Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

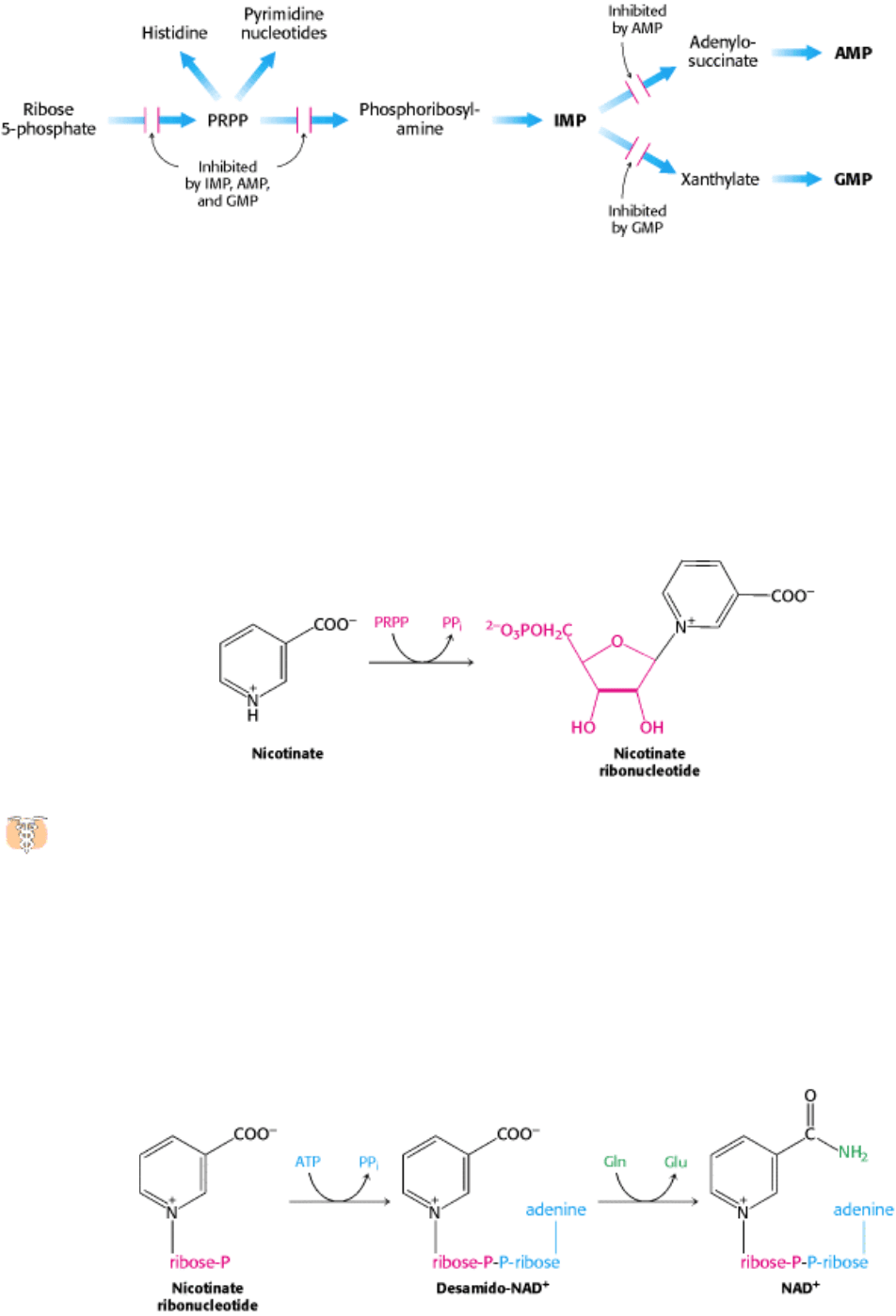
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.4. Key Steps in Nucleotide Biosynthesis Are Regulated by Feedback Inhibition
Figure 25.16. Control of Purine Biosynthesis. Feedback inhibition controls both the overall rate of purine biosynthesis
and the balance between AMP and GMP production.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
25.5. NAD
+
, FAD, and Coenzyme A Are Formed from ATP
Nucleotides are important constituents not only of RNA and DNA, but also of a number of key biomolecules considered
many times in our study of biochemistry. NAD
+
and NADP
+
, coenzymes that function in oxidation-reduction reactions,
are metabolites of ATP. The first step in the synthesis of nicotinamide adenine dinucleotide (NAD
+
) is the formation of
nicotinate ribonucleotide from nicotinate and PRPP.
