Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

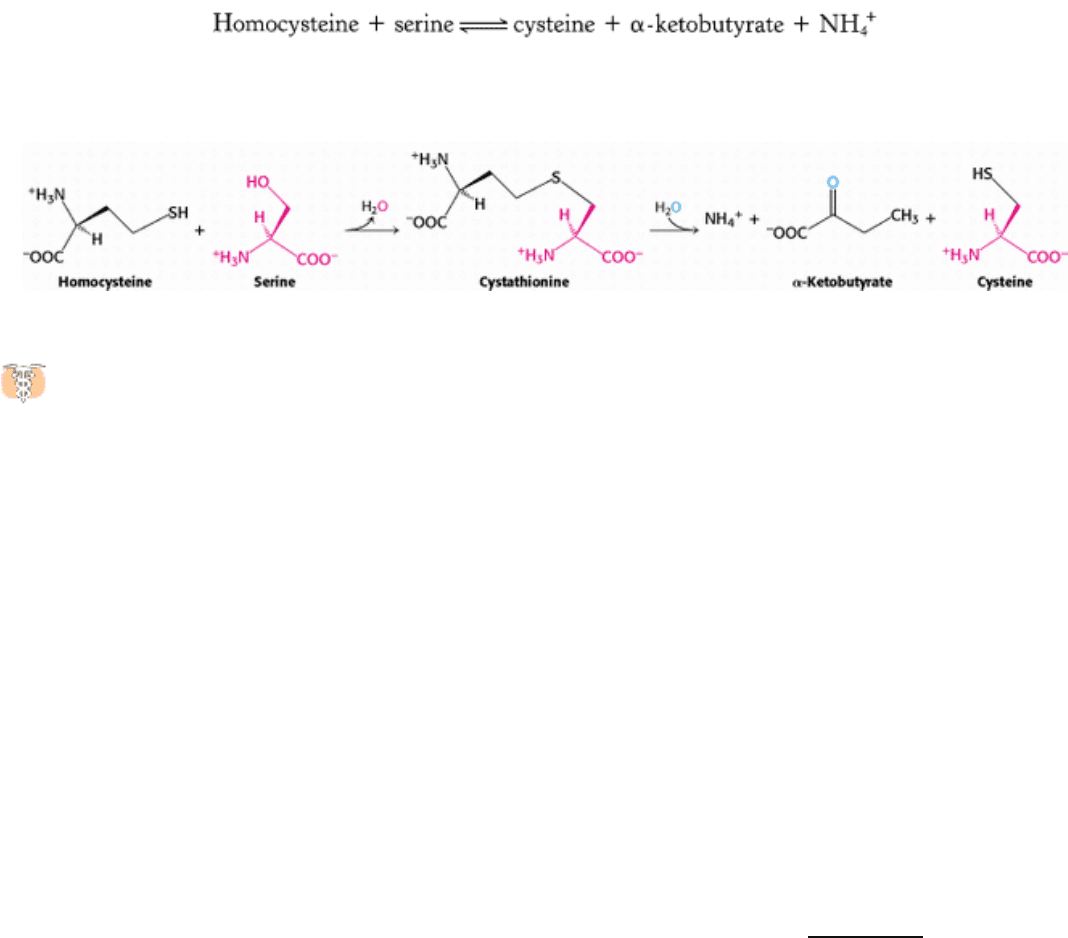
In addition to being a precursor of methionine in the activated methyl cycle, homocysteine is an intermediate in the
synthesis of cysteine. Serine and homocysteine condense to form cystathionine. This reaction is catalyzed by
cystathionine β-synthase. Cystathionine is then deaminated and cleaved to cysteine and α-ketobutyrate by
cystathioninase. Both of these enzymes utilize PLP and are homologous to aspartate aminotransferase. The net reaction is
Note that the sulfur atom of cysteine is derived from homocysteine, whereas the carbon skeleton comes from serine.
