Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


given in Table 25.1.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
Figure 25.1. Salvage and de Novo Pathways. In a salvage pathway, a base is reattached to a ribose, activated in the
form of 5-phosphoribosyl-1-pyrophosphate (PRPP). In de novo synthesis, the base itself is synthesized from simpler
starting materials, including amino acids. ATP hydrolysis is required for de novo synthesis.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
Table 25.1. Nomenclature of bases, nucleosides, and nucleotides
RNA
Base Ribonucleoside Ribonucleotide (5 -monophosphate)
Adenine (A) Adenosine Adenylate (AMP)
Guanine (G) Guanosine Guanylate (GMP)
Uracil (U) Uridine Uridylate (UMP)
Cytosine (C) Cytidine Cytidylate (CMP)
DNA
Base Deoxyribonucleoside Deoxyribonucleotide (5 -monophosphate)
Adenine (A) Deoxyadenosine Deoxyadenylate (dAMP)
Guanine (G) Deoxyguanosine Deoxyguanylate (dGMP)
Thymine (T) Thymidine Thymidylate (TMP)
Cytosine (C) Deoxycytidine Deoxycytidylate (dCMP)
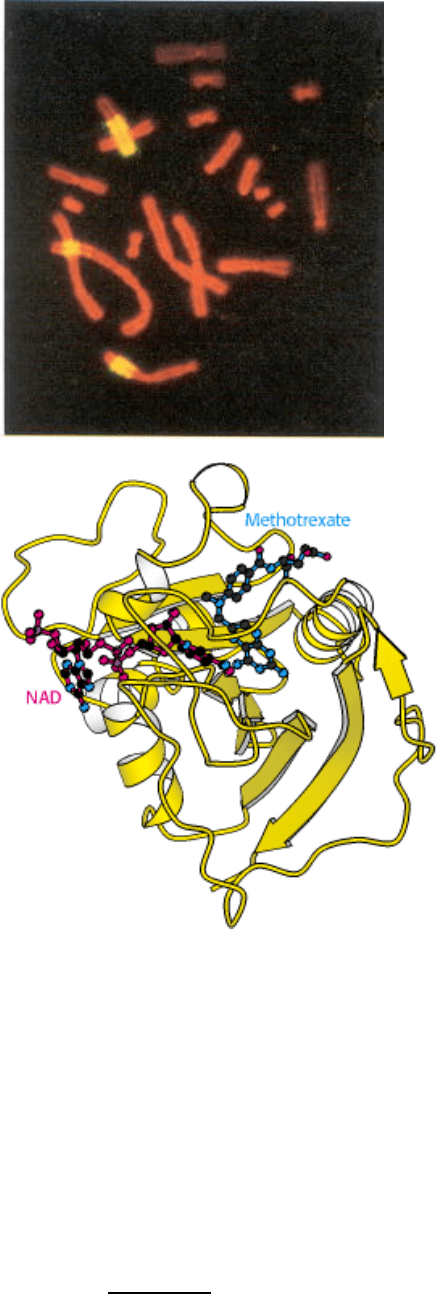
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
Nucleotides are required for cell growth and replication. A key enzyme for the synthesis of one nucleotide is
dihydrofolate reductase (right). Cells grown in the presence of methotrexate, a reductase inhibitor, respond by increasing
the number of copies of the reductase gene. The bright yellow regions visible on three of the chromosomes in the
fluorescence micrograph (left), which were grown in the presence of methotrexate, contain hundreds of copies of the
reductase gene. [(Left) Courtesy of Dr. Barbara Trask and Dr. Joyce Hamlin.]
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
25.1. In de Novo Synthesis, the Pyrimidine Ring Is Assembled from Bicarbonate,
Aspartate, and Glutamine
In de novo synthesis of pyrimidines, the ring is synthesized first and then it is attached to ribose to form a pyrimidine
nucleotide (Figure 25.2). Pyrimidine rings are assembled from bicarbonate, aspartic acid, and ammonia. Although
ammonia can be used directly, it is usually produced from the hydrolysis of the side chain of glutamine.