Nicotinate (also called niacin or vitamin B
6
) is derived from tryptophan. Human beings can synthesize the
required amount of nicotinate if the supply of tryptophan in the diet is adequate. However, nicotinate must be
obtained directly if the dietary intake of tryptophan is low. A dietary deficiency of tryptophan and nicotinate can lead to
pellagra, a disease characterized by dermatitis, diarrhea, and dementia. An endocrine tumor that consumes large amounts
of tryptophan in synthesizing the hormone and neurotransmitter serotonin (5-hydroxytryptamine) can lead to pellagra-
like symptoms.
An AMP moiety is transferred from ATP to nicotinate ribonucleotide to form desamido-NAD
+
. The final step is the
transfer of the ammonia generated from the amide group of glutamine to the nicotinate carboxyl group to form NAD
+
.
NADP
+
is derived from NAD
+
by phosphorylation of the 2 -hydroxyl group of the adenine ribose moiety. This transfer
of a phosphoryl group from ATP is catalyzed by NAD
+
kinase.
Flavin adenine dinucleotide (FAD) is synthesized from riboflavin and two molecules of ATP. Riboflavin is
phosphorylated by ATP to give riboflavin 5
-phosphate (also called flavin mononucleotide, FMN). FAD is then formed
from FMN by the transfer of an AMP moiety from a second molecule of ATP.
The AMP moiety of coenzyme A also comes from ATP. A common feature of the biosyntheses of NAD
+
, FAD, and
CoA is the transfer of the AMP moiety of ATP to the phosphate group of a phosphorylated intermediate. The
pyrophosphate formed in these condensations is then hydrolyzed to orthophosphate. As in many other biosyntheses,
much of the thermodynamic driving force comes from the hydrolysis of the released pyrophosphate.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
25.6. Disruptions in Nucleotide Metabolism Can Cause Pathological Conditions
Nucleotides are vital to a host of biochemical processes. It is not surprising, then, that disruption in nucleotide
metabolism would have a variety of physiological effects.
25.6.1. Purines Are Degraded to Urate in Human Beings
The nucleotides of a cell undergo continual turnover. Nucleotides are hydrolytically degraded to nucleosides by
nucleotidases. The phosphorolytic cleavage of nucleosides to free bases and ribose 1-phosphate (or deoxyribose 1-
phosphate) is catalyzed by nucleoside phosphorylases. Ribose 1-phosphate is isomerized by phosphoribomutase to
ribose 5-phosphate, a substrate in the synthesis of PRPP. Some of the bases are reused to form nucleotides by salvage
pathways. Others are degraded to products that are excreted (Figure 25.17). For example, AMP is degraded to the free
base hypoxanthine through deamination and hydrolytic cleavage of the glycosidic bond. Xanthine oxidase, a
molybdenum- and iron-containing flavoprotein, oxidizes hypoxanthine to xanthine and then to uric acid. Molecular
oxygen, the oxidant in both reactions, is reduced to H
2
O
2
, which is decomposed to H
2
O and O
2
by catalase. Uric acid
