Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

25.2.4. AMP and GMP Are Formed from IMP
A few steps convert inosinate into either AMP or GMP (Figure 25.9). Adenylate is synthesized from inosinate by the
substitution of an amino group for the carbonyl oxygen atom at C-6. Again, the addition of aspartate followed by the
elimination of fumarate contributes the amino group. GTP, rather than ATP, is the phosphoryl-group donor in the
synthesis of the adenylosuccinate intermediate from inosinate and aspartate. In accord with the use of GTP, the enzyme
that promotes this conversion, adenylsuccinate synthase, is structurally related to the G-protein family and does not
contain an ATP-grasp domain. The same enzyme catalyzes the removal of fumarate from adenylosuccinate in the
synthesis of adenylate and from 5-aminoimidazole-4-N-succinocarboxamide ribonucleotide in the synthesis of inosinate.
Guanylate (GMP) is synthesized by the oxidation of inosinate to xanthylate (XMP), followed by the incorporation of an
amino group at C-2. NAD
+
is the hydrogen acceptor in the oxidation of inosinate. Xanthylate is activated by the transfer
of an AMP group (rather than a phosphoryl group) from ATP to the oxygen atom in the newly formed carbonyl group.
Ammonia, generated by the hydrolysis of glutamine, then displaces the AMP group to form guanylate, in a reaction
catalyzed by GMP synthetase. Note that the synthesis of adenylate requires GTP, whereas the synthesis of guanylate
requires ATP. This reciprocal use of nucleotides by the pathways creates an important regulatory opportunity (Section
25.4).
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.2. Purine Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways
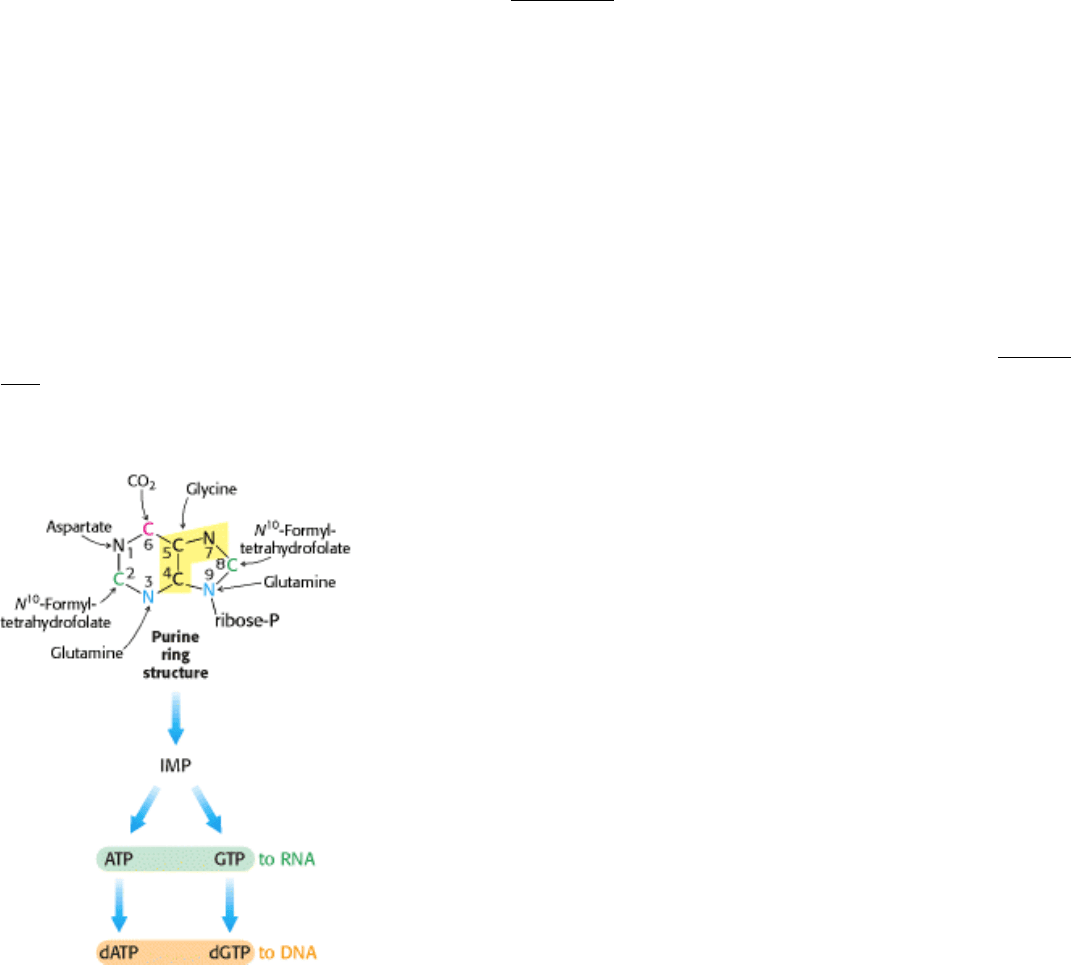
Figure 25.6. de Novo Pathway for Purine Nucleotide Synthesis. The origins of the atoms in the purine ring are
indicated.
