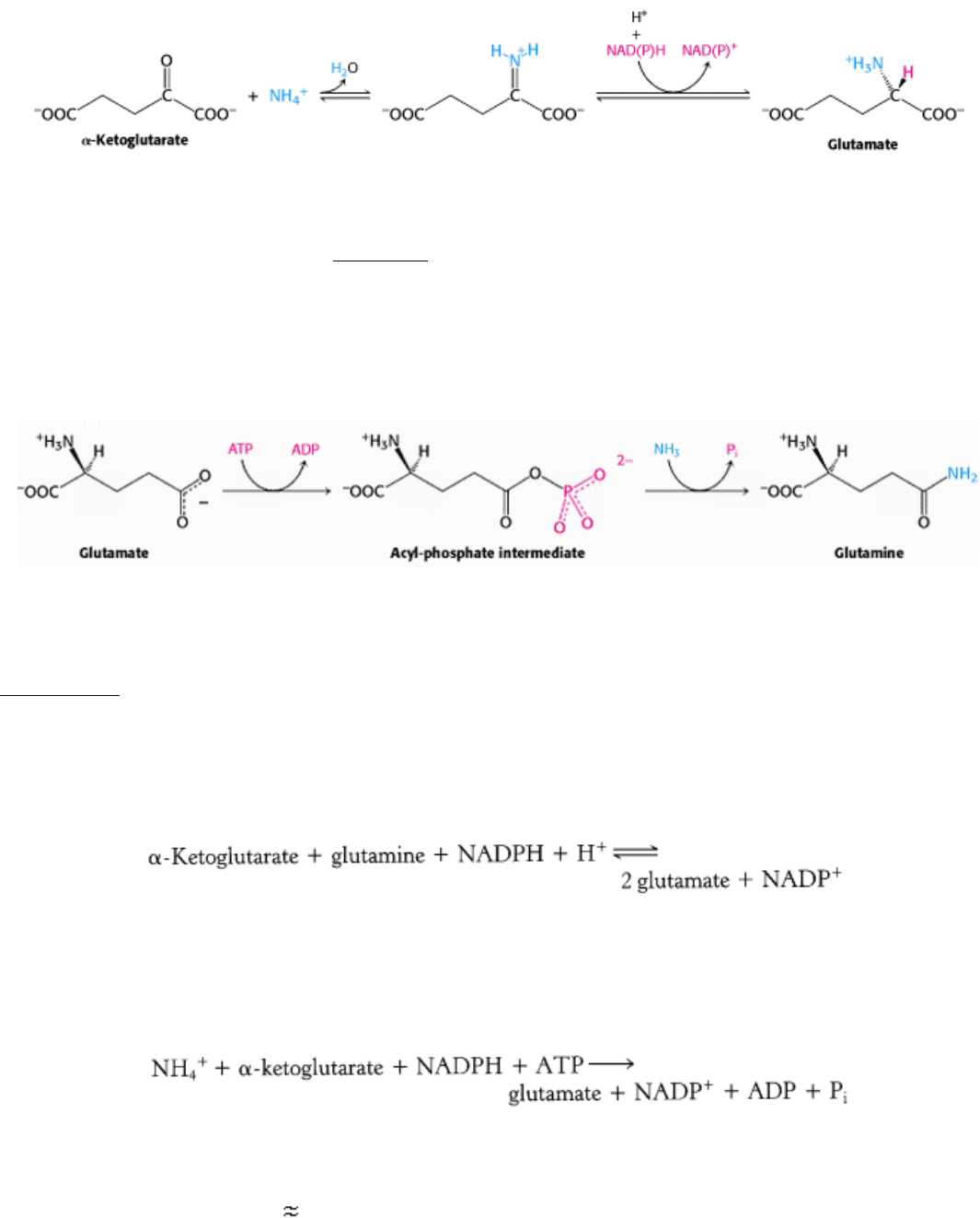
Figure 24.2. Nitrogen Fixation. Electrons flow from ferredoxin to the reductase (iron protein, or Fe protein) to
nitrogenase (molybdenum-iron protein, or MoFe protein) to reduce nitrogen to ammonia. ATP hydrolysis within the
reductase drives conformational changes necessary for the efficient transfer of electrons.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.1. Nitrogen Fixation: Microorganisms Use ATP and a Powerful Reductant to Reduce Atmospheric Nitrogen to Ammonia
Figure 24.3. Fe Protein.