loses a proton at physiological pH to form urate. In human beings, urate is the final product of purine degradation and is
excreted in the urine. High serum levels of urate induce gout, a disease in which salts of urate crystallize and damage
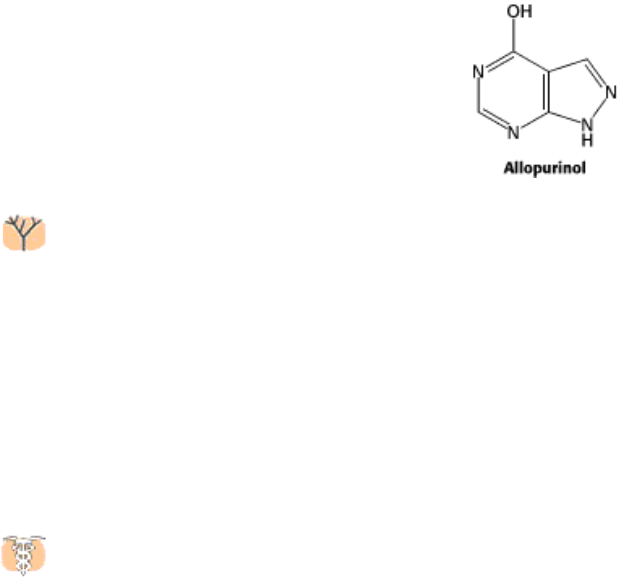
joints and kidneys (Figure 25.18). Allopurinol, an inhibitor of xanthine oxidase, is used to treat gout in some cases.
The average serum level of urate in humans is close to the solubility limit. In contrast, prosimians (such as lemurs)
have tenfold lower levels. A striking increase in urate levels occurred in the evolution of primates. What is the
selective advantage of a urate level so high that it teeters on the brink of gout in many people? It turns out that urate has a
markedly beneficial action. Urate is a highly effective scavenger of reactive oxygen species. Indeed, urate is about as
effective as ascorbate (vitamin C) as an antioxidant. The increased level of urate is humans compared with prosimians
and other lower primates may contribute significantly to the longer life span of humans and to lowering the incidence of
human cancer.
25.6.2. Lesch-Nyhan Syndrome Is a Dramatic Consequence of Mutations in a Salvage-
Pathway Enzyme
Mutations in genes that encode nucleotide biosynthetic enzymes can reduce levels of needed nucleotides and can
lead to an accumulation of intermediates. A nearly total absence of hypoxanthine-guanine
phosphoribosyltransferase has unexpected and devastating consequences. The most striking expression of this inborn
error of metabolism, called the Lesch-Nyhan syndrome, is compulsive self-destructive behavior. At age 2 or 3, children
with this disease begin to bite their fingers and lips and will chew them off if unrestrained. These children also behave
aggressively toward others. Mental deficiency and spasticity are other characteristics of the Lesch-Nyhan syndrome.
Elevated levels of urate in the serum lead to the formation of kidney stones early in life, followed by the symptoms of
gout years later. The disease is inherited as a sex-linked recessive disorder.
The biochemical consequences of the virtual absence of hypoxanthine-guanine phosphoribosyl transferase are an
elevated concentration of PRPP, a marked increase in the rate of purine biosynthesis by the de novo pathway, and an
overproduction of urate. The relation between the absence of the transferase and the bizarre neurologic signs is an
enigma. Specific cells in the brain may be dependent on the salvage pathway for the synthesis of IMP and GMP. Indeed,
transporters of the neurotransmitter dopamine are present at lower levels in affected individuals. Alternatively, cells may
be damaged by the accumulation of intermediates to abnormal levels. The Lesch-Nyhan syndrome demonstrates that the
salvage pathway for the synthesis of IMP and GMP is not gratuitous. Moreover, the LeschNyhan syndrome reveals that
abnormal behavior such as self-mutilation and extreme hostility can be caused by the absence of a single enzyme.