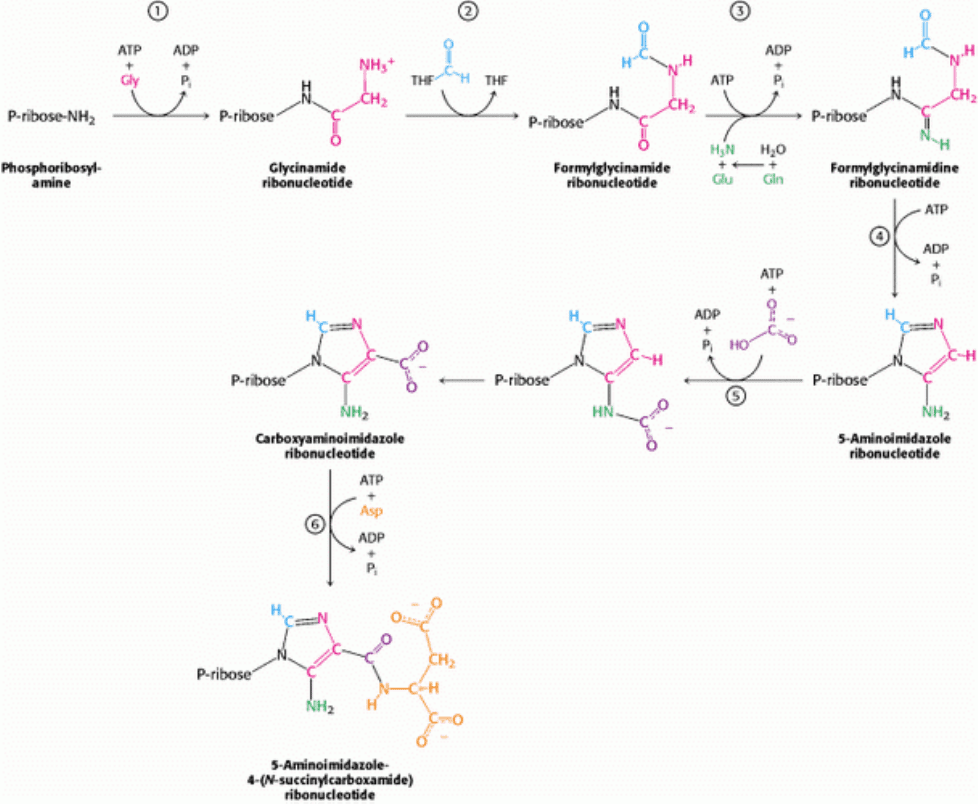
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.2. Purine Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways
Figure 25.7. de Novo Purine Biosynthesis. 1. Glycine is coupled to the amino group of phosphoribosylamine. 2. N
10
-
Formyltetrahydrofolate transfers a formyl group to the amino group of the glycine residue. 3. The inner amide group is
phosphorylated and converted into an amidine by the addition of ammonia derived from glutamine. 4. An intramolecular
coupling reaction forms the five-membered imidazole ring. 5. Bicarbonate adds first to the exocyclic amino group and
then to a carbon atom of the imidazole ring. 6. The imidazole carboxylate is phosphorylated, and the phosphate is
displaced by the amino group of aspartate.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.2. Purine Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways
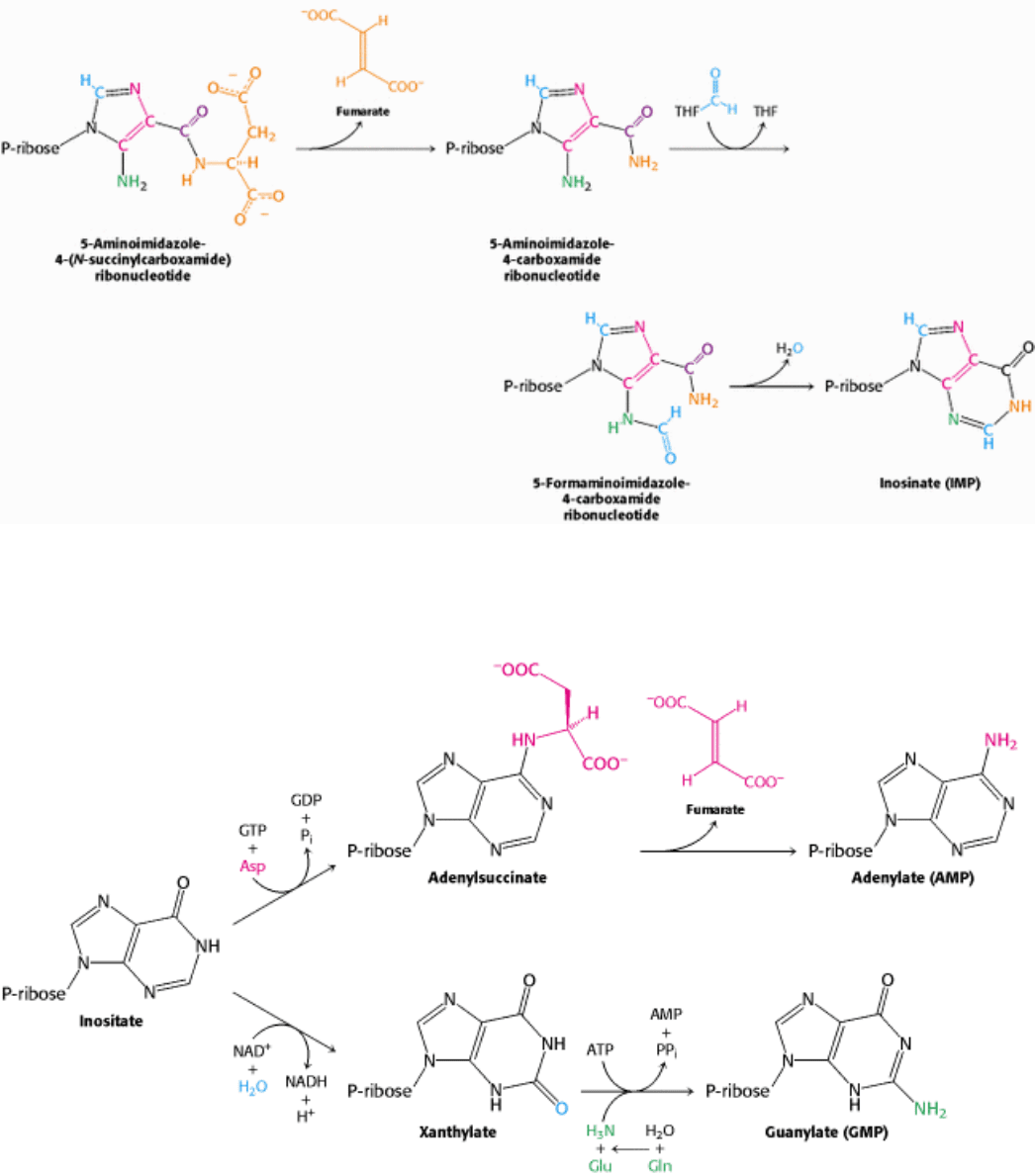
Figure 25.8. Inosinate Formation. The removal of fumarate, the addition of a second formyl group from N
10
-
formyltetrahydrofolate, and cyclization completes the synthesis of inosinate (IMP), a purine nucleotide.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.2. Purine Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways
Figure 25.9. Generating AMP and GMP. Inosinate is the precursor of AMP and GMP. AMP is formed by the addition
of aspartate followed by the release of fumarate. GMP is generated by the addition of water, dehydrogenation by NAD
+
,
and the replacement of the carbonyl oxygen atom by -NH
2
derived by the hydrolysis of glutamine.