25.1.1. Bicarbonate and Other Oxygenated Carbon Compounds Are Activated by
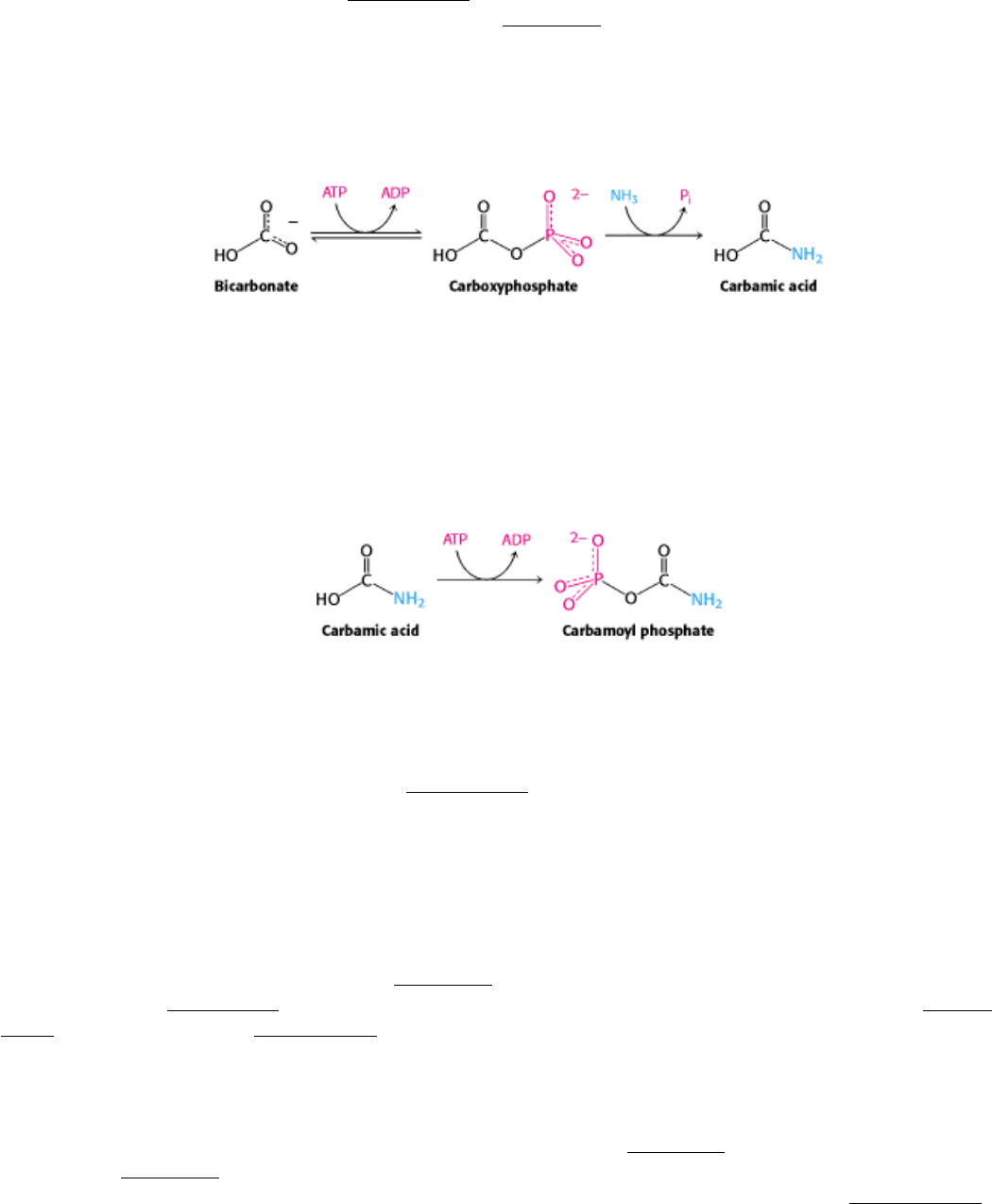
Phosphorylation
The first step in de novo pyrimidine biosynthesis is the synthesis of carbamoyl phosphate from bicarbonate and
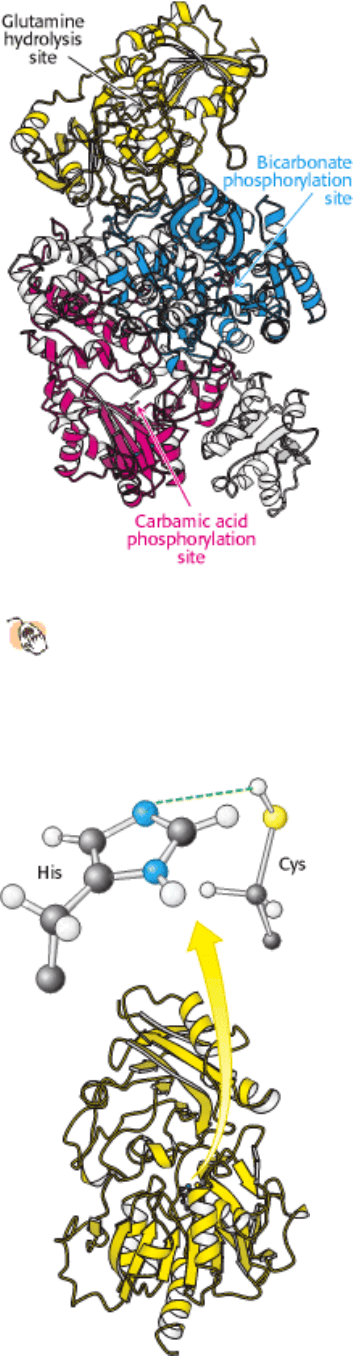
ammonia in a multistep process, requiring the cleavage of two molecules of ATP. This reaction is catalyzed by
carbamoyl phosphate synthetase (CPS) (Section 23.4.1). Analysis of the structure of CPS reveals two homologous
domains, each of which catalyzes an ATP-dependent step (Figure 25.3).