Psychiatry will no doubt benefit from the unraveling of the molecular basis of such mental disorders.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.6. Disruptions in Nucleotide Metabolism Can Cause Pathological Conditions
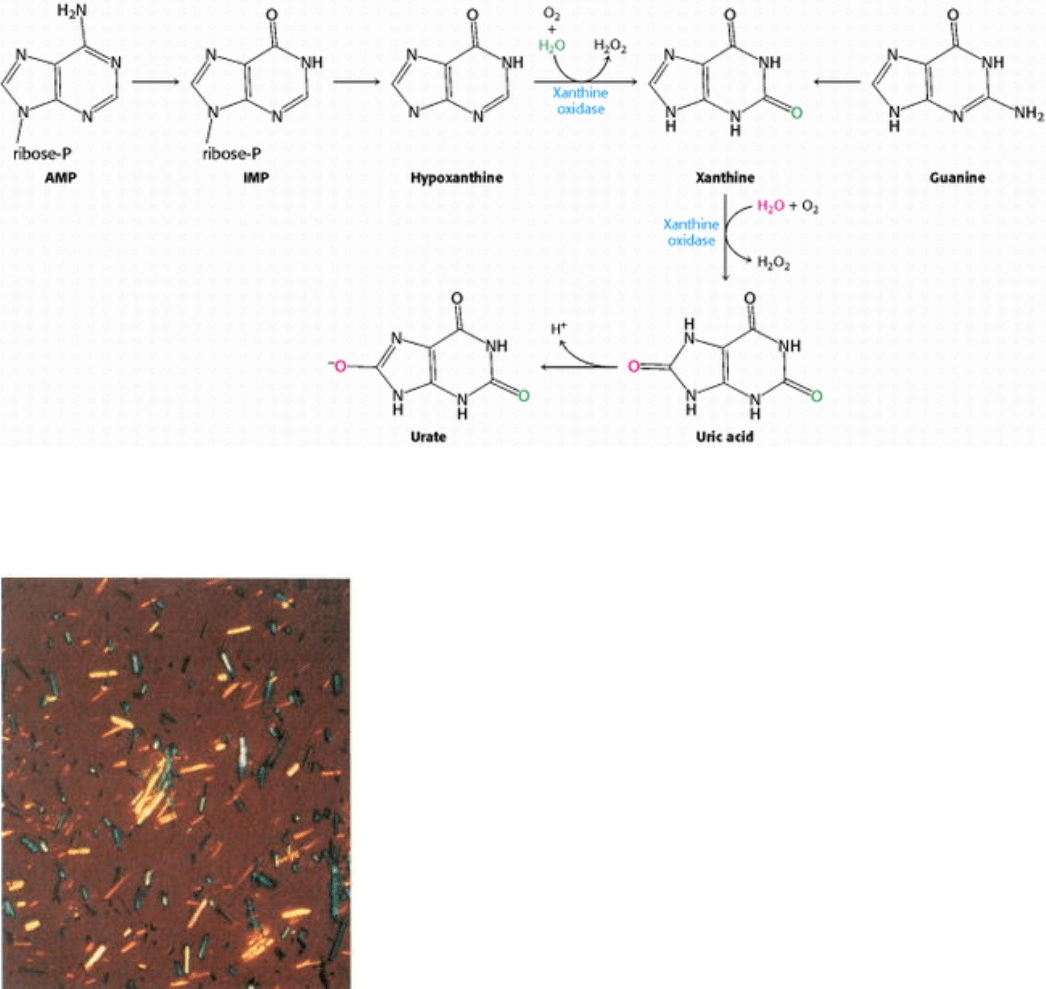
Figure 25.17. Purine Catabolism. Purine bases are converted first into xanthine and then into urate for excretion.
Xanthine oxidase catalyzes two steps in this process.
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis 25.6. Disruptions in Nucleotide Metabolism Can Cause Pathological Conditions
Figure 25.18. Urate Crystals. Micrograph of sodium urate crystals. Joints and kidneys are damaged by these crystals in
gout. [Courtesy of Dr. James McGuire.]