III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
25.3. Deoxyribonucleotides Synthesized by the Reduction of Ribonucleotides
Through a Radical Mechanism
We turn now to the synthesis of deoxyribonucleotides. These precursors of DNA are formed by the reduction of
ribonucleotides
specifically, the 2 -hydroxyl group on the ribose moiety is replaced by a hydrogen atom. The
substrates are ribonucleoside diphosphates or triphosphates, and the ultimate reductant is NADPH. The enzyme
ribonucleotide reductase is responsible for the reduction reaction for all four ribonucleotides. The ribonucleotide
reductases of different organisms are a remarkably diverse set of enzymes. The results of detailed studies have revealed
that they have a common reaction mechanism, and their three-dimensional structural features indicate that these enzymes
are homologous. We will focus on the best understood of these enzymes, that of E. coli living aerobically. This
ribonucleotide reductase consists of two subunits: R1 (an 87-kd dimer) and R2 (a 43-kd dimer).
The R1 subunit contains the active site as well as two allosteric control sites (Section 25.4). This subunit includes three
conserved cysteine residues and a glutamate residue, all four of which participate in the reduction of ribose to
deoxyribose (Figure 25.10).
The R2 subunit's role in catalysis is to generate a remarkable free radical in each of its two chains. Each R2 chain
contains a stable tyrosyl radical with an unpaired electron delocalized onto its aromatic ring (Figure 25.11). This very
unusual free radical is generated by a nearby iron center consisting of two ferric (Fe
3+
) ions bridged by an oxide (O
2-
)
ion.
In the synthesis of a deoxyribonucleotide, the hydroxyl group bonded to C-2
of the ribose ring is replaced by H, with
retention of the configuration at the C-2
carbon atom (Figure 25.12).
1. The reaction begins with the transfer of an electron from a cysteine residue on R1 to the tyrosyl radical on R2. The
loss of an electron generates a highly reactive cysteine thiyl radical within the active site of R1.
2. This radical then abstracts a hydrogen atom from C-3 of the ribose unit, generating a radical at that carbon atom.
3. The radical at C-3 promotes the release of the hydroxide ion on the carbon-2 atom. Protonated by a second cysteine
residue, the departing hydroxide ion leaves as a water molecule.
4. A hydride ion (a proton on two electrons) is then transferred from a third cysteine residue to complete the reduction of
the C-2 position, form a disulfide bond, and reform a C-3 radical.
5. This C-3 radical recaptures the same hydrogen atom originally abstracted by the first cysteine residue, and the
deoxyribonucleotide is free to leave the enzyme.
6. The disulfide bond generated in the enzyme's active site is then reduced by specific disulfide-containing proteins, such
as thioredoxin, to regenerate the active enzyme.
To complete the overall reaction, the oxidized thioredoxin generated by this process is reduced by NADH in a reaction
catalyzed by thioredoxin reductase.
Ribonucleotide reductases that do not contain tyrosyl radicals have been characterized in other organisms. Instead,
these enzymes contain other stable radicals that are generated by other processes. For example, in one class of
reductases, the coenzyme adenosylcobalamin is the radical source. Despite differences in the stable radical employed,
the active sites of these enzymes are similar to that of the E. coli ribonucleotide reductase, and they appear to act by the
same mechanism, based on the exceptional reactivity of cysteine radicals. Thus, these enzymes have a common ancestor
but evolved a range of mechanisms for generating stable radical species that function well under different growth
conditions. It appears that the primordial enzymes were inactivated by oxygen, whereas enzymes such as the E. coli
enzyme make use of oxygen to generate the initial tyrosyl radical. Note that the reduction of ribonucleotides to
deoxyribonucleotides is a difficult reaction chemically, likely to require a sophisticated catalyst. The existence of a
common protein enzyme framework for this process strongly suggests that proteins joined the RNA world before the
evolution of DNA as a stable storage form for genetic information.
25.3.1. Thymidylate Is Formed by the Methylation of Deoxyuridylate
Uracil, produced by the pyrimidine synthesis pathway, is not a component of DNA. Rather, DNA contains thymine, a
methylated analog of uracil. Another step is required to generate thymidylate from uracil. Thymidylate synthase catalyzes
this finishing touch: deoxyuridylate (dUMP) is methylated to thymidylate (TMP). As will be discussed in Chapter 27,
the methylation of this nucleotide facilitates the identification of DNA damage for repair and, hence, helps preserve the
integrity of the genetic information stored in DNA. The methyl donor in this reaction is N
5
,N
10
-
methylenetetrahydrofolate rather than S-adenosylmethionine.