This protein is a dimer composed of two polypeptide chains linked by a 4Fe-4S cluster. Each
monomer is a member of the P-loop NTPase family and contains an ATP-binding site.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.1. Nitrogen Fixation: Microorganisms Use ATP and a Powerful Reductant to Reduce Atmospheric Nitrogen to Ammonia
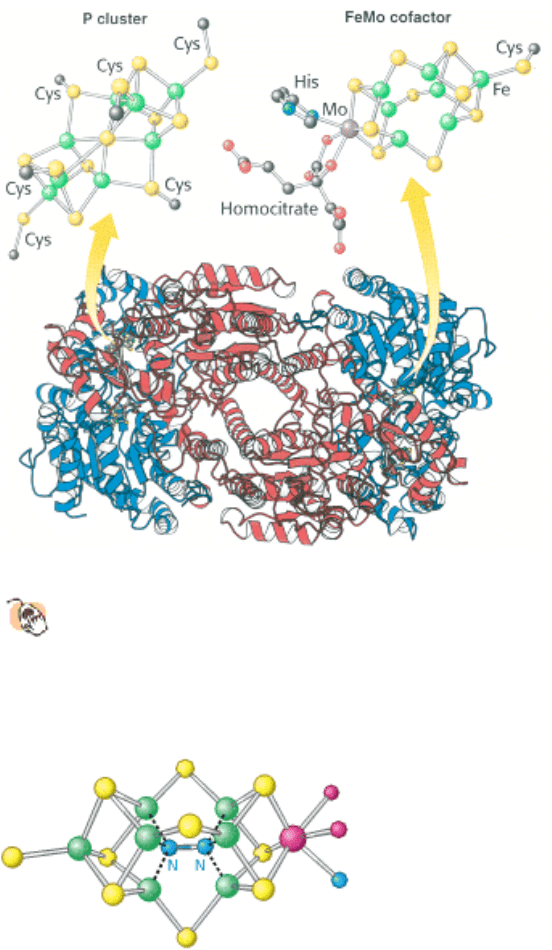
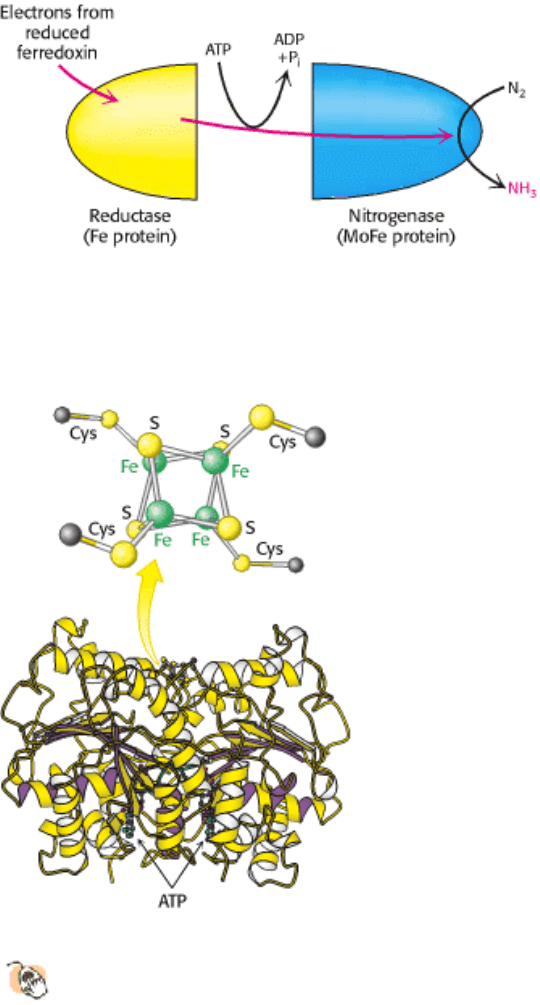
Figure 24.4. MOFe Protein.