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
Summary
In de Novo Synthesis, the Pyrimidine Ring Is Assembled from Bicarbonate, Aspartate,
and Glutamine
The pyrimidine ring is assembled first and then linked to ribose phosphate to form a pyrimidine nucleotide. PRPP is the
donor of the ribose phosphate moiety. The synthesis of the pyrimidine ring starts with the formation of
carbamoylaspartate from carbamoyl phosphate and aspartate, a reaction catalyzed by aspartate transcarbamoylase.
Dehydration, cyclization, and oxidation yield orotate, which reacts with PRPP to give orotidylate. Decarboxylation of
this pyrimidine nucleotide yields UMP. CTP is then formed by the amination of UTP.

Purines Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways
The purine ring is assembled from a variety of precursors: glutamine, glycine, aspartate, N
10
-formyltetrahydrofolate,
and CO
2
. The committed step in the de novo synthesis of purine nucleotides is the formation of 5-phosphoribosylamine
from 5-phosphoribosyl-1-pyrophosphate (PRPP) and glutamine. The purine ring is assembled on ribose phosphate, in
contrast with the de novo synthesis of pyrimidine nucleotides. The addition of glycine, followed by formylation,
amination, and ring closure, yields 5-aminoimidazole ribonucleotide. This intermediate contains the completed five-
membered ring of the purine skeleton. The addition of CO
2
, the nitrogen atom of aspartate, and a formyl group, followed
by ring closure, yields inosinate (IMP), a purine ribonucleotide. AMP and GMP are formed from IMP. Purine
ribonucleotides can also be synthesized by a salvage pathway in which a preformed base reacts directly with PRPP.
Deoxyribonucleotides Are Synthesized by the Reduction of Ribonucleotides Through a
Radical Mechanism
Deoxyribonucleotides, the precursors of DNA, are formed in E. coli by the reduction of ribonucleoside diphosphates.
These conversions are catalyzed by ribonucleotide reductase. Electrons are transferred from NADPH to sulfhydryl
groups at the active sites of this enzyme by thioredoxin or glutaredoxin. A tyrosyl free radical generated by an iron
center in the reductase initiates a radical reaction on the sugar, leading to the exchange of H for OH at C-2
. TMP is
formed by methylation of dUMP. The donor of a methylene group and a hydride in this reaction is N
5
,N
10
-
methylenetetrahydrofolate, which is converted into dihydrofolate. Tetrahydrofolate is regenerated by the reduction of
dihydrofolate by NADPH. Dihydrofolate reductase, which catalyzes this reaction, is inhibited by folate analogs such as
aminopterin and methotrexate. These compounds and fluorouracil, an inhibitor of thymidylate synthase, are used as
anticancer drugs.
Key Steps in Nucleotide Biosynthesis Are Regulated by Feedback Inhibition
Pyrimidine biosynthesis in E. coli is regulated by the feedback inhibition of aspartate transcarbamoylase, the enzyme that
catalyzes the committed step. CTP inhibits and ATP stimulates this enzyme. The feedback inhibition of glutamine-PRPP
amidotransferase by purine nucleotides is important in regulating their biosynthesis.
NAD
+
, FAD, and Coenzyme A Are Formed from ATP
Nucleotides are important constituents not only of RNA and DNA, but also of a number of other key biomolecules.
Coenzymes NAD
+
and FAD, prominent in oxidation-reduction reactions, have ADP as an important constituent. The
acyl-group activation compound, coenzyme A, is also derived from ATP.
Disruptions in Nucleotide Metabolism Can Cause Pathological Conditions
Purines are degraded to urate in human beings. Gout, a disease that affects joints and leads to arthritis, is associated with
the excessive accumulation of urate. The Lesch-Nyhan syndrome, a genetic disease characterized by self-mutilation,
mental deficiency, and gout, is caused by the absence of hypoxanthine-guanine phosphoribosyltransferase. This enzyme
is essential for the synthesis of purine nucleotides by the salvage pathway.