The methyl group becomes attached to the C-5 atom within the aromatic ring of dUMP, but this carbon atom is not a
good nucleophile and cannot itself attack the appropriate group on the methyl donor. Thymidylate synthase promotes the
methylation by adding a thiolate from a cysteine side chain to this ring to generate a nucleophilic species that can attack
the methylene group of N
5
,N
10
-methylenetetrahydrofolate (Figure 25.13). This methylene group, in turn, is activated by
distortions imposed by the enzyme that favor opening the open five-membered ring. The activated UMP's attack on the
methylene group forms the new carbon-carbon bond. The intermediate formed is then converted into product: a hydride
ion is transferred from the tetrahydrofolate ring to transform the methylene group into a methyl group, and a proton is
abstracted from the carbon atom bearing the methyl group to eliminate the cysteine and regenerate the aromatic ring.
Thus, the tetrahydrofolate derivative loses both its methylene group and a hydride ion and, hence, is oxidized to
dihydrofolate. For the synthesis of more thymidylate, tetrahydrofolate must be regenerated.
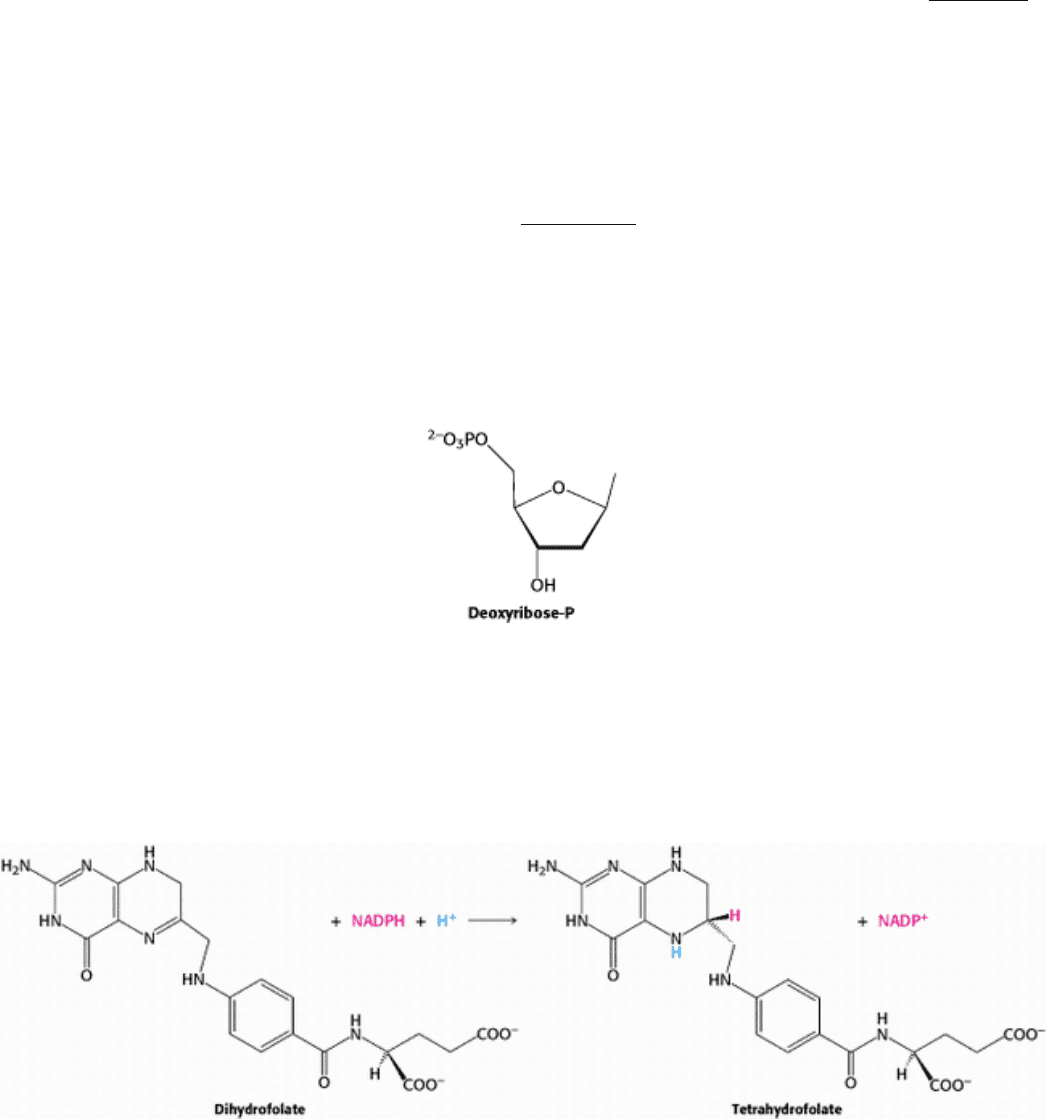
25.3.2. Dihydrofolate Reductase Catalyzes the Regeneration of Tetrahydrofolate, a One-
Carbon Carrier
Tetrahydrofolate is regenerated from the dihydrofolate that is produced in the synthesis of thymidylate. This regeneration
is accomplished by dihydrofolate reductase with the use of NADPH as the reductant.
A hydride ion is directly transferred from the nicotinamide ring of NADPH to the pteridine ring of dihydrofolate. The
bound dihydrofolate and NADPH are held in close proximity to facilitate the hydride transfer.
25.3.3. Several Valuable Anticancer Drugs Block the Synthesis of Thymidylate
Rapidly dividing cells require an abundant supply of thymidylate for the synthesis of DNA. The vulnerability of
these cells to the inhibition of TMP synthesis has been exploited in cancer chemotherapy. Thymidylate synthase
and dihydrofolate reductase are choice targets of chemotherapy (Figure 25.14).