24.2.9. High Homocysteine Levels Are Associated with Vascular Disease
People with elevated serum levels of homocysteine or the disulfide-linked dimer homocystine have an unusually
high risk for coronary heart disease and arteriosclerosis. The most common genetic cause of high homocysteine
levels is a mutation within the gene encoding cystathionine β-synthase. The molecular basis of homocysteine's action has
not been clearly identified, although it appears to damage cells lining blood vessels and to increase the growth of
vascular smooth muscle. The amino acid raises oxidative stress as well. Vitamin treatments are effective in reducing
homocysteine levels in some people. Treatment with vitamins maximizes the activity of the two major metabolic
pathways processing homocysteine. Pyridoxal phosphate, a vitamin B
6
derivative, is necessary for the activity of
cystathionine β-synthase, which converts homocysteine into cystathione; tetrahydrofolate, and vitamin B
12
, supports the
methylation of homocysteine to methionine.
24.2.10. Shikimate and Chorismate Are Intermediates in the Biosynthesis of Aromatic
Amino Acids
We turn now to the biosynthesis of essential amino acids. These amino acids are synthesized by plants and
microorganisms, and those in the human diet are ultimately derived primarily from plants. The essential amino acids are
formed by much more complex routes than are the nonessential amino acids. The pathways for the synthesis of aromatic
amino acids in bacteria have been selected for discussion here because they are well understood and exemplify recurring
mechanistic motifs.
Phenylalanine, tyrosine, and tryptophan are synthesized by a common pathway in E. coli (Figure 24.16). The initial step
is the condensation of phosphoenolpyruvate (a glycolytic intermediate) with erythrose 4-phosphate (a pentose phosphate
pathway intermediate). The resulting seven-carbon open-chain sugar is oxidized, loses its phosphoryl group, and cyclizes
to 3-dehydroquinate. Dehydration then yields 3-dehydroshikimate, which is reduced by NADPH to shikimate.
Phosphorylation of shikimate by ATP gives shikimate 3-phosphate, which condenses with a second molecule of
phosphoenolpyruvate. This 5-enolpyruvyl intermediate loses its phosphoryl group, yielding chorismate, the common
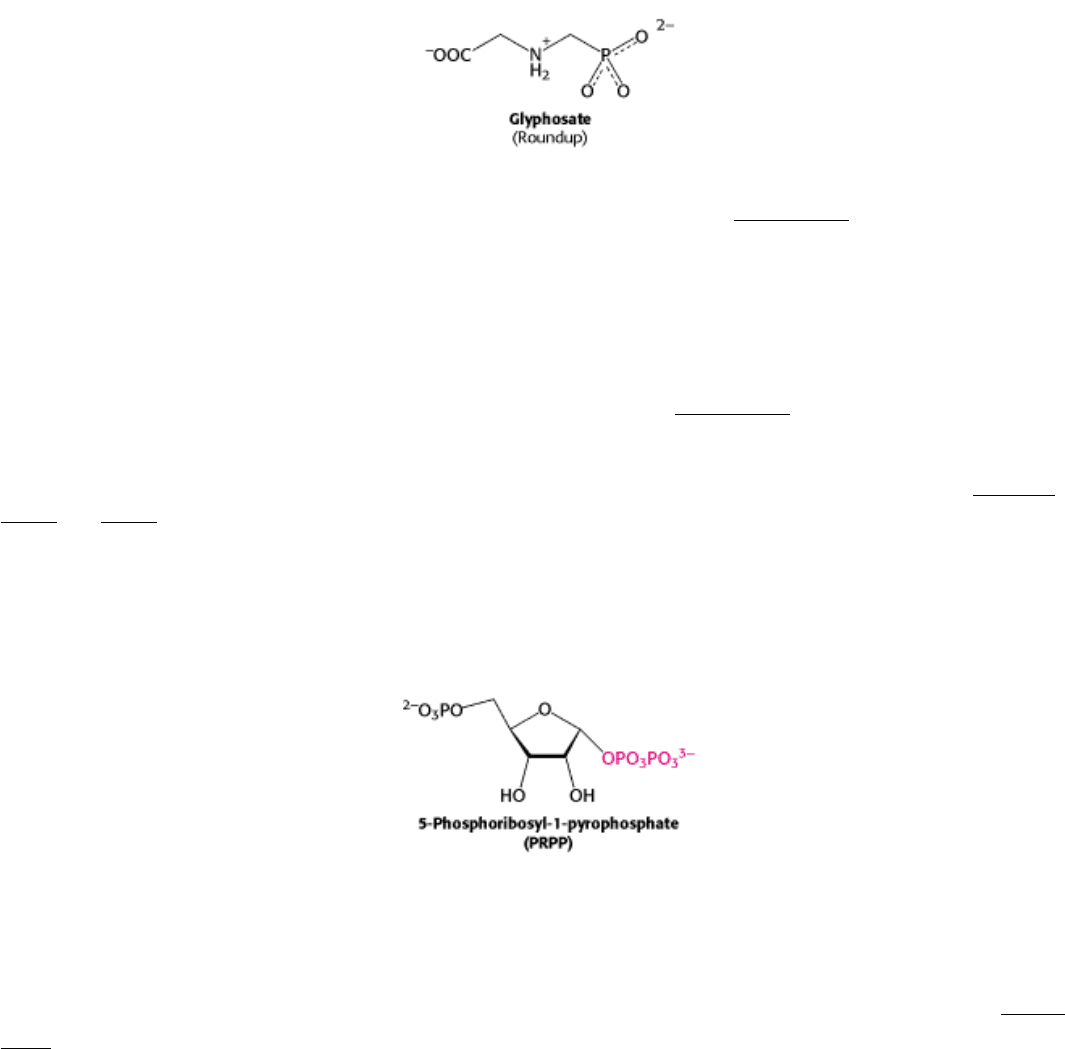
precursor of all three aromatic amino acids. The importance of this pathway is revealed by the effectiveness of
glyphosate (Roundup), a broad-spectrum herbicide. This compound inhibits the enzyme that produces 5-
enolpyruvylshikimate 3-phosphate and, hence, blocks aromatic amino acid biosynthesis in plants. Because animals lack