Key Terms
nucleoside
nucleotide

pyrimidine
carbamoyl phosphate synthetase (CSP)
ATP-grasp fold
5-phosphoribosyl-1-pyrophosphate (PRPP)
orotidylate
purine
salvage pathway
inosinate
hypoxanthine
glutamine phosphoribosyl amidotransferase
ribonucleotide reductase
thymidylate synthase
dihydrofolate reductase
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
Problems
1.
Activated ribose phosphate. Write a balanced equation for the synthesis of PRPP from glucose through the oxidative
branch of the pentose phosphate pathway.
See answer
2.
Making a pyrimidine. Write a balanced equation for the synthesis of orotate from glutamine, CO
2
, and aspartate.
See answer
3.
Identifying the donor. What is the activated reactant in the biosynthesis of each of these compounds?
(a) Phosphoribosylamine
(b)Carbamoylaspartate
(c) Orotidylate (from orotate)
(d) Nicotinate ribonucleotide
(e) Phosphoribosylanthranilate

See answer
4.
Inhibiting purine biosynthesis. Amidotransferases are inhibited by the antibiotic azaserine (O-diazoacetyl-L-serine),
which is an analog of glutamine.
Which intermediates in purine biosynthesis would accumulate in cells treated with azaserine?
See answer
5.
The price of methylation. Write a balanced equation for the synthesis of TMP from dUMP that is coupled to the
conversion of serine into glycine.
See answer
6.
Sulfa action. Bacterial growth is inhibited by sulfanilamide and related sulfa drugs, and there is a concomitant
accumulation of 5-aminoimidazole-4-carboxamide ribonucleotide. This inhibition is reversed by the addition of p-
aminobenzoate.
Propose a mechanism for the inhibitory effect of sulfanilamide.
See answer
7.
A generous donor. What major biosynthetic reactions utilize PRPP?
See answer
8.
HAT medium. Mutant cells unable to synthesize nucleotides by salvage pathways are very useful tools in molecular
and cell biology. Suppose that cell A lacks thymidine kinase, the enzyme catalyzing the phosphorylation of
thymidine to thymidylate, and that cell B lacks hypoxanthine-guanine phosphoribosyl transferase.
(a) Cell A and cell B do not proliferate in a HAT medium containing hypoxanthine, aminopterin or amethopterin
(methotrexate), and thymine. However, cell C formed by the fusion of cells A and B grows in this medium. Why?
(b) Suppose that you wanted to introduce foreign genes into cell A. Devise a simple means of distinguishing between
cells that have taken up foreign DNA and those that have not.
See answer

9.
Adjunct therapy. Allopurinol is sometimes given to patients with acute leukemia who are being treated with
anticancer drugs. Why is allopurinol used?
See answer
10.
A hobbled enzyme. Both side-chain oxygen atoms of aspartate 27 at the active site of dihydrofolate reductase form
hydrogen bonds with the pteridine ring of folates. The importance of this interaction was assessed by studying two
mutants at this position, Asn 27 and Ser 27. The dissociation constant of methotrexate was 0.07 nM for the wild
type, 1.9 nM for the Asn 27 mutant, and 210 nM for the Ser 27 mutant, at 25°C. Calculate the standard free energy
of binding of methotrexate by these three proteins. What is the decrease in binding energy resulting from each
mutation?
See answer
11.
Correcting deficiencies. Suppose that a person is found who is deficient in an enzyme required for IMP synthesis.
How might this person be treated?
See answer
12.
Labeled nitrogen. Purine biosynthesis is allowed to take place in the presence of [
15
N]aspartate, and the newly
synthesized GTP and ATP are isolated. What positions are labeled in the two nucleotides?
See answer
13.
Changed inhibitor. Xanthine oxidase treated with allopurinol results in the formation of a new compound that is an
extremely potent inhibitor of the enzyme. Propose a structure for this compound.
See answer
Mechanism Problems
14.