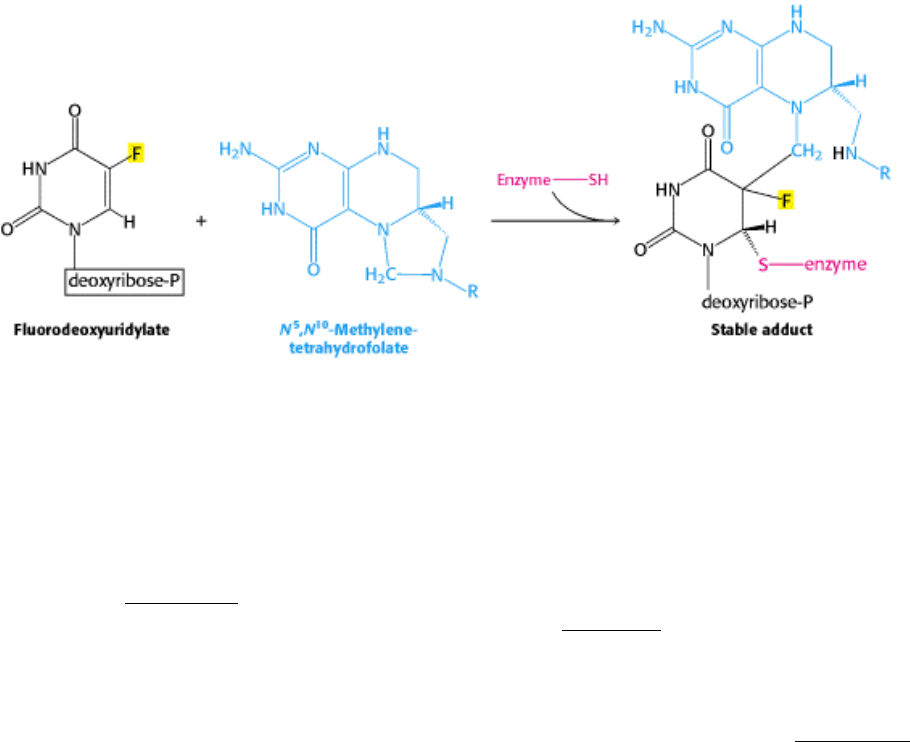
Fluorouracil, a clinically useful anticancer drug, is converted in vivo into fluorodeoxyuridylate (F-dUMP). This analog
of dUMP irreversibly inhibits thymidylate synthase after acting as a normal substrate through part of the catalytic cycle.
Recall that the formation of TMP requires the removal of a proton (H
+
) from C-5 of the bound nucleotide (see Figure
25.13). However, the enzyme cannot abstract F
+
from F-dUMP, and so catalysis is blocked at the stage of the covalent
complex formed by F-dUMP, methylenetetrahydrofolate, and the sulfhydryl group of the enzyme (Figure 25.15). We see
here an example of suicide inhibition, in which an enzyme converts a substrate into a reactive inhibitor that halts the
enzyme's catalytic activity (Section 8.5.2).
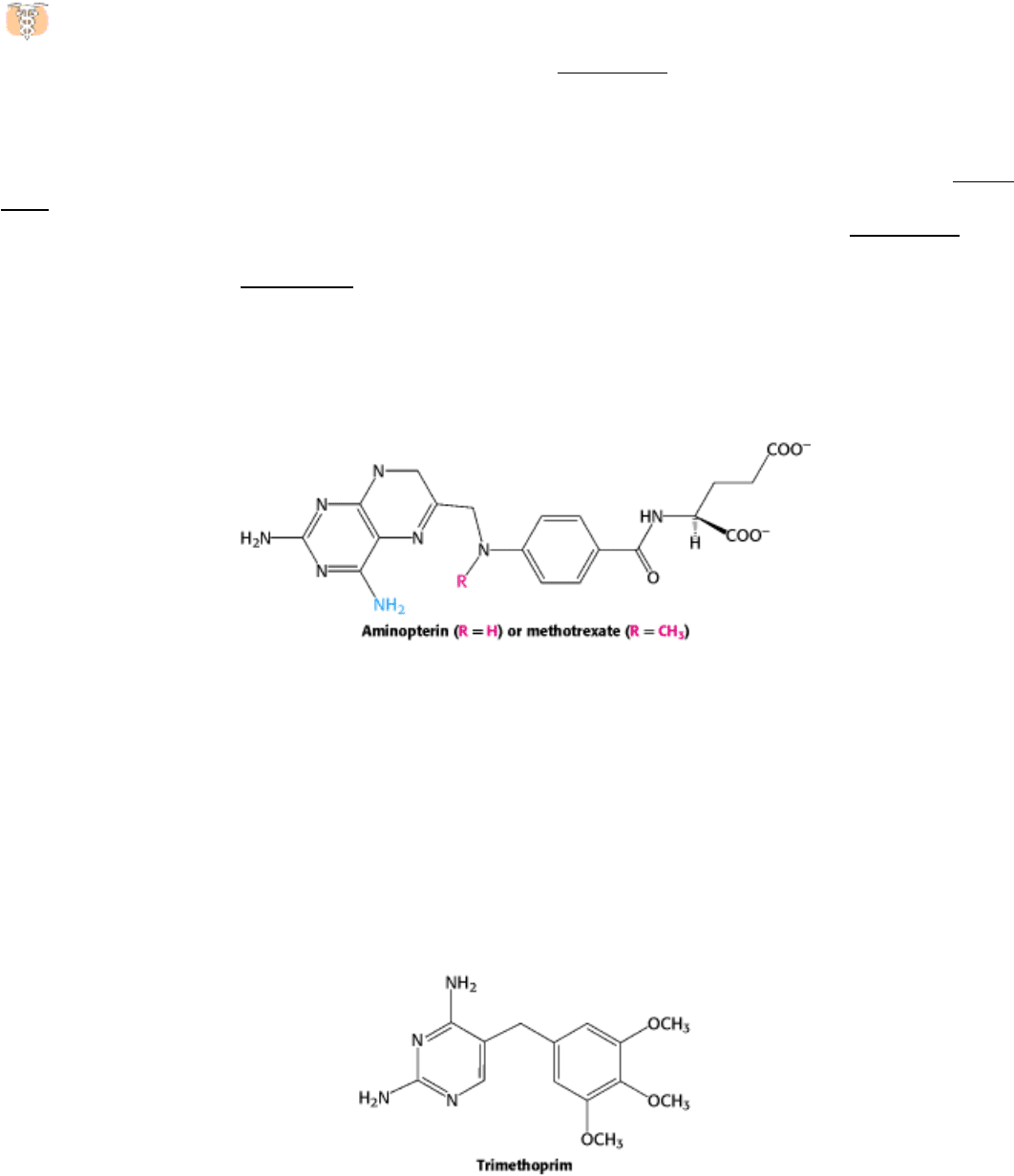
The synthesis of TMP can also be blocked by inhibiting the regeneration of tetrahydrofolate. Analogs of dihydrofolate,
such as aminopterin and methotrexate (amethopterin), are potent competitive inhibitors (K
i
< 1 nM) of dihydrofolate
reductase.