this enzyme, the herbicide is fairly nontoxic.
The pathway bifurcates at chorismate. Let us first follow the prephenate branch (Figure 24.17). A mutase converts
chorismate into prephenate, the immediate precursor of the aromatic ring of phenylalanine and tyrosine. This fascinating
conversion is a rare example of an electrocyclic reaction in biochemistry, mechanistically similar to the well-known
Diels-Alder reaction from organic chemistry. Dehydration and decarboxylation yield phenylpyruvate. Alternatively,
prephenate can be oxidatively decarboxylated to p-hydroxyphenylpyruvate. These α-ketoacids are then transaminated to
form phenylalanine and tyrosine.
The branch starting with anthranilate leads to the synthesis of tryptophan (Figure 24.18). Chorismate acquires an amino
group derived from the hydrolysis of the side chain of glutamine and releases pyruvate to form anthranilate. Then
anthranilate condenses with 5-phosphoribosyl-1-pyrophosphate (PRPP), an activated form of ribose phosphate. PRPP is
also an important intermediate in the synthesis of histidine, purine nucleotides, and pyrimidine nucleotides (Sections
25.1.4 and 25.2.2). The C-1 atom of ribose 5-phosphate becomes bonded to the nitrogen atom of anthranilate in a
reaction that is driven by the release and hydrolysis of pyrophosphate. The ribose moiety of phosphoribosylanthranilate
undergoes rearrangement to yield 1-(o-carboxyphenylamino)-1-deoxyribulose 5-phosphate. This intermediate is
dehydrated and then decarboxylated to indole-3-glycerol phosphate, which is cleaved to indole. Then indole reacts with
serine to form tryptophan. In these final steps, which are catalyzed by tryptophan synthetase, the side chain of indole-3-
glycerol phosphate is removed as glyceraldehyde 3-phosphate and replaced by the carbon skeleton of serine.
24.2.11. Tryptophan Synthetase Illustrates Substrate Channeling in Enzymatic
Catalysis
Tryptophan synthetase of E. coli, an α
2
β
2
tetramer, can be dissociated into two α subunits and a β
2
subunit (Figure
24.19). The α subunit catalyzes the formation of indole from indole-3-glycerol phosphate, whereas each β subunit has a
PLP-containing active site that catalyzes the condensation of indole and serine to form tryptophan. The overall three-
dimensional structure of this enzyme is distinct from that of aspartate aminotransferase and the other PLP enzymes
already discussed. Serine forms a Schiff base with this PLP, which is then dehydrated to give the Schiff base of
aminoacrylate. This reactive intermediate is attacked by indole to give tryptophan.