The same but not the same. Write out mechanisms for the conversion of phosphoribosylamine into glycinamide
ribonucleotide and of xanthylate into guanylate.
See answer
15.
Closing the ring. Propose a mechanism for the conversion of 5-formamidoimidazole-4-carboxamide ribonucleotide
into inosinate.
See answer
Chapter Integration Problems

16.
They're everywhere! Nucleotides play a variety of roles in the cell. Give an example of a nucleotide that acts in
each of the following roles or processes.
(a) Second messenger
(b) Phosphoryl-group transfer
(c) Activation of carbohydrates
(d) Activation of acetyl groups
(e) Transfer of electrons
(f) DNA sequencing
(g) Chemotherapy
(h) Allosteric effector
See answer
17.
Pernicious anemia. Purine biosynthesis is impaired by vitamin B
12
deficiency. Why? How might fatty acid and
amino acid metabolism also be affected by a vitamin B
12
deficiency?
See answer
18.
Hyperuricemia. Many patients with glucose 6-phosphatase deficiency have high serum levels of urate.
Hyperuricemia can be induced in normal people by the ingestion of alcohol or by strenuous exercise. Propose a
common mechanism that accounts for these findings.
See answer
19.
Labeled carbon. Succinate uniformly labeled with
14
C is added to cells actively engaged in pyrimidine
biosynthesis. Propose a mechanism by which carbon atoms from succinate could be incorporated into a pyrimidine.
At what positions is the pyrimidine labeled?
See answer
20.
Exercising muscle. Some interesting reactions take place in muscle tissue to facilitate the generation of ATP for
contraction.
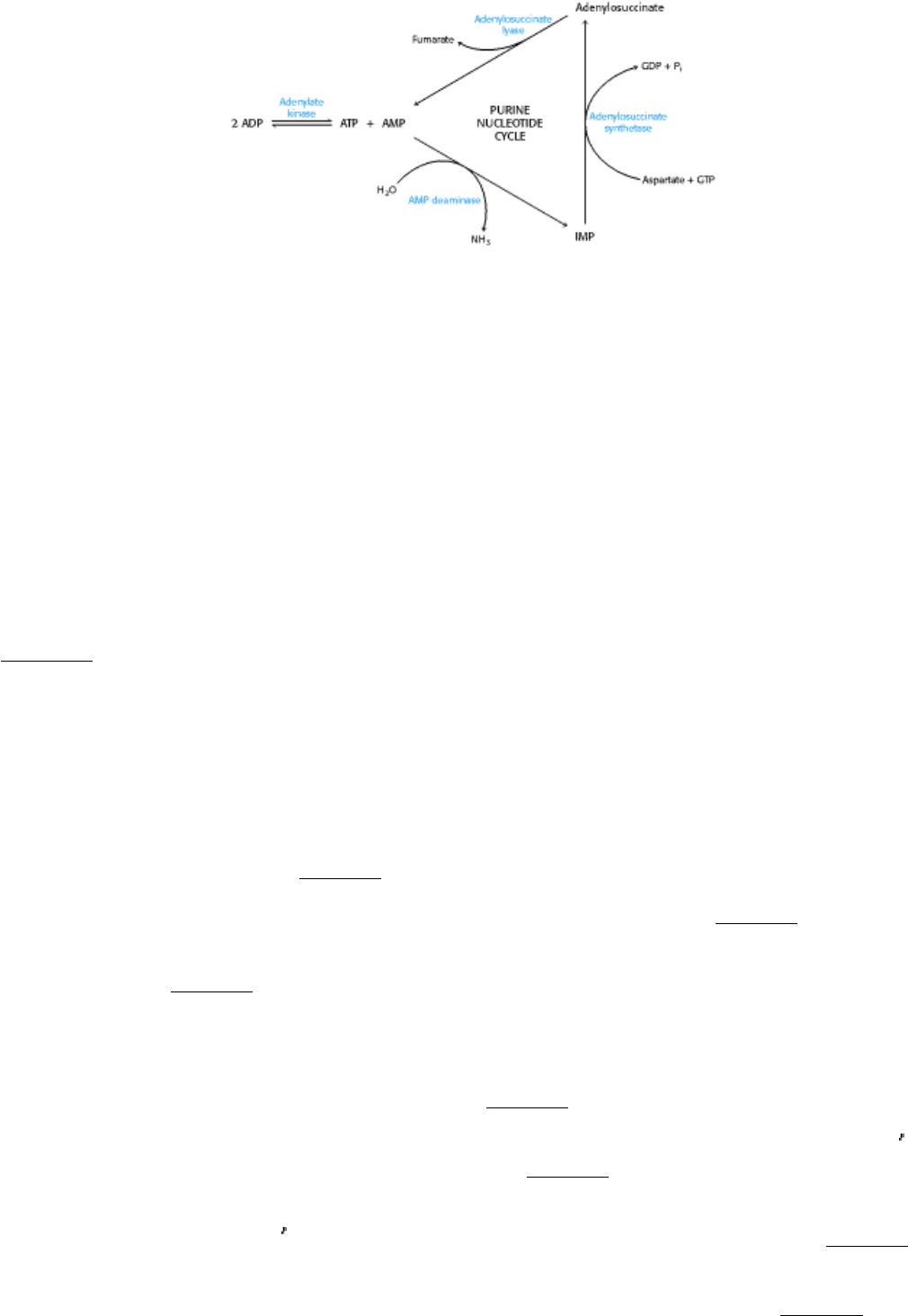
In muscle contraction, ATP is converted into ADP. Adenylate kinase converts two molecules of ADP into a
molecule of ATP and AMP.
(a) Why is this reaction beneficial to contracting muscle?