This protein is an heterotetramer composed of two α subunits (red) and two β subunits
(blue). The protein contains two copies each of two types of clusters: P clusters and FeMo cofactors. Each P
cluster contains eight iron atoms and seven sulfides linked to the protein by six cysteinate residues. Each FeMo
cofactor contains one molybdenum atom, seven iron atoms, nine sulfides, and a homocitrate, and is linked to the protein
by one cysteinate residue and one histidine residue.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.1. Nitrogen Fixation: Microorganisms Use ATP and a Powerful Reductant to Reduce Atmospheric Nitrogen to Ammonia
Figure 24.5. Nitrogen-Reduction Site. The FeMo cofactor contains an open center that is the likely site of nitrogen
binding and reduction.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.1. Nitrogen Fixation: Microorganisms Use ATP and a Powerful Reductant to Reduce Atmospheric Nitrogen to Ammonia

Figure 24.6. Establishment of Chirality. In the active site of glutamate dehydrogenase, hydride transfer from NAD(P)
H to a specific face of the achiral protonated Schiff base of α-ketoglutarate establishes the
l configuration of glutamate.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids
24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other
Major Pathways
Thus far, we have considered the conversion of N
2
into NH
4
+
and the assimilation of NH
4
+
into glutamate and
glutamine. We turn now to the biosynthesis of the other amino acids. The pathways for the biosynthesis of amino acids
are diverse. However, they have an important common feature: their carbon skeletons come from intermediates of
glycolysis, the pentose phosphate pathway, or the citric acid cycle. On the basis of these starting materials, amino acids
can be grouped into six biosynthetic families (Figure 24.7).
24.2.1. Human Beings Can Synthesize Some Amino Acids but Must Obtain Others
from the Diet
Most microorganisms such as E. coli can synthesize the entire basic set of 20 amino acids, whereas human beings cannot
make 9 of them. The amino acids that must be supplied in the diet are called essential amino acids, whereas the others
are termed nonessential amino acids (Table 24.1). These designations refer to the needs of an organism under a
particular set of conditions. For example, enough arginine is synthesized by the urea cycle to meet the needs of an adult
but perhaps not those of a growing child. A deficiency of even one amino acid results in a negative nitrogen balance. In
this state, more protein is degraded than is synthesized, and so more nitrogen is excreted than is ingested.
The nonessential amino acids are synthesized by quite simple reactions, whereas the pathways for the formation of the
essential amino acids are quite complex. For example, the nonessential amino acids alanine and aspartate are
synthesized in a single step from pyruvate and oxaloacetate, respectively. In contrast, the pathways for the essential
amino acids require from 5 to 16 steps (Figure 24.8). The sole exception to this pattern is arginine, inasmuch as the
synthesis of this nonessential amino acid de novo requires 10 steps. Typically, though, it is made in only 3 steps from
ornithine as part of the urea cycle. Tyrosine, classified as a nonessential amino acid because it can be synthesized in 1
step from phenylalanine, requires 10 steps to be synthesized from scratch and is essential if phenylalanine is not
abundant. We begin with the biosynthesis of nonessential amino acids.
24.2.2. A Common Step Determines the Chirality of All Amino Acids
Three α-ketoacids
α-ketoglutarate, oxaloacetate, and pyruvate can be converted into amino acids in one step

through the addition of an amino group. We have seen that α-ketoglutarate can be converted into glutamate by reductive
amination (Section 24.1.2). The amino group from glutamate can be transferred to other α-ketoacids by transamination
reactions. Thus, aspartate and alanine can be made from the addition of an amino group to oxaloacetate and pyruvate,
respectively.
These reactions are carried out by pyridoxal phosphate-dependent transaminases. Transamination reactions participate in
the synthesis of most amino acids. We shall review the transaminase mechanism (Section 23.3.1) as it applies to amino
acid biosynthesis (see Figure 23.10).