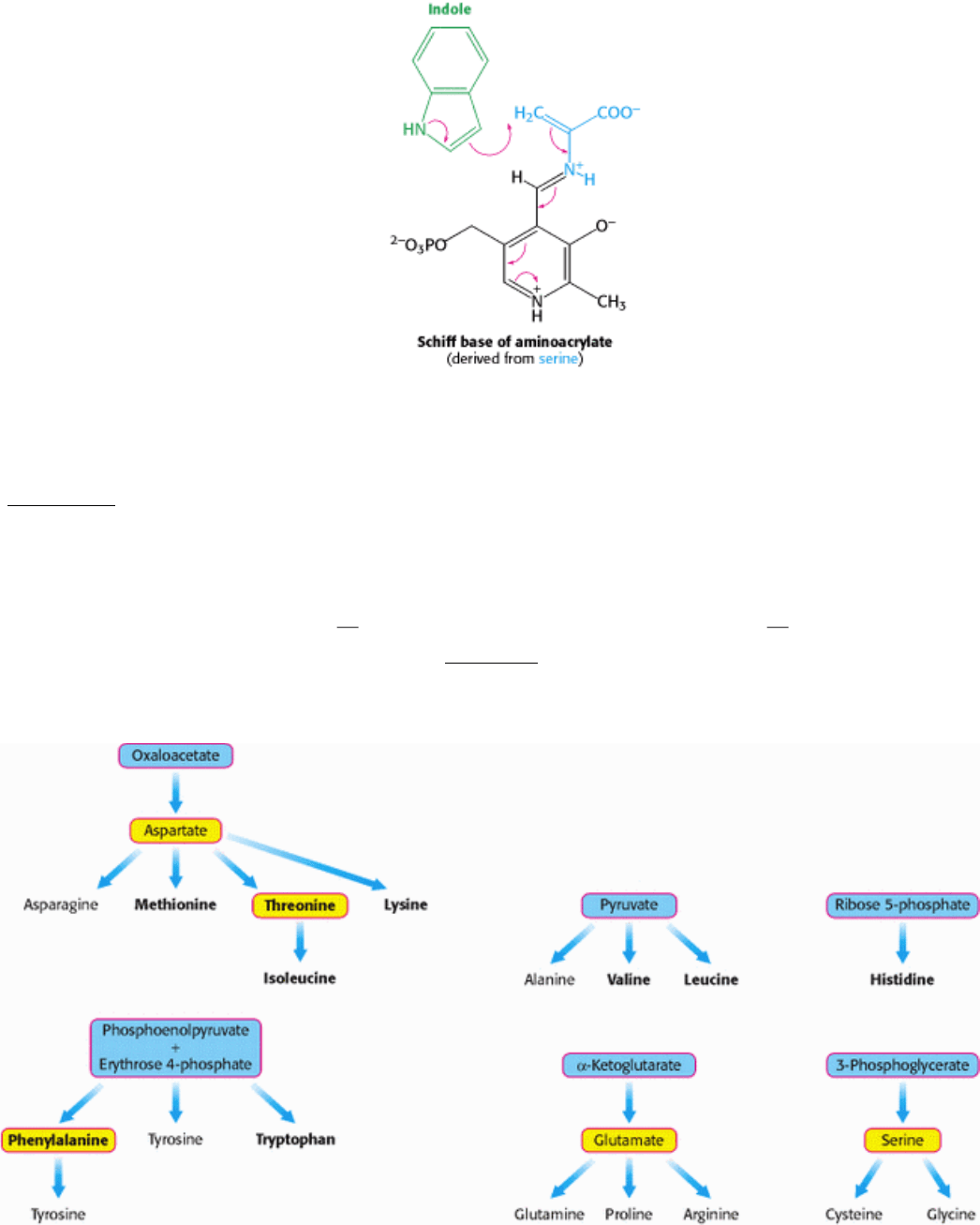
The synthesis of tryptophan poses a challenge. Indole, a hydrophobic molecule, readily traverses membranes and would
be lost from the cell if it were allowed to diffuse away from the enzyme. This problem is solved in an ingenious way. A
25-Å-long channel connects the active site of the α subunit with that of the adjacent β subunit in the α
2
β
2
tetramer
(Figure 24.20). Thus, indole can diffuse from one active site to the other without being released into bulk solvent.