(b) Why is the equilibrium for the adenylate kinase approximately equal to 1?
Muscle can metabolize AMP by using the purine nucleotide cycle. The initial step in this cycle, catalyzed by AMP
deaminase, is the conversion of AMP into IMP.
(c) Why might the deamination of AMP facilitate ATP formation in muscle?
(d) How does the purine nucleotide cycle assist the aerobic generation of ATP?
See answer
III. Synthesizing the Molecules of Life 25. Nucleotide Biosynthesis
Selected Readings
Where to start
M.Y. Galperin and E.V. Koonin. 1997. A diverse superfamily of enzymes with ATP-dependent carboxylate-amine/thiol
ligase activity Protein Sci. 6: 2639-2643. (PubMed)
A. Jordan and P. Reichard. 1998. Ribonucleotide reductases Annu. Rev. Biochem. 67: 71-98. (PubMed)
J.E. Seegmiller. 1989. Contributions of Lesch-Nyhan syndrome to the understanding of purine metabolism J. Inherited
Metab. Dis. 12: 184-196. (PubMed)
Pyrimidine biosynthesis
F.M. Raushel, J.B. Thoden, G.D. Reinhart, and H.M. Holden. 1998. Carbamoyl phosphate synthetase: A crooked path
from substrates to products Curr. Opin. Chem. Biol. 2: 624-632. (PubMed)
T.P. Begley, T.C. Appleby, and S.E. Ealick. 2000. The structural basis for the remarkable proficiency of orotidine 5
-
monophosphate decarboxylase Curr. Opin. Struct. Biol. 10: 711-718. (PubMed)
T.W. Traut and B.R. Temple. 2000. The chemistry of the reaction determines the invariant amino acids during the
evolution and divergence of orotidine 5 -monophosphate decarboxylase J. Biol. Chem. 275: 28675-28681. (PubMed)
L. Lee, R.E. Kelly, S.C. Pastra-Landis, and D.R. Evans. 1985. Oligomeric structure of the multifunctional protein CAD
that initiates pyrimidine biosynthesis in mammalian cells Proc. Natl. Acad. Sci. USA 82: 6802-6806. (PubMed)