The reaction pathway begins with pyridoxal phosphate in a Schiff-base linkage with lysine at the transaminase active
site, forming an internal aldimine (Figure 24.9). An external aldimine forms between PLP and the amino-group donor,
glutamate, which displaces the lysine residue. The transfer of the amino group from glutamate to pyridoxal phosphate
forms pyridoxamine phosphate, the actual amino donor. When the amino group has been incorporated into
pyridoxamine, the reaction pathway proceeds in reverse, and the amino group is transferred to an α-ketoacid to form an
amino acid.
Aspartate aminotransferase is the prototype of a large family of PLP-dependent enzymes. Comparisons of amino acid
sequences as well as several three-dimensional structures reveal that almost all transaminases having roles in amino acid
biosynthesis are related to aspartate aminotransferase by divergent evolution. An examination of the aligned amino acid
sequences reveals that two residues are completely conserved. These residues are the lysine residue that forms the Schiff
base with the pyridoxal phosphate cofactor (lysine 258 in aspartate aminotransferase) and an arginine residue that
interacts with the α-carboxylate group of the ketoacid (see Figure 23.11).
An essential step in the transamination reaction is the protonation of the quinonoid intermediate to form the external
aldimine. The chirality of the amino acid formed is determined by the direction from which this proton is added to the
quinonoid form. (Figure 24.10). This protonation step determines the l configuration of the amino acids produced. The
interaction between the conserved arginine residue and the α-carboxylate group helps orient the substrate so that, when
the lysine residue transfers a proton to the face of the quinonoid intermediate, it generates an aldimine with an
l
configuration at the C
α
center.
24.2.3. An Adenylated Intermediate Is Required to Form Asparagine from Aspartate
The formation of asparagine from aspartate is chemically analogous to the formation of glutamine from glutamate. Both
transformations are amidation reactions and both are driven by the hydrolysis of ATP. The actual reactions are different,
however. In bacteria, the reaction for the asparagine synthesis is
Thus, the products of ATP hydrolysis are AMP and PP
i
rather than ADP and P
i
. Aspartate is activated by adenylation
rather than by phosphorylation.
We have encountered this mode of activation in fatty acid degradation and will see it again in lipid and protein synthesis.
In mammals, the nitrogen donor for asparagine is glutamine rather than ammonia as in bacteria. Ammonia is generated
by hydrolysis of the side chain of glutamine and directly transferred to activated aspartate, bound in the active site. An
advantage is that the cell is not directly exposed to NH
4
+
, which is toxic at high levels to human beings and other
mammals. The use of glutamine hydrolysis as a mechanism for generating ammonia for use within the same enzyme is a
motif common throughout biosynthetic pathways.
24.2.4. Glutamate Is the Precursor of Glutamine, Proline, and Arginine
The synthesis of glutamate by the reductive amination of α-ketoglutarate has already been discussed, as has the
conversion of glutamate into glutamine (Section 24.1.2). Glutamate is the precursor of two other nonessential amino
acids: proline and arginine. First, the γ-carboxyl group of glutamate reacts with ATP to form an acyl phosphate. This
mixed anhydride is then reduced by NADPH to an aldehyde.
Glutamic γ-semialdehyde cyclizes with a loss of H
2
O in a nonenzymatic process to give ∆
1
-pyrroline-5-carboxylate,
which is reduced by NADPH to proline. Alternatively, the semialdehyde can be transaminated to ornithine, which is
converted in several steps into arginine (Section 23.4.1).