Methotrexate is a valuable drug in the treatment of many rapidly growing tumors, such as those in acute leukemia and
choriocarcinoma, a cancer derived from placental cells. However, methotrexate kills rapidly replicating cells whether
they are malignant or not. Stem cells in bone marrow, epithelial cells of the intestinal tract, and hair follicles are
vulnerable to the action of this folate antagonist, accounting for its toxic side effects, which include weakening of the
immune system, nausea, and hair loss.
Folate analogs such as trimethoprim have potent antibacterial and antiprotozoal activity. Trimethoprim binds 10
5
-fold
less tightly to mammalian dihydrofolate reductase than it does to reductases of susceptible microorganisms. Small
differences in the active-site clefts of these enzymes account for its highly selective antimicrobial action. The
combination of trimethoprim and sulfamethoxazole (an inhibitor of folate synthesis) is widely used to treat infections.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.3. Deoxyribonucleotides Synthesized by the Reduction of Ribonucleotides Through a Radical Mechanism
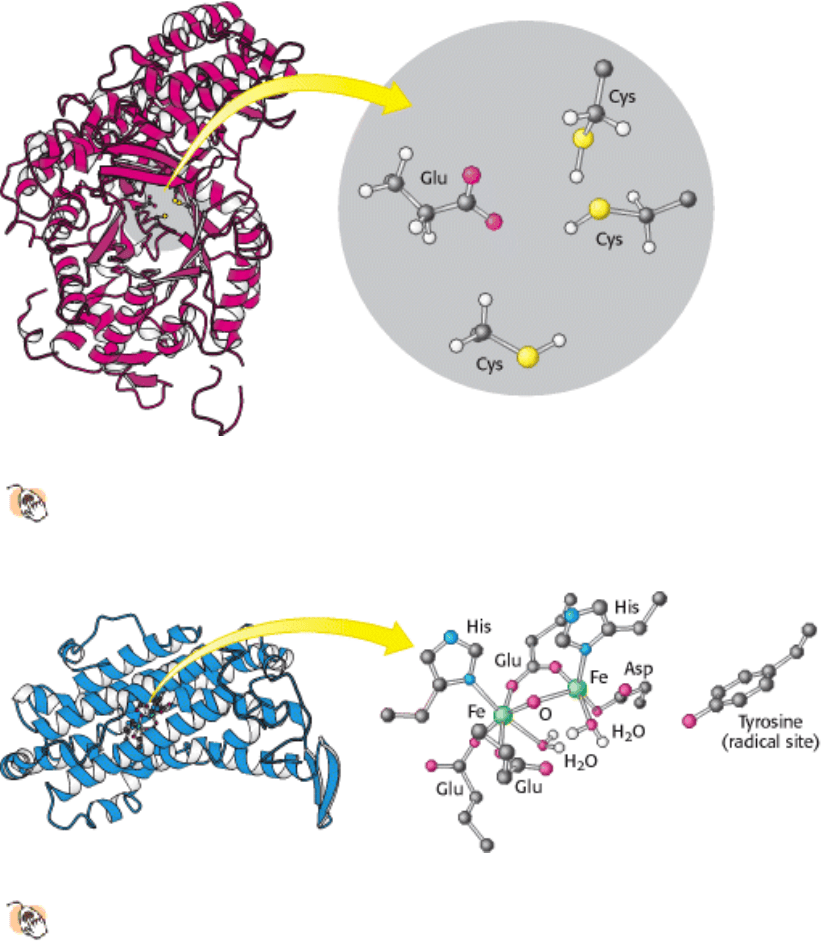
Figure 25.10. Ribonucleotide Reductase R1 Subunit.
Ribonucleotide reductase reduces ribonucleotides to
deoxyribonucleotides in its R1 subunit in an active site that contains three key cysteine residues and one glutamate
residue. Two R1 subunits come together to form a dimer.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.3. Deoxyribonucleotides Synthesized by the Reduction of Ribonucleotides Through a Radical Mechanism
Figure 25.11. Ribonucleotide Reductase R2 Subunit.
This subunit contains a stable free radical on a tyrosine residue.