Indeed, the results of isotopic-labeling experiments showed that indole formed by the α subunit does not leave the
enzyme when serine is present. Furthermore, the two partial reactions are coordinated. Indole is not formed by the α
subunit until the highly reactive aminoacrylate is ready and waiting in the β subunit. We see here a clear-cut example of
substrate channeling in catalysis by a multienzyme complex. Channeling substantially increases the catalytic rate.
Furthermore, a deleterious side reaction
in this case, the potential loss of an intermediate is prevented. We shall
encounter other examples of substrate channeling in Chapter 25.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Figure 24.7. Biosynthetic Families of Amino Acids in Bacteria and Plants. Major metabolic precursors are shaded
blue. Amino acids that give rise to other amino acids are shaded yellow. Essential amino acids are in boldface type.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Table 24.1. Basic set of 20 amino acids
Nonessential Essential
Alanine Histidine
Arginine Isoleucine
Asparagine Leucine
Aspartate Lysine
Cysteine Methionine
Glutamate Phenylalanine
Glutamine Threonine
Glycine Tryptophan
Proline Valine
Serine
Tyrosine
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
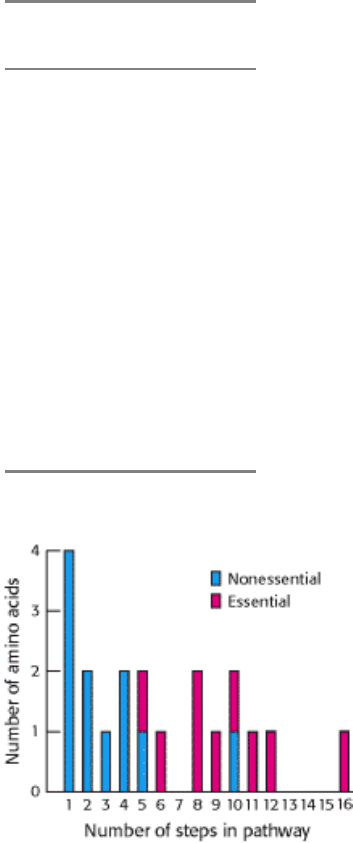
Figure 24.8. Essential and Nonessential Amino Acids. Some amino acids are nonessential to human beings because
they can be biosynthesized in a small number of steps. Those amino acids requiring a large number of steps for their
synthesis are essential in the diet because some of the enzymes for these steps have been lost in the course of evolution.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways

Figure 24.9. Amino Acid Biosynthesis by Transamination. Within a transaminase, the internal aldimine is converted
into pyridoxamine phosphate (PMP) by reaction with glutamate. PMP then reacts with an α-ketoacid to generate a
ketimine. This intermediate is converted into a quinonoid intermediate, which in turn yields an external aldimine. The
aldimine is cleaved to release the newly formed amino acid to complete the cycle.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Figure 24.10. Stereochemistry of Proton Addition. In a transaminase active site, the addition of a proton from the
lysine residue to the bottom face of the quinonoid intermediate determines the
l configuration of the amino acid product.
The conserved arginine residue interacts with the α-carboxylate group and helps establish the appropriate geometry of
the quinonoid intermediate.

III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Figure 24.11. Structure of Serine Hydroxymethyltransferase.