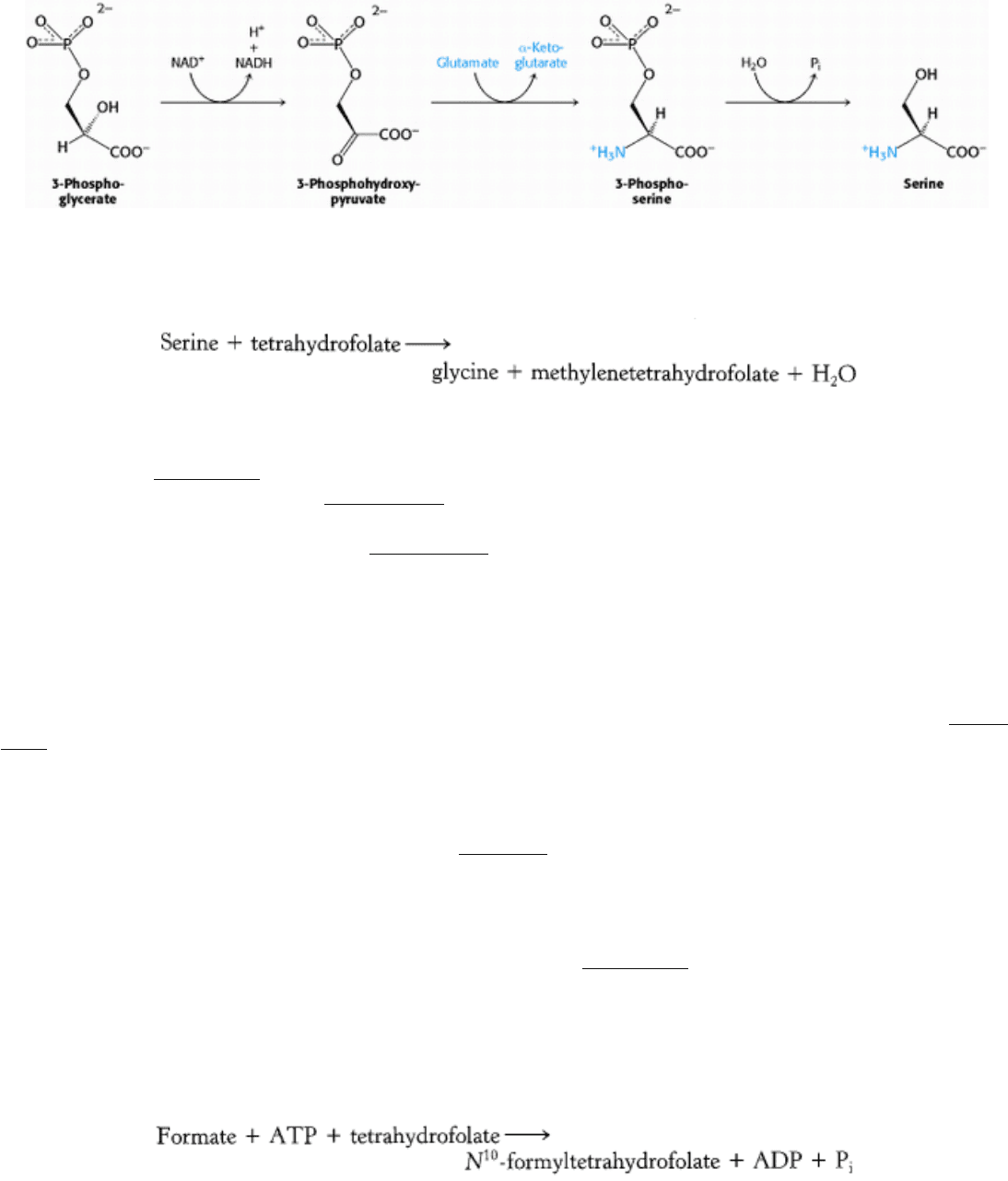
24.2.5. Serine, Cysteine, and Glycine Are Formed from 3-Phosphoglycerate
Serine is synthesized from 3-phosphoglycerate, an intermediate in glycolysis. The first step is an oxidation to 3-
phosphohydroxypyruvate. This α-ketoacid is transaminated to 3-phosphoserine, which is then hydrolyzed to serine.
Serine is the precursor of glycine and cysteine. In the formation of glycine, the side-chain methylene group of serine is
transferred to tetrahydrofolate, a carrier of one-carbon units that will be discussed shortly.
This interconversion is catalyzed by serine transhydroxymethylase, another PLP enzyme that is homologous to aspartate
aminotransferase (Figure 24.11). The bond between the α- and β-carbon atoms of serine is labilized by the formation of
a Schiff base between serine and PLP (Section 23.3.3). The side-chain methylene group of serine is then transferred to
tetrahydrofolate. The conversion of serine into cysteine requires the substitution of a sulfur atom derived from
methionine for the side-chain oxygen atom (Section 24.2.8).
