Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

Purine biosynthesis
J.B. Thoden, S. Firestine, A. Nixon, S.J. Benkovic, and H.M. Holden. 2000. Molecular structure of Escherichia coli
PurT-encoded glycinamide ribonucleotide transformylase Biochemistry 39: 8791-8802. (PubMed)
F.M. McMillan, M. Cahoon, A. White, L. Hedstrom, G.A. Petsko, and D. Ringe. 2000. Crystal structure at 2.4 Å
resolution of Borrelia burgdorferi inosine 5
-monophosphate dehydrogenase: Evidence of a substrate-induced hinged-lid
motion by loop 6 Biochemistry 39: 4533-4542. (PubMed)
E.J. Mueller, S. Oh, E. Kavalerchik, T.J. Kappock, E. Meyer, C. Li, S.E. Ealick, and J. Stubbe. 1999. Investigation of the
ATP binding site of Escherichia coli aminoimidazole ribonucleotide synthetase using affinity labeling and site-directed
mutagenesis Biochemistry 38: 9831-9839. (PubMed)
V.M. Levdikov, V.V. Barynin, A.I. Grebenko, W.R. Melik-Adamyan, V.S. Lamzin, and K.S. Wilson. 1998. The
structure of SAICAR synthase: An enzyme in the de novo pathway of purine nucleotide biosynthesis Structure 6: 363-
376. (PubMed)
J.L. Smith, E.J. Zaluzec, J.P. Wery, L. Niu, R.L. Switzer, H. Zalkin, and Y. Satow. 1994. Structure of the allosteric
regulatory enzyme of purine biosynthesis Science 264: 1427-1433. (PubMed)
G. Weber, M. Nagai, Y. Natsumeda, S. Ichikawa, H. Nakamura, J.N. Eble, H.N. Jayaram, W.N. Zhen, E. Paulik, and R.
Hoffman. 1991. Regulation of de novo and salvage pathways in chemo-therapy Adv. Enzyme Regul. 31: 45-67.
(PubMed)
Ribonucleotide reductases
P. Reichard. 1997. The evolution of ribonucleotide reduction Trends Biochem. Sci. 22: 81-85. (PubMed)
J. Stubbe. 2000. Ribonucleotide reductases: The link between an RNA and a DNA world? Curr. Opin. Struct. Biol. 10:
731-736. (PubMed)
D.T. Logan, J. Andersson, B.M. Sjoberg, and P. Nordlund. 1999. A glycyl radical site in the crystal structure of a class
III ribonucleotide reductase Science 283: 1499-1504. (PubMed)
A. Tauer and S.A. Benner. 1997. The B
12
-dependent ribonucleotide reductase from the archaebacterium Thermoplasma
acidophila: An evolutionary solution to the ribonucleotide reductase conundrum Proc. Natl. Acad. Sci. USA 94: 53-58.
(PubMed) (Full Text in PMC)
A. Jordan, E. Torrents, C. Jeanthon, R. Eliasson, U. Hellman, C. Wernstedt, J. Barbe, I. Gibert, and P. Reichard. 1997.
B
12
-dependent ribonucleotide reductases from deeply rooted eubacteria are structurally related to the aerobic enzyme
from Escherichia coli Proc. Natl. Acad. Sci. USA 94: 13487-13492. (PubMed) (Full Text in PMC)
J. Stubbe and P. Riggs-Gelasco. 1998. Harnessing free radicals: Formation and function of the tyrosyl radical in
ribonucleotide reductase Trends Biochem. Sci. 23: 438-443. (PubMed)
J.A. Stubbe. 1989. Protein radical involvement in biological catalysis? Annu. Rev. Biochem 58: 257-285. (PubMed)
Thymidylate synthase and dihydrofolate reductase
R. Li, R. Sirawaraporn, P. Chitnumsub, W. Sirawaraporn, J. Wooden, F. Athappilly, S. Turley, and W.G. Hol. 2000.
Three-dimensional structure of M. tuberculosis dihydrofolate reductase reveals opportunities for the design of novel
tuberculosis drugs J. Mol. Biol. 295: 307-323. (PubMed)
P.H. Liang and K.S. Anderson. 1998. Substrate channeling and domain-domain interactions in bifunctional thymidylate
synthase-dihydrofolate reductase Biochemistry 37: 12195-12205. (PubMed)
G.P. Miller and S.J. Benkovic. 1998. Stretching exercises: Flexibility in dihydrofolate reductase catalysis Chem. Biol. 5:
R105-R113. (PubMed)
R.L. Blakley. 1995. Eukaryotic dihydrofolate reductase Adv. Enzymol. Relat. Areas Mol. Biol. 70: 23-102. (PubMed)
C.W. Carreras and D.V. Santi. 1995. The catalytic mechanism and structure of thymidylate synthase Annu. Rev.
Biochem. 64: 721-762. (PubMed)
B.I. Schweitzer, A.P. Dicker, and J.R. Bertino. 1990. Dihydrofolate reductase as a therapeutic target FASEB J. 4: 2441-
2452. (PubMed)
K.A. Brown and J. Kraut. 1992. Exploring the molecular mechanism of dihydrofolate reductase Faraday Discuss. 1992:
217-224.
C. Bystroff, S.J. Oatley, and J. Kraut. 1990. Crystal structures of Escherichia coli dihydrofolate reductase: The NADP
+
holoenzyme and the folate NADP
+
ternary complex Substrate binding and a model for the transition state
Biochemistry 29: 3263-3277. (PubMed)
Genetic diseases
Striver, C. R., Beaudet, A. L., Sly, W. S., Valle, D., Stanbury, J. B., Wyngaarden, J. B., and Fredrickson, D. S. (Eds.),
1995. The Metabolic Basis of Inherited Diseases (7th ed., pp. 1655
1840). McGraw-Hill.