This enzyme transfers a one-carbon unit from the side
chain of serine to tetrahydrofolate. One subunit of the dimeric enzyme is shown.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Figure 24.12. Tetrahydrofolate. This cofactor includes three components: a pteridine ring, p-aminobenzoate, and one
or more glutamate residues.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Table 24.2. One-carbon groups carried by tetrahydrofolate
Oxidation state Group
Most reduced ( = methanol) -CH
3
Methyl
Intermediate ( = formaldehyde) -CH
2
- Methylene
Most oxidized ( = formic acid) -CHO Formyl
-CHNH Formimino
-CH Methenyl

III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Figure 24.13. Conversions of One-Carbon Units Attached to Tetrahydrofolate.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Figure 24.14. Activated Methyl Cycle. The methyl group of methionine is activated by the formation of S-
adenosylmethionine.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
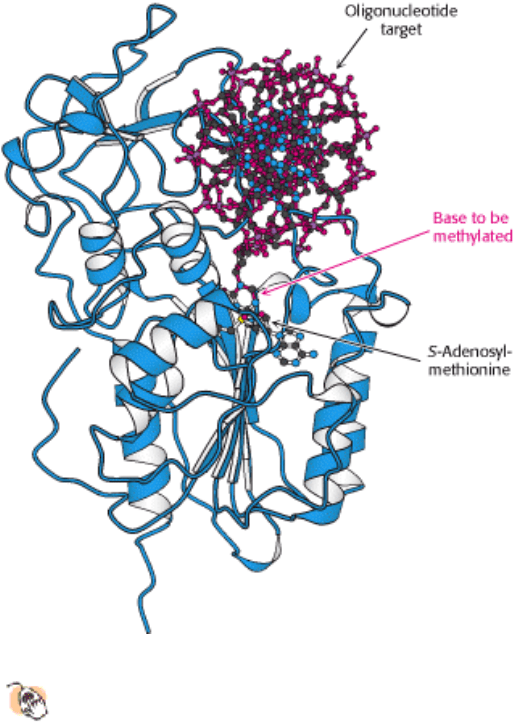