In the first step of the carbamoyl phosphate synthesis pathway, bicarbonate is phosphorylated by ATP to form
carboxyphosphate and ADP. Ammonia then reacts with carboxyphosphate to form carbamic acid and inorganic
phosphate.
The active site for this reaction lies in a domain formed by the aminoterminal third of CPS. This domain forms a
structure, called an ATP-grasp fold, that surrounds ATP and holds it in an orientation suitable for nucleophilic attack at
the γ phosphoryl group. Proteins containing ATP-grasp folds catalyze the formation of carbon-nitrogen bonds through
acyl-phosphate intermediates and are widely used in nucleotide biosynthesis. In the final step catalyzed by carbamoyl
phosphate synthetase, carbamic acid is phosphorylated by another molecule of ATP to form carbamoyl phosphate.
This reaction takes place in a second ATP-grasp domain within the enzyme. The active sites leading to carbamic acid
formation and carbamoyl phosphate formation are very similar, revealing that this enzyme evolved by a gene duplication
event. Indeed, duplication of a gene encoding an ATP-grasp domain followed by specialization was central to the
evolution of nucleotide biosynthetic processes (Section 25.2.3).
25.1.2. The Side Chain of Glutamine Can Be Hydrolyzed to Generate Ammonia
Carbamoyl phosphate synthetase primarily uses glutamine as a source of ammonia. In this case, a second polypeptide
component of the carbamoyl phosphate synthetase enzyme hydrolyzes glutamine to form ammonia and glutamate. The
active site of the glutamine-hydrolyzing component of carbamoyl phosphate synthetase contains a catalytic dyad
comprising a cysteine and a histidine residue (Figure 25.4). Such a catalytic dyad, reminiscent of the active site of
cysteine proteases (Section 9.1.6), is conserved in a family of amidotransferases, including CTP synthetase (Section
25.1.6) and GMP synthetase (Section 25.2.4).
25.1.3. Intermediates Can Move Between Active Sites by Channeling
Carbamoyl phosphate synthetase contains three different active sites (see Figure 25.3), separated from one another by a
total of 80 Å (Figure 25.5). Intermediates generated at one site move to the next without leaving the enzyme; that is, they