This radical is generated by the reaction of oxygen at a nearby site containing two iron atoms. Two R2 subunits
come together to form a dimer.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.3. Deoxyribonucleotides Synthesized by the Reduction of Ribonucleotides Through a Radical Mechanism
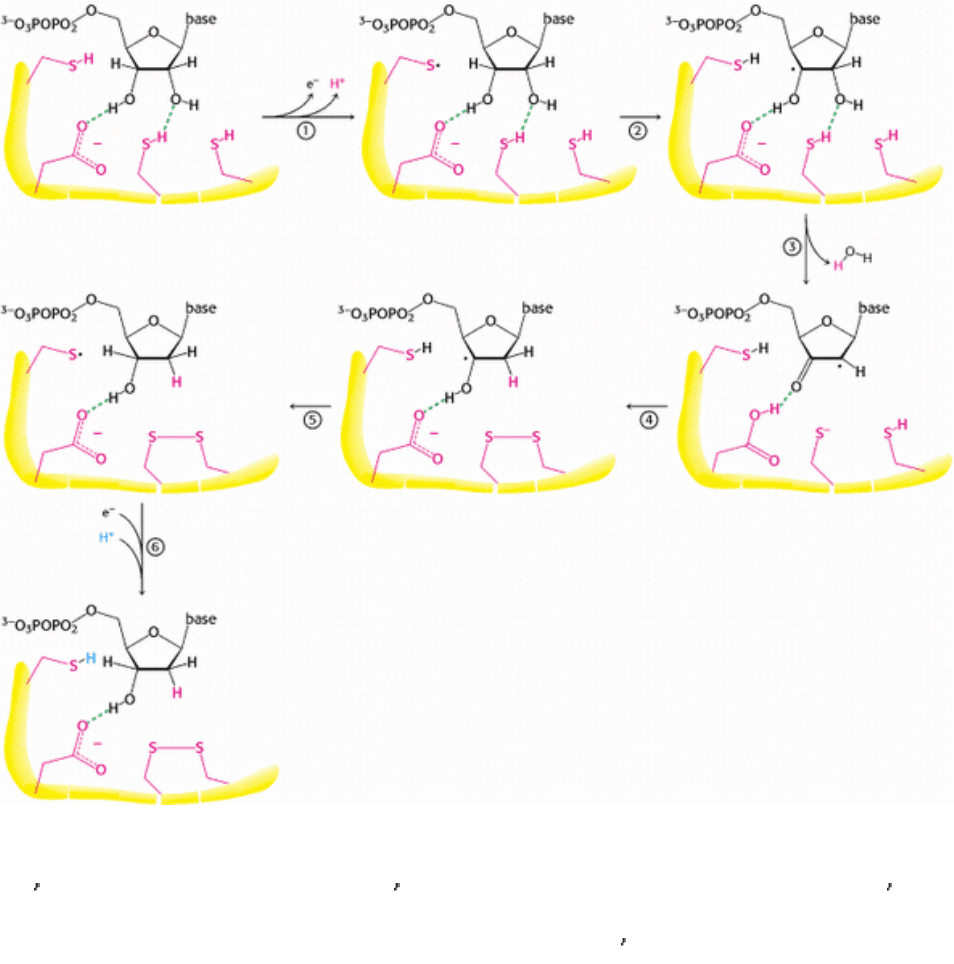
Figure 25.12. Ribonucleotide Reductase Mechanism. 1. An electron is transferred from a cysteine residue on R1 to a
tyrosine radical on R2, generating a highly reactive cysteine thiyl radical. 2. This radical abstracts a hydrogen atom from
C-3
of the ribose unit. 3. The radical at C-3 causes the removal of the hydroxide ion from the C-2 carbon atom.
Combined with a hydrogen atom from a second cysteine residue, the hydroxide ion is eliminated as water. 4. A
hydroxide ion is transferred from a third cysteine residue. 5. The C-3
radical recaptures the originally abstracted
hydrogen atom. 6. An electron is transferred from R2 to reduce the thiyl radical. The deoxyribonucleotide is free to leave
R1. The disulfide formed in the active site must be reduced to begin another reaction cycle.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.3. Deoxyribonucleotides Synthesized by the Reduction of Ribonucleotides Through a Radical Mechanism
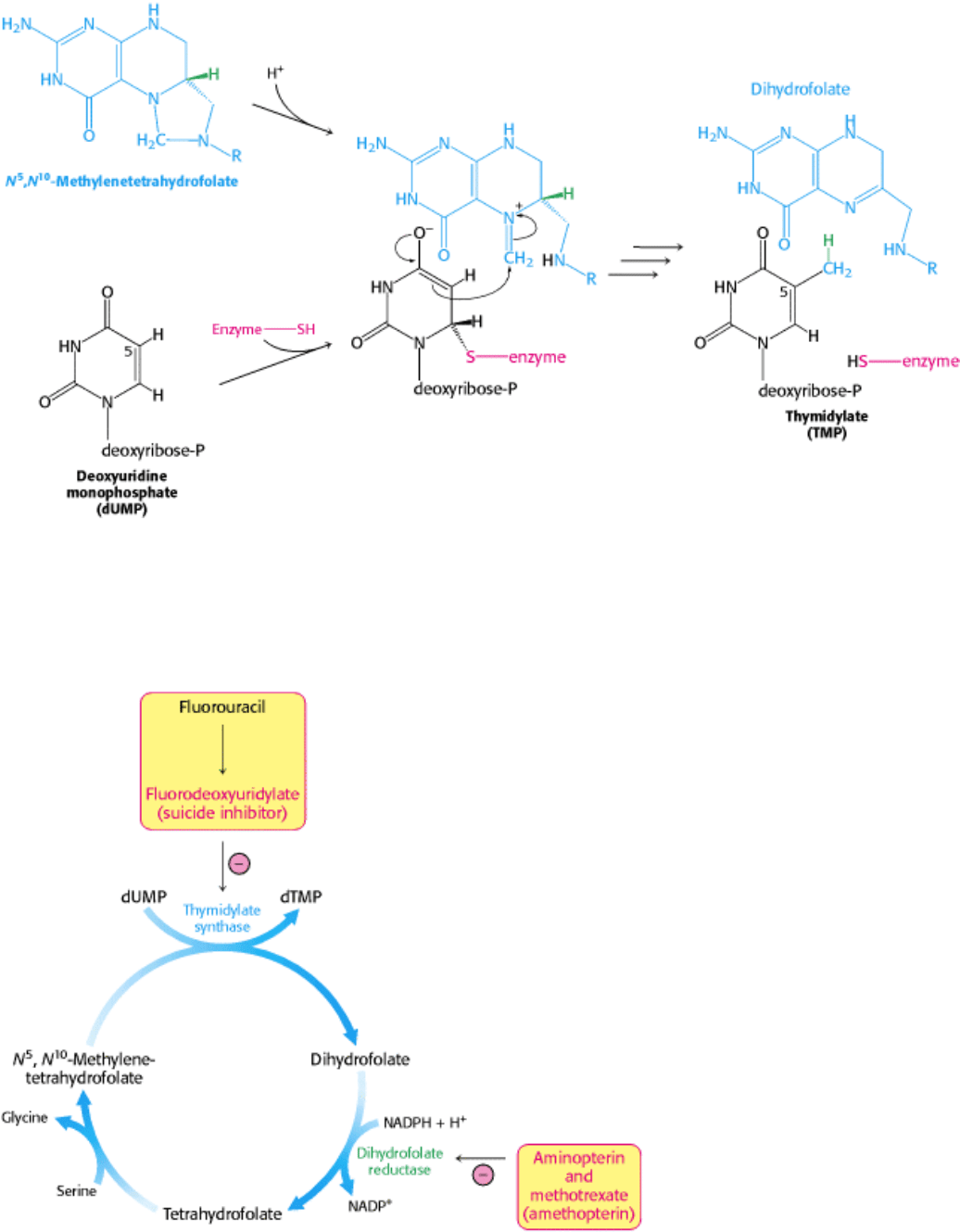
Figure 25.13. Thymidylate Synthesis. Thymidylate synthase catalyzes the addition of a methyl group (derived from N
5
,
N
10
-methylenetertahydrofolate to dUMP to form TMP. The addition of a thiolate from the enzyme activates dUMP.
Opening the five-membered ring of the THF derivative prepares the methylene group for nucleophilic attack by the
activated dUMP. The reaction is completed by the transfer of a hydride ion to form dihydrofolate.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.3. Deoxyribonucleotides Synthesized by the Reduction of Ribonucleotides Through a Radical Mechanism
Figure 25.14. Anticancer Drug Targets. Thymidylate synthase and dihydrofolate reductase are choice targets in cancer
chemotherapy because the generation of large quantities of precursors for DNA synthesis is required for rapidly dividing
cancer cells.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.3. Deoxyribonucleotides Synthesized by the Reduction of Ribonucleotides Through a Radical Mechanism
Figure 25.15. Suicide Inhibition. Fluorodeoxyuridylate (generated from fluorouracil) traps thymidylate synthase in a
form that cannot proceed down the reaction pathway.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