24.2.6. Tetrahydrofolate Carries Activated One-Carbon Units at Several Oxidation
Levels
Tetrahydrofolate (also called tetrahydropteroylglutamate), a highly versatile carrier of activated one-carbon units,
consists of three groups: a substituted pteridine, p-aminobenzoate, and a chain of one or more glutamate residues (Figure
24.12). Mammals can synthesize the pteridine ring, but they are unable to conjugate it to the other two units. They obtain
tetrahydrofolate from their diets or from microorganisms in their intestinal tracts.
The one-carbon group carried by tetrahydrofolate is bonded to its N-5 or N-10 nitrogen atom (denoted as N
5
and N
10
)
or to both. This unit can exist in three oxidation states (Table 24.2). The most-reduced form carries a methyl group,
whereas the intermediate form carries a methylene group. More-oxidized forms carry a formyl, formimino, or methenyl
group. The fully oxidized one-carbon unit, CO
2
, is carried by biotin rather than by tetrahydrofolate.
The one-carbon units carried by tetrahydrofolate are interconvertible (Figure 24.13). N
5
,N
10
-Methylenetetrahydrofolate
can be reduced to N
5
-methyltetrahydrofolate or oxidized to N
5
,N
10
-methenyltetrahydrofolate. N
5
, N
10
-
Methenyltetrahydrofolate can be converted into N
5
-formiminotetrahydrofolate or N
10
-formyltetrahydrofolate, both of
which are at the same oxidation level. N
10
-Formyltetrahydrofolate can also be synthesized from tetrahydrofolate,
formate, and ATP.
N
5
-Formyltetrahydrofolate can be reversibly isomerized to N
10
-formyltetrahydrofolate or it can be converted into N
5
,
N
10
-methenyltetrahydrofolate.
These tetrahydrofolate derivatives serve as donors of one-carbon units in a variety of biosyntheses. Methionine is
regenerated from homocysteine by transfer of the methyl group of N
5
-methyltetrahydrofolate, as will be discussed
shortly. We shall see in Chapter 25 that some of the carbon atoms of purines are acquired from derivatives of N
10
-
formyltetrahydrofolate. The methyl group of thymine, a pyrimidine, comes from N
5
,N
10
-methylenetetrahydrofolate.
This tetrahydrofolate derivative can also donate a one-carbon unit in an alternative synthesis of glycine that starts with
CO
2
and NH
4
+
, a reaction catalyzed by glycine synthase (called the glycine cleavage enzyme when it operates in the
reverse direction).
Thus, one-carbon units at each of the three oxidation levels are utilized in biosyntheses. Furthermore, tetrahydrofolate
serves as an acceptor of one-carbon units in degradative reactions. The major source of one-carbon units is the facile
conversion of serine into glycine, which yields N
5
,N
10
-methylenetetrahydrofolate. Serine can be derived from 3-
phosphoglycerate, and so this pathway enables one-carbon units to be formed de novo from carbohydrates.