move by means of substrate channeling, similar to the process described for tryptophan synthetase (Section 24.2.11). The
ammonia generated in the glutamine-hydrolysis active site travels 45 Å through a channel within the enzyme to reach the
site at which carboxyphosphate has been generated. The carbamic acid generated at this site diffuses an additional 35 Å
through an extension of the channel to reach the site at which carbamoyl phosphate is generated. This channeling serves
two roles: (1) intermediates generated at one active site are captured with no loss caused by diffusion; and (2) labile
intermediates, such as carboxyphosphate and carbamic acid (which decompose in less than 1 s at pH 7), are protected
from hydrolysis.
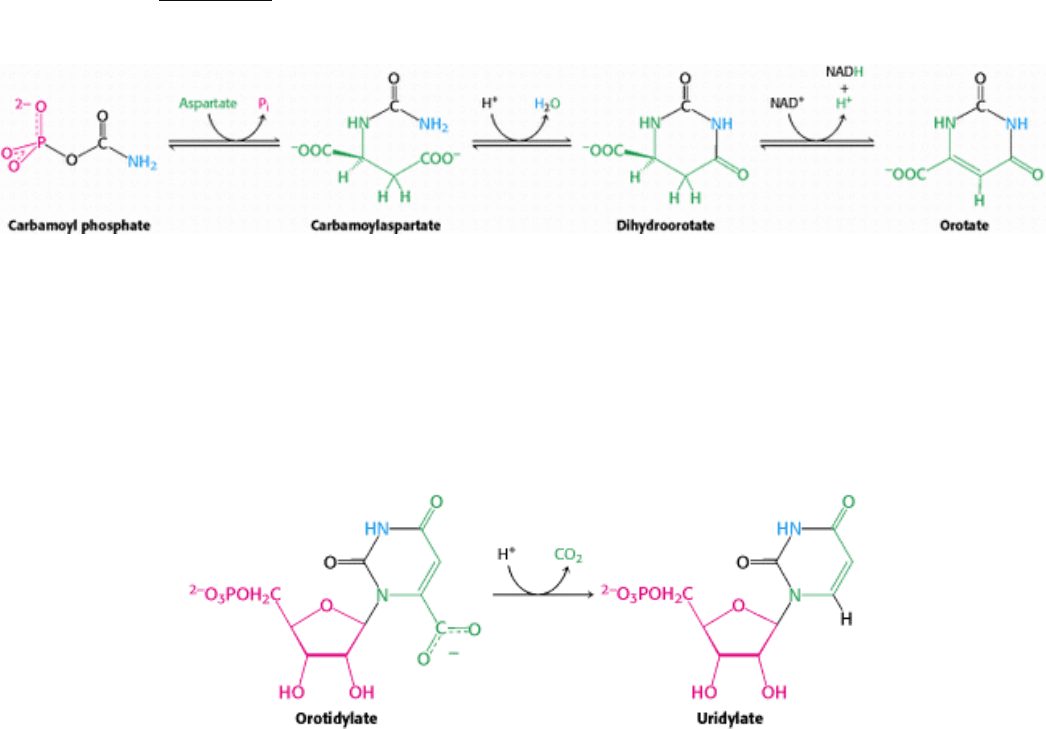
25.1.4. Orotate Acquires a Ribose Ring from PRPP to Form a Pyrimidine Nucleotide
and Is Converted into Uridylate
Carbamoyl phosphate reacts with aspartate to form carbamoylaspartate in a reaction catalyzed by aspartate
transcarbamoylase (Section 10.1). Carbamoylaspartate then cyclizes to form dihydroorotate which is then oxidized by
NAD
+
to form orotate.
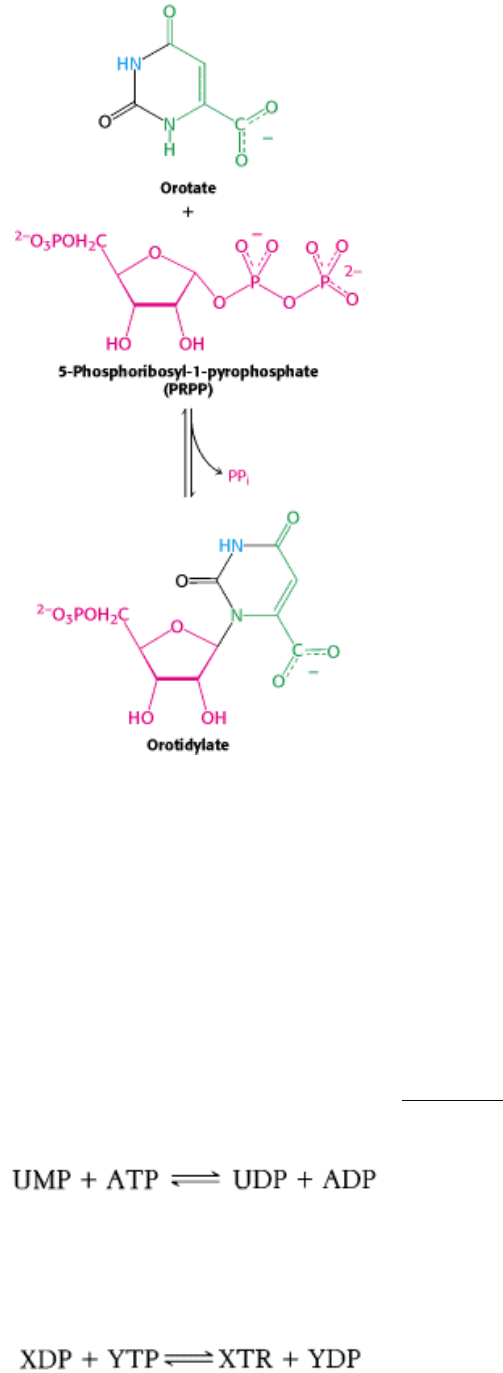
At this stage, orotate couples to ribose, in the form of 5-phosphoribosyl-1-pyrophosphate (PRPP), a form of ribose
activated to accept nucleotide bases. PRPP is synthesized from ribose-5-phosphate, formed by the pentose phosphate
pathway, by the addition of pyrophosphate from ATP. Orotate reacts with PRPP to form orotidylate, a pyrimidine
nucleotide. This reaction is driven by the hydrolysis of pyrophosphate. The enzyme that catalyzes this addition,
pyrimidine phosphoribosyltransferase, is homologous to a number of other phosphoribosyltransferases that add different
groups to PRPP to form the other nucleotides. Orotidylate is then decarboxylated to form uridylate (UMP), a major
pyrimidine nucleotide that is a precursor to RNA. This reaction is catalyzed by orotidylate decarboxylase.
This enzyme is one of the most proficient enzymes known. In its absence, decarboxylation is extremely slow and is
estimated to take place once every 78 million years; with the enzyme present, it takes place approximately once per
second, a rate enhancement of 10
17
-fold!