25.4. Key Steps in Nucleotide Biosynthesis Are Regulated by Feedback Inhibition
Nucleotide biosynthesis is regulated by feedback inhibition in a manner similar to the regulation of amino acid
biosynthesis (Section 24.3). Indeed, aspartate transcarbamoylase, one of the key enzymes for the regulation of
pyrimidine biosynthesis in bacteria, was described in detail in Chapter 10. Recall that ATCase is inhibited by CTP, the
final product of pyrimidine biosynthesis, and stimulated by ATP. Carbamoyl phosphate synthetase is a site of feedback
inhibition in both prokaryotes and eukaryotes.
The synthesis of purine nucleotides is controlled by feedback inhibition at several sites (Figure 25.16).
1. The committed step in purine nucleotide biosynthesis is the conversion of PRPP into phosphoribosylamine by
glutamine phosphoribosyl amidotransferase. This important enzyme is feedback-inhibited by many purine
ribonucleotides. It is noteworthy that AMP and GMP, the final products of the pathway, are synergistic in inhibiting the
amidotransferase.
2. Inosinate is the branch point in the synthesis of AMP and GMP. The reactions leading away from inosinate are sites
of feedback inhibition. AMP inhibits the conversion of inosinate into adenylosuccinate, its immediate precursor.
Similarly, GMP inhibits the conversion of inosinate into xanthylate, its immediate precursor.
3. As already noted, GTP is a substrate in the synthesis of AMP, whereas ATP is a substrate in the synthesis of GMP.
This reciprocal substrate relation tends to balance the synthesis of adenine and guanine ribonucleotides.
The reduction of ribonucleotides to deoxyribonucleotides is precisely controlled by allosteric interactions. Each
polypeptide of the R1 subunit of the aerobic E. coli ribonucleotide reductase contains two allosteric sites: one of them
controls the overall activity of the enzyme, whereas the other regulates substrate specificity. The overall catalytic activity
of ribonucleotide reductase is diminished by the binding of dATP, which signals an abundance of deoxyribonucleotides.
The binding of ATP reverses this feedback inhibition. The binding of dATP or ATP to the substrate-specificity control
sites enhances the reduction of UDP and CDP, the pyrimidine nucleotides. The binding of thymidine triphosphate (TTP)
promotes the reduction of GDP and inhibits the further reduction of pyrimidine ribonucleotides. The subsequent increase
in the level of dGTP stimulates the reduction of ATP to dATP. This complex pattern of regulation supplies the
appropriate balance of the four deoxyribonucleotides needed for the synthesis of DNA.