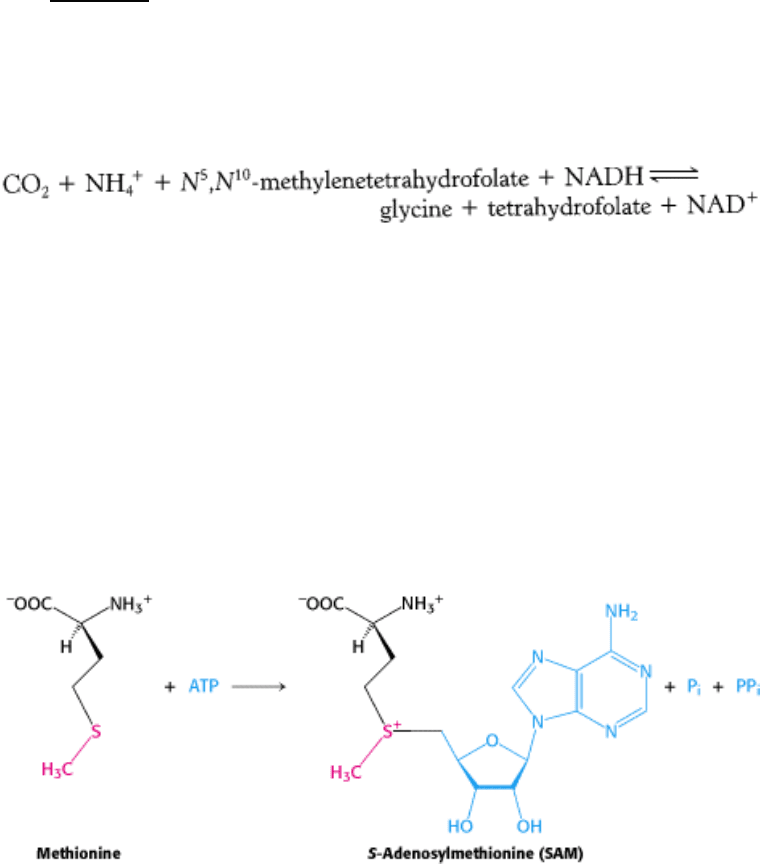
24.2.7. S-Adenosylmethionine Is the Major Donor of Methyl Groups
Tetrahydrofolate can carry a methyl group on its N-5 atom, but its transfer potential is not sufficiently high for most
biosynthetic methylations. Rather, the activated methyl donor is usually S-adenosylmethionine (SAM), which is
synthesized by the transfer of an adenosyl group from ATP to the sulfur atom of methionine.
The methyl group of the methionine unit is activated by the positive charge on the adjacent sulfur atom, which makes the
molecule much more reactive than N
5
-methyltetrahydrofolate. The synthesis of S-adenosylmethionine is unusual in that
the triphosphate group of ATP is split into pyrophosphate and orthophosphate; the pyrophosphate is subsequently
hydrolyzed to two molecules of P
i
. S-Adenosylhomocysteine is formed when the methyl group of S-adenosylmethionine
is transferred to an acceptor. S-Adenosylhomocysteine is then hydrolyzed to homocysteine and adenosine.
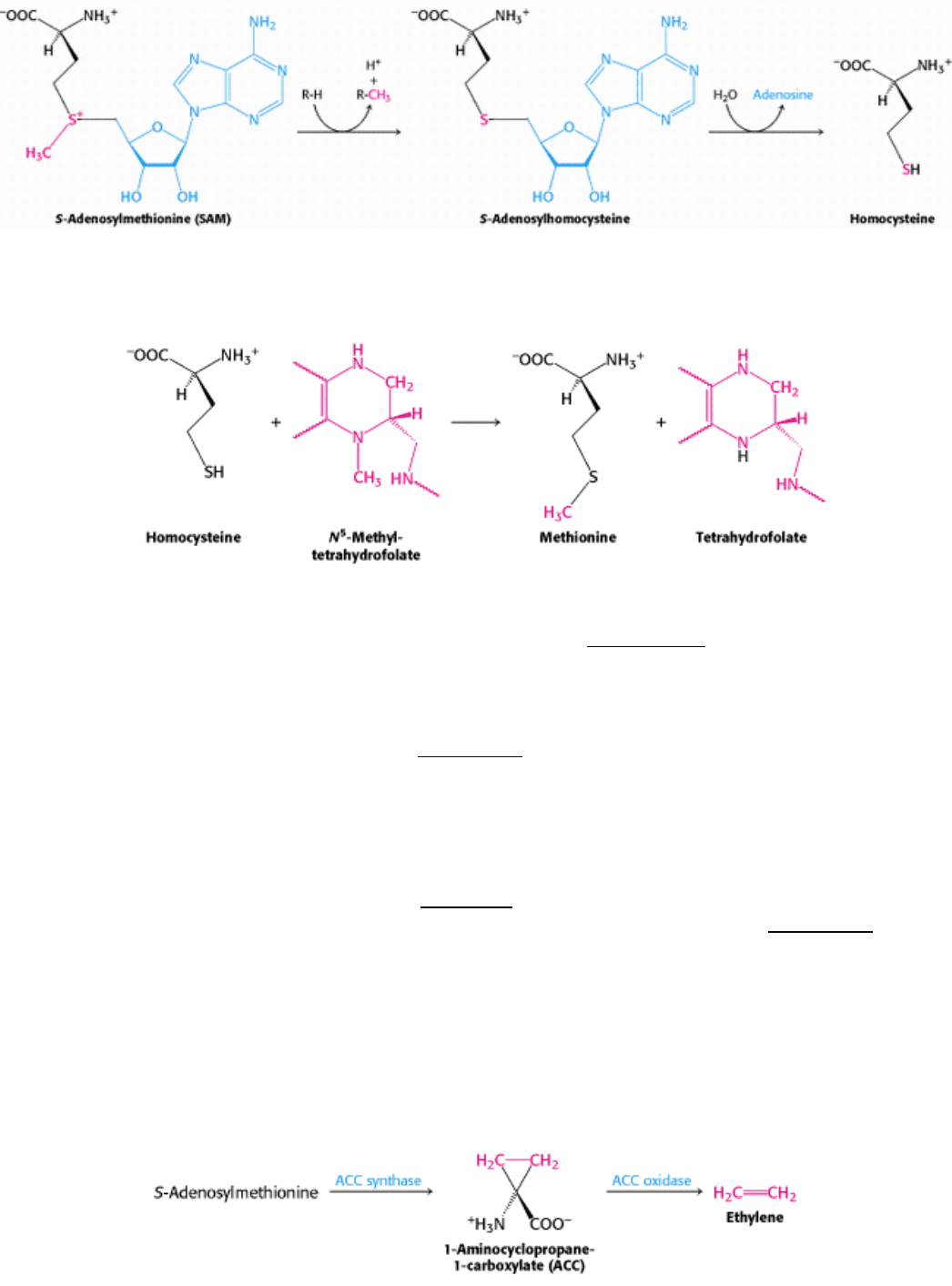
Methionine can be regenerated by the transfer of a methyl group to homocysteine from N
5
-methyltetrahydrofolate, a
reaction catalyzed by methionine synthase (also known as homocysteine methyltransferase).
The coenzyme that mediates this transfer of a methyl group is methylcobalamin, derived from vitamin B
12
. In fact, this
reaction and the rearrangement of l-methylmalonyl CoA to succinyl CoA (Section 23.5.4), catalyzed by a homologous
enzyme, are the only two B
12
-dependent reactions known to take place in mammals. Another enzyme that converts