Figure 24.15. DNA Methylation.
The structure of a DNA methylase bound to an oligonucleotide target shows that the
base to be methylated is flipped out of the DNA helix into the active site of a SAM-dependent methylase.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
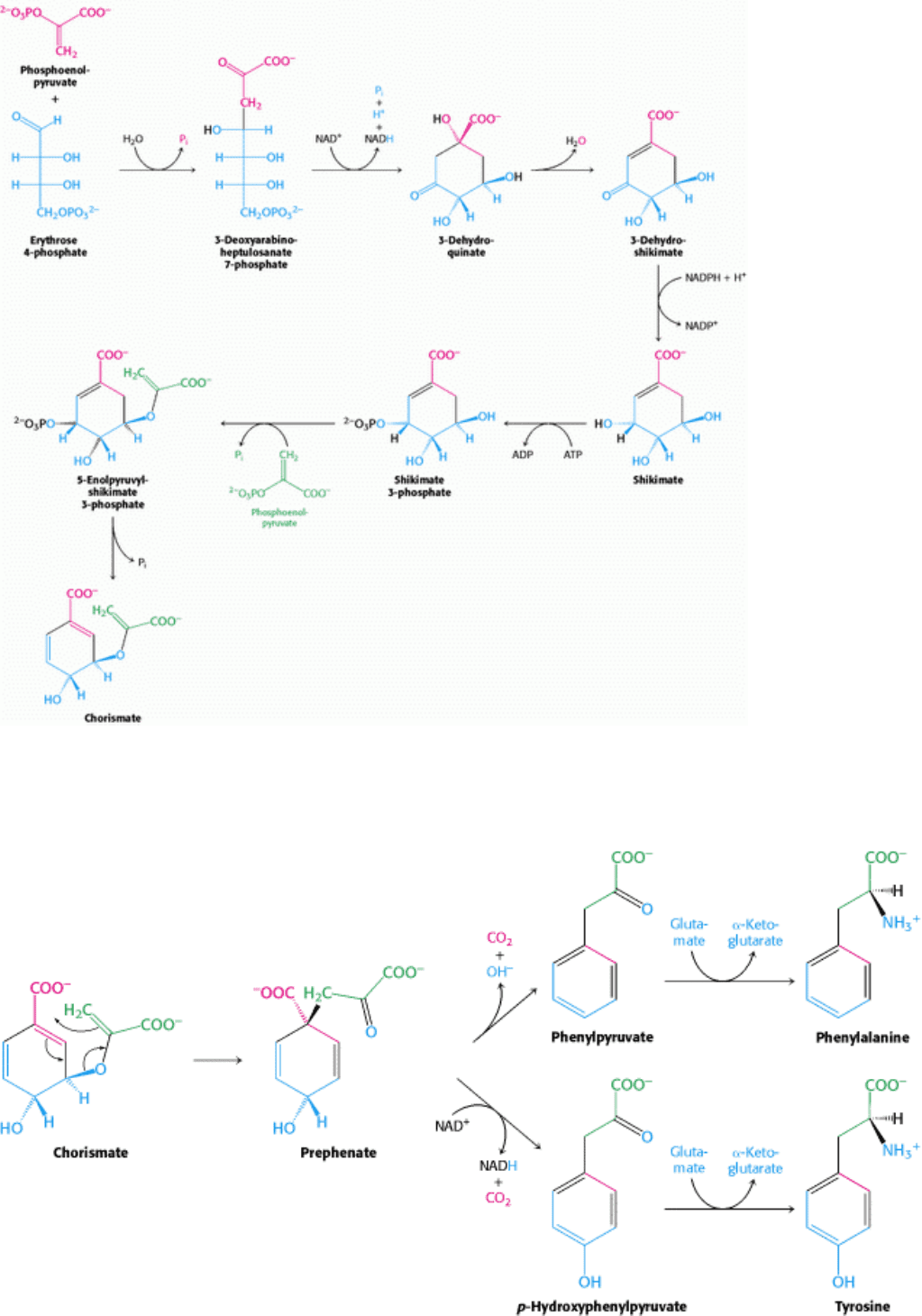
Figure 24.16. Pathway to Chorismate. Chorismate is an intermediate in the biosynthesis of phenylalanine, tyrosine,
and tryptophan.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
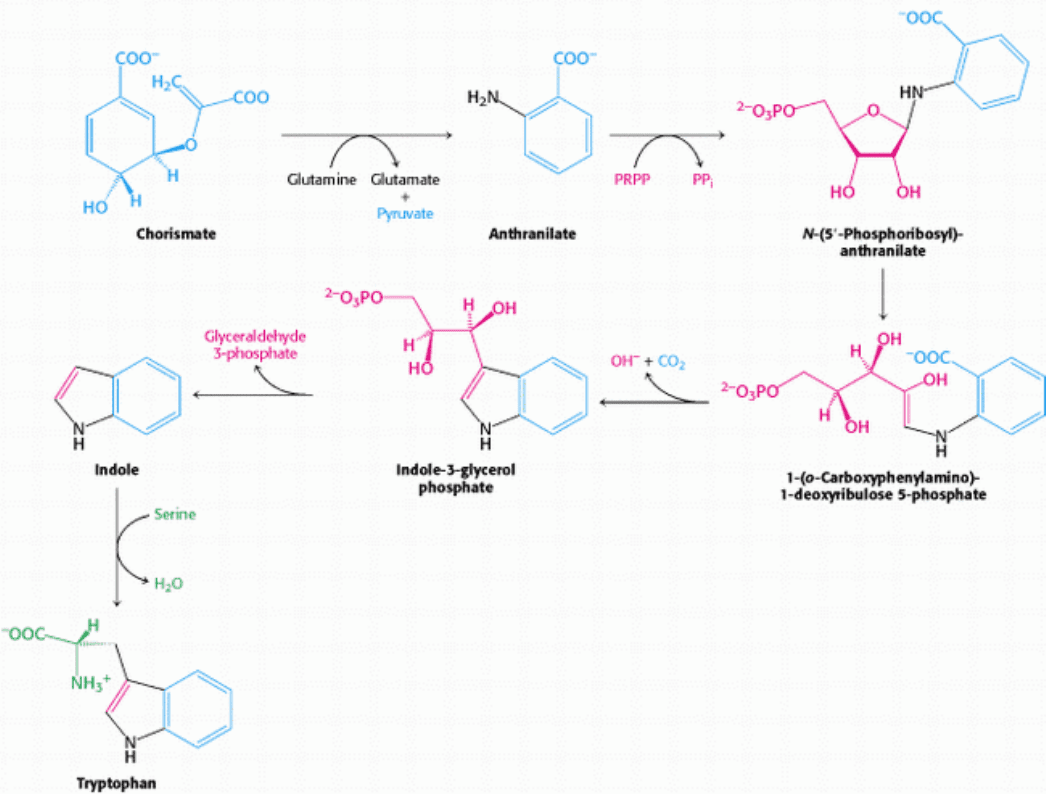
Figure 24.17. Synthesis of Phenylalanine and Tyrosine. Chorismate can be converted into prephenate, which is
subsequently converted into phenylalanine and tyrosine.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Figure 24.18. Synthesis of Tryptophan. Chorismate can be converted into anthranilate, which is subsequently
converted into tryptophan.
III. Synthesizing the Molecules of Life 24. The Biosynthesis of Amino Acids 24.2. Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