25.1.5. Nucleotide Mono-, Di-, and Triphosphates Are Interconvertible
How is the other major pyrimidine ribonucleotide, cytidine, formed? It is synthesized from the uracil base of UMP, but
UMP is converted into UTP before the synthesis can take place. Recall that the diphosphates and triphosphates are the
active forms of nucleotides in biosynthesis and energy conversions. Nucleoside monophosphates are converted into
nucleoside triphosphates in stages. First, nucleoside monophosphates are converted into diphosphates by specific
nucleoside monophosphate kinases that utilize ATP as the phosphoryl-group donor (Section 9.4). For example, UMP is
phosphorylated to UDP by UMP kinase.
Nucleoside diphosphates and triphosphates are interconverted by nucleoside diphosphate kinase, an enzyme that has
broad specificity, in contrast with the monophosphate kinases. X and Y can represent any of several ribonucleosides or
even deoxyribonucleosides.
25.1.6. CTP Is Formed by Amination of UTP
After uridine triphosphate has been formed, it can be transformed into cytidine triphosphate by the replacement of a
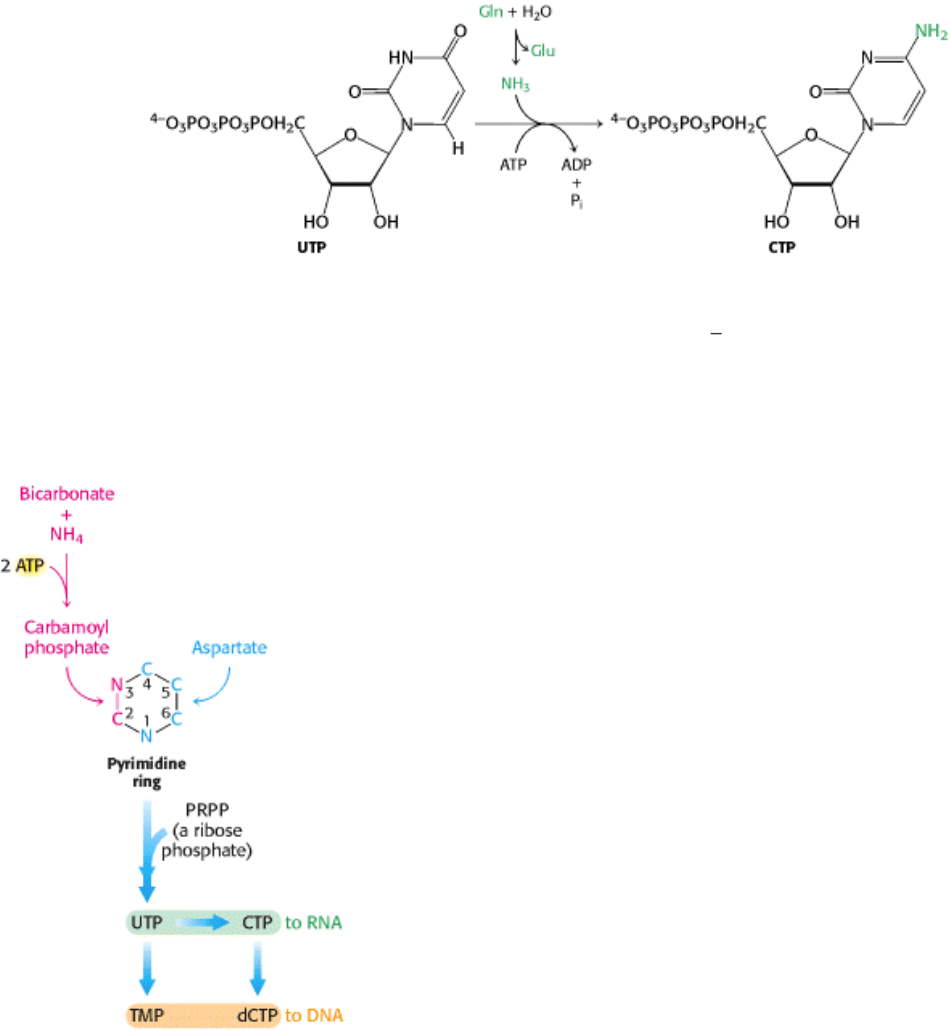
carbonyl group by an amino group.
Like the synthesis of carbamoyl phosphate, this reaction requires ATP and uses glutamine as the source of the amino
group. The reaction proceeds through an analogous mechanism in which the O
4 atom is phosphorylated to form a
reactive intermediate, and then the phosphate is displaced by ammonia, freed from glutamine by hydrolysis. CTP can
then be used in many biochemical processes, including RNA synthesis.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.1. In de Novo Synthesis, the Pyrimidine Ring Is Assembled from Bicarbonate, Aspartate, and Glutamine
Figure 25.2. de Novo Pathway for Pyrimidine Nucleotide Synthesis. The C-2 and N-3 atoms in the pyrimidine ring
come from carbamoyl phosphate, whereas the other atoms of the ring come from aspartate.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.1. In de Novo Synthesis, the Pyrimidine Ring Is Assembled from Bicarbonate, Aspartate, and Glutamine
Figure 25.3. Structure of Carbamoyl Phosphate Synthetase.