homocysteine into methionine without vitamin B
12
also is present in many organisms.
These reactions constitute the activated methyl cycle (Figure 24.14). Methyl groups enter the cycle in the conversion of
homocysteine into methionine and are then made highly reactive by the addition of adenosyl groups, which make the
sulfur atoms positively charged and the methyl groups much more electrophilic. The high transfer potential of the S-
methyl group enables it to be transferred to a wide variety of acceptors.
Among the acceptors modified by S-adenosylmethionine are specific bases in DNA. The methylation of DNA protects
bacterial DNA from cleavage by restriction enzymes (Section 9.3). The base to be methylated is flipped out of the DNA
double helix into the active site where it can accept a methyl group from S-adenosylmethionine (Figure 24.15). A
recurring S-adenosylmethionine-binding domain is present in many SAM-dependent methylases.
S-Adenosylmethionine is also the precursor of ethylene, a gaseous plant hormone that induces the ripening of fruit. S-
Adenosylmethionine is cyclized to a cyclopropane derivative that is then oxidized to form ethylene. The Greek
philosopher Theophrastus recognized more than 2000 years ago that sycamore figs do not ripen unless they are scraped
with an iron claw. The reason is now known: wounding triggers ethylene production, which in turn induces ripening.
Much effort is being made to understand this biosynthetic pathway because ethylene is a culprit in the spoilage of fruit.
24.2.8. Cysteine Is Synthesized from Serine and Homocysteine