This enzyme consists of two chains. The smaller chain
(yellow) contains a site for glutamine hydrolysis to generate ammonia. The larger chain includes two ATP-grasp
domains (blue and red). In one ATP-grasp domain (blue), bicarbonate is phosphorylated to carboxyphosphate,
which then reacts with ammonia to generate carbamic acid. In the other ATP-grasp domain, the carbamic acid is
phosphorylated to produce carbamoyl phosphate.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.1. In de Novo Synthesis, the Pyrimidine Ring Is Assembled from Bicarbonate, Aspartate, and Glutamine
Figure 25.4. Ammonia-Generation Site. The smaller domain of carbamoyl phosphate synthetase contains an active site
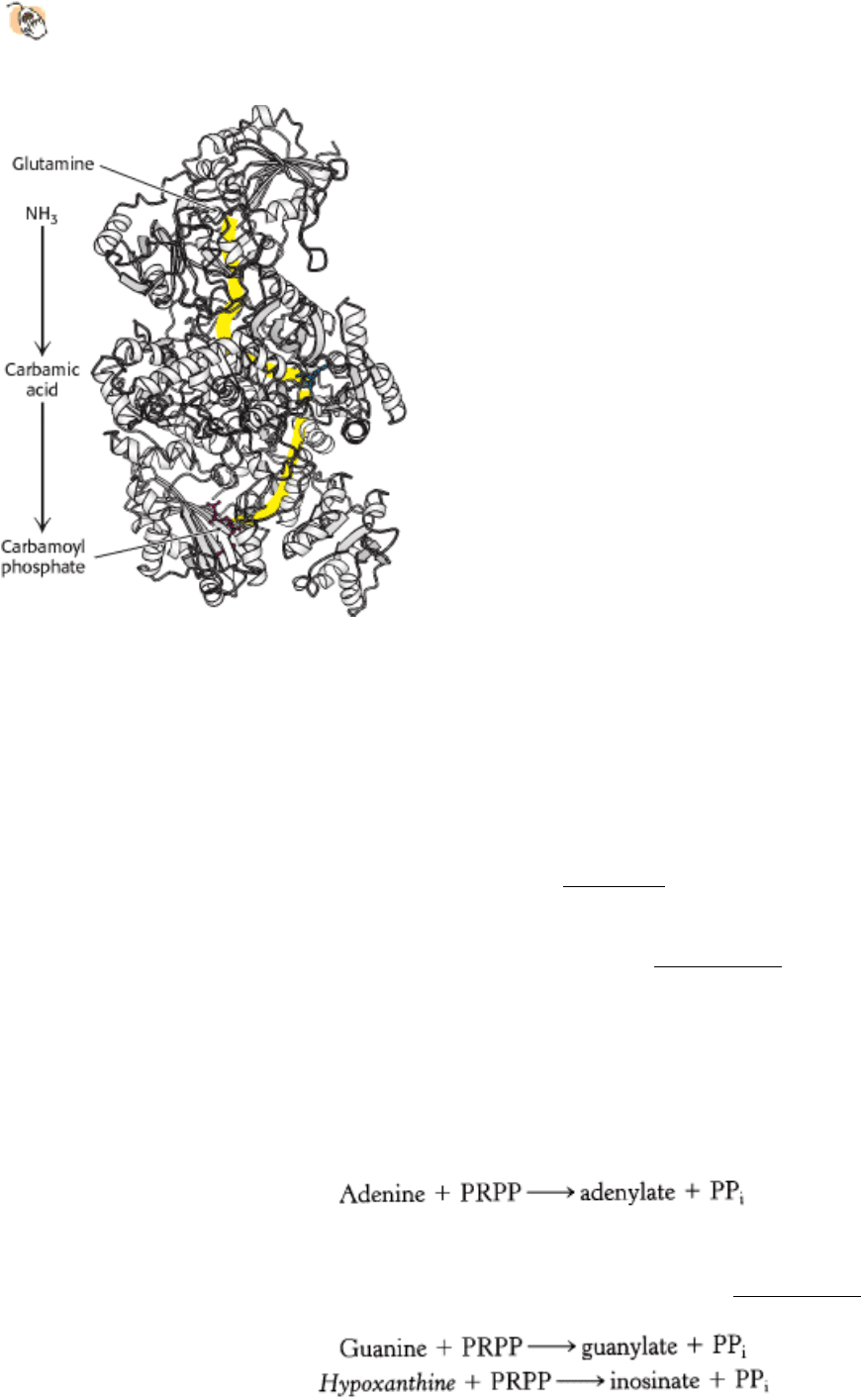
for the hydrolysis of the side chain carboxamide of glutamine to generate ammonia. Key residues in this active site
include a cysteine residue and a histidine residue.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.1. In de Novo Synthesis, the Pyrimidine Ring Is Assembled from Bicarbonate, Aspartate, and Glutamine
Figure 25.5. Substrate Channeling. The three active sites of carbamoyl phosphate synthetase are linked by a channel
(yellow) through which intermediates pass. Glutamine enters one active site, and carbamoyl phosphate, which includes
the nitrogen atom from the glutamine side chain, leaves another 80 Å away.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
25.2. Purine Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways
Purine nucleotides can be synthesized in two distinct pathways. First, purines are synthesized de novo, beginning with
simple starting materials such as amino acids and bicarbonate (Figure 25.6). Unlike the case for pyrimidines, the purine
bases are assembled already attached to the ribose ring. Alternatively, purine bases, released by the hydrolytic
degradation of nucleic acids and nucleotides, can be salvaged and recycled. Purine salvage pathways are especially noted
for the energy that they save and the remarkable effects of their absence (Section 25.6.2).
25.2.1. Salvage Pathways Economize Intracellular Energy Expenditure
Free purine bases, derived from the turnover of nucleotides or from the diet, can be attached to PRPP to form purine
nucleoside monophosphates, in a reaction analogous to the formation of orotidylate. Two salvage enzymes with different
specificities recover purine bases. Adenine phosphoribosyltransferase catalyzes the formation of adenylate
whereas hypoxanthine-guanine phosphoribosyltransferase (HGPRT) catalyzes the formation of guanylate as well as
inosinate (inosine monophosphate, IMP), a precursor of guanylate and adenylate (Section 25.2.4).
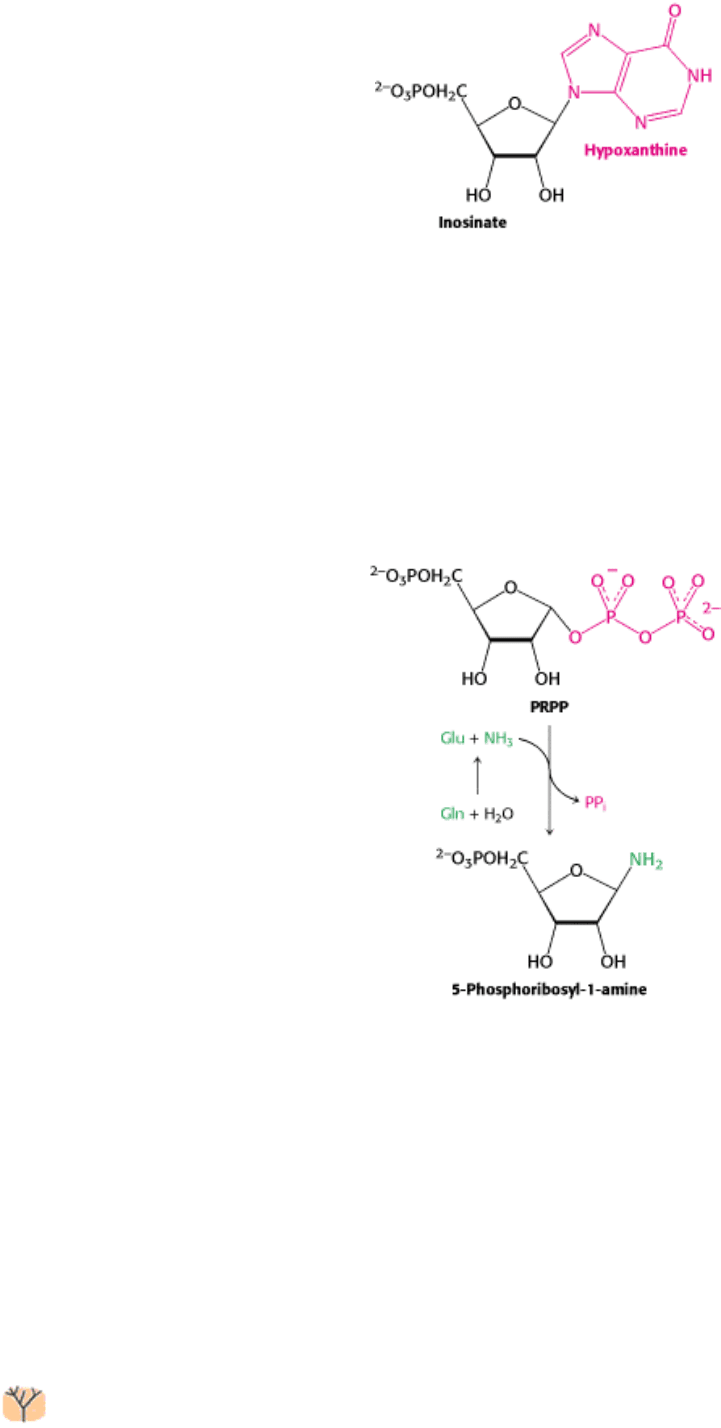
Similar salvage pathways exist for pyrimidines. Pyrimidine phosphoribosyltransferase will reconnect uracil, but not
cytosine, to PRPP.
25.2.2. The Purine Ring System Is Assembled on Ribose Phosphate
De novo purine biosynthesis, like pyrimidine biosynthesis, requires PRPP, but for purines, PRPP provides the foundation
on which the bases are constructed step by step. The initial committed step is the displacement of pyrophosphate by
ammonia, rather than by a preassembled base, to produce 5-phosphoribosyl-1-amine, with the amine in the β
configuration.
Glutamine phosphoribosyl amidotransferase catalyzes this reaction. This enzyme comprises two domains: the first is
homologous to the phosphoribosyltransferases in salvage pathways, whereas the second produces ammonia from
glutamine by hydrolysis. However, this glutamine-hydrolysis domain is distinct from the domain that performs the same
function in carbamoyl phosphate synthetase. In glutamine phosphoribosyl amidotransferase, a cysteine residue located at
the amino terminus facilitates glutamine hydrolysis. To prevent wasteful hydrolysis of either substrate, the
amidotransferase assumes the active configuration only on binding of both PRPP and glutamine. As is the case with
carbamoyl phosphate synthetase, the ammonia generated at the glutamine-hydrolysis active site passes through a channel
to reach PRPP without being released into solution.
25.2.3. The Purine Ring Is Assembled by Successive Steps of Activation by
Phosphorylation Followed by Displacement
Nine additional steps are required to assemble the purine ring. Remarkably, the first six steps are analogous
reactions. Most of these steps are catalyzed by enzymes with ATP-grasp domains that are homologous to those in
carbamoyl phosphate synthetase. Each step consists of the activation of a carbon-bound oxygen atom (typically a

carbonyl oxygen atom) by phosphorylation, followed by the displacement of a phosphoryl group by ammonia or an
amine group acting as a nucleophile (Nu).
De novo purine biosynthesis proceeds as follows (Figure 25.7).
1. The carboxylate group of a glycine residue is activated by phosphorylation and then coupled to the amino group of
phosphoribosylamine. A new amide bond is formed while the amino group of glycine is free to act as a nucleophile in
the next step.
2. Formate is activated and then added to this amino group to form formylglycinamide ribonucleotide. In some
organisms, two distinct enzymes can catalyze this step. One enzyme transfers the formyl group from N
10
-
formyltetrahydrofolate (Section 24.2.6). The other enzyme activates formate as formyl phosphate, which is added
directly to the glycine amino group.
3. The inner amide group is activated and then converted into an amidine by the addition of ammonia derived from
glutamine.
4. The product of this reaction, formylglycinamidine ribonucleotide, cyclizes to form the five-membered imidazole ring
found in purines. Although this cyclization is likely to be favorable thermodynamically, a molecule of ATP is consumed
to ensure irreversibility. The familiar pattern is repeated: a phosphoryl group from the ATP molecule activates the
carbonyl group and is displaced by the nitrogen atom attached to the ribose molecule. Cyclization is thus an
intramolecular reaction in which the nucleophile and phosphate-activated carbon atom are present within the same
molecule.
5. Bicarbonate is activated by phosphorylation and then attacked by the exocyclic amino group. The product of the
reaction in step 5 rearranges to transfer the carboxylate group to the imidazole ring. Interestingly, mammals do not
require ATP for this step; bicarbonate apparently attaches directly to the exocyclic amino group and is then transferred to
the imidazole ring.
6. The imidazole carboxylate group is phosphorylated again and the phosphate group is displaced by the amino group of
aspartate. Thus, a six-step process links glycine, formate, ammonia, bicarbonate, and aspartate to form an intermediate
that contains all but two of the atoms necessary for the formation of the purine ring.
Three more steps complete the ring construction (Figure 25.8). Fumarate, an intermediate in the citric acid cycle, is
eliminated, leaving the nitrogen atom from aspartate joined to the imidazole ring. The use of aspartate as an amino-group
donor and the concomitant release of fumarate are reminiscent of the conversion of citrulline into arginine in the urea
cycle and these steps are catalyzed by homologous enzymes in the two pathways (Section 23.4.2). A formyl group from
N
10
-formyltetrahydrofolate is added to this nitrogen atom to form a final intermediate that cyclizes with the loss of
water to form inosinate.