W.L. Nyhan. 1997. The recognition of Lesch-Nyhan syndrome as an inborn error of purine metabolism J. Inherited
Metab. Dis. 20: 171-178. (PubMed)
D.F. Wong, J.C. Harris, S. Naidu, F. Yokoi, S. Marenco, R.F. Dannals, H.T. Ravert, M. Yaster, A. Evans, O. Rousset, R.
N. Bryan, A. Gjedde, M.J. Kuhar, and G.R. Breese. 1996. Dopamine transporters are markedly reduced in Lesch-Nyhan
disease in vivo Proc. Natl. Acad. Sci. USA 93: 5539-5543. (PubMed) (Full Text in PMC)
R. Resta and L.F. Thompson. 1997. SCID: The role of adenosine deaminase deficiency Immunol. Today 18: 371-374.
(PubMed)
B.L. Davidson, M. Pashmforoush, W.N. Kelley, and T.D. Palella. 1989. Human hypoxanthine-guanine
phosphoribosyltransferase deficiency: The molecular defect in a patient with gout (HPRTAshville) J. Biol. Chem. 264:
520-525. (PubMed)
D.G. Sculley, P.A. Dawson, B.T. Emerson, and R.B. Gordon. 1992. A review of the molecular basis of hypoxanthine-
guanine phosphoribosyltransferase (HPRT) deficiency Hum. Genet. 90: 195-207. (PubMed)
III. Synthesizing the Molecules of Life
26. The Biosynthesis of Membrane Lipids and Steroids
This chapter examines the biosynthesis of three important components of biological membranes
phospholipids,
sphingolipids, and cholesterol (Chapter 12). Triacylglycerols also are considered here because the pathway for their
synthesis overlaps that of phospholipids. Cholesterol is of interest both as a membrane component and as a precursor of
many signal molecules, including the steroid hormones progesterone, testosterone, estrogen, and cortisol. The
biosynthesis of cholesterol exemplifies a fundamental mechanism for the assembly of extended carbon skeletons from
five-carbon units.
The transport of cholesterol in blood by the low-density lipoprotein and its uptake by a specific receptor on the cell
surface vividly illustrate a recurring mechanism for the entry of metabolites and signal molecules into cells. The absence
of this receptor in people with familial hypercholesterolemia, a genetic disease, leads to markedly elevated cholesterol
levels in the blood, cholesterol deposits on blood vessels, and childhood heart attacks. Indeed, cholesterol is implicated
in the development of atherosclerosis in individuals without genetic defects. Thus, the regulation of cholesterol synthesis
and transport can be a source of especially clear insight into the role that our understanding of biochemistry plays in
medicine.
III. Synthesizing the Molecules of Life 26. The Biosynthesis of Membrane Lipids and Steroids
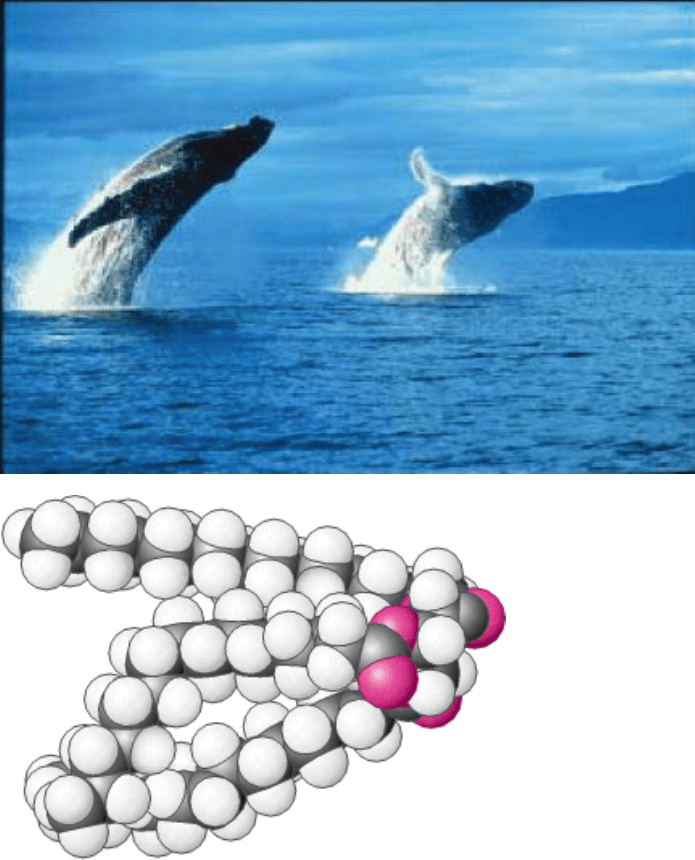
Fats such as the triacylglycerol molecule (below) are widely used to store excess energy for later use and to fulfill
other purposes, illustrated by the insulating blubber of whales. The natural tendency of fats to exist in nearly water-
free forms makes these molecules well-suited for these roles. [(Left) François Cohier/Photo Researchers.]
III. Synthesizing the Molecules of Life 26. The Biosynthesis of Membrane Lipids and Steroids
26.1. Phosphatidate Is a Common Intermediate in the Synthesis of Phospholipids and
Triacylglycerols
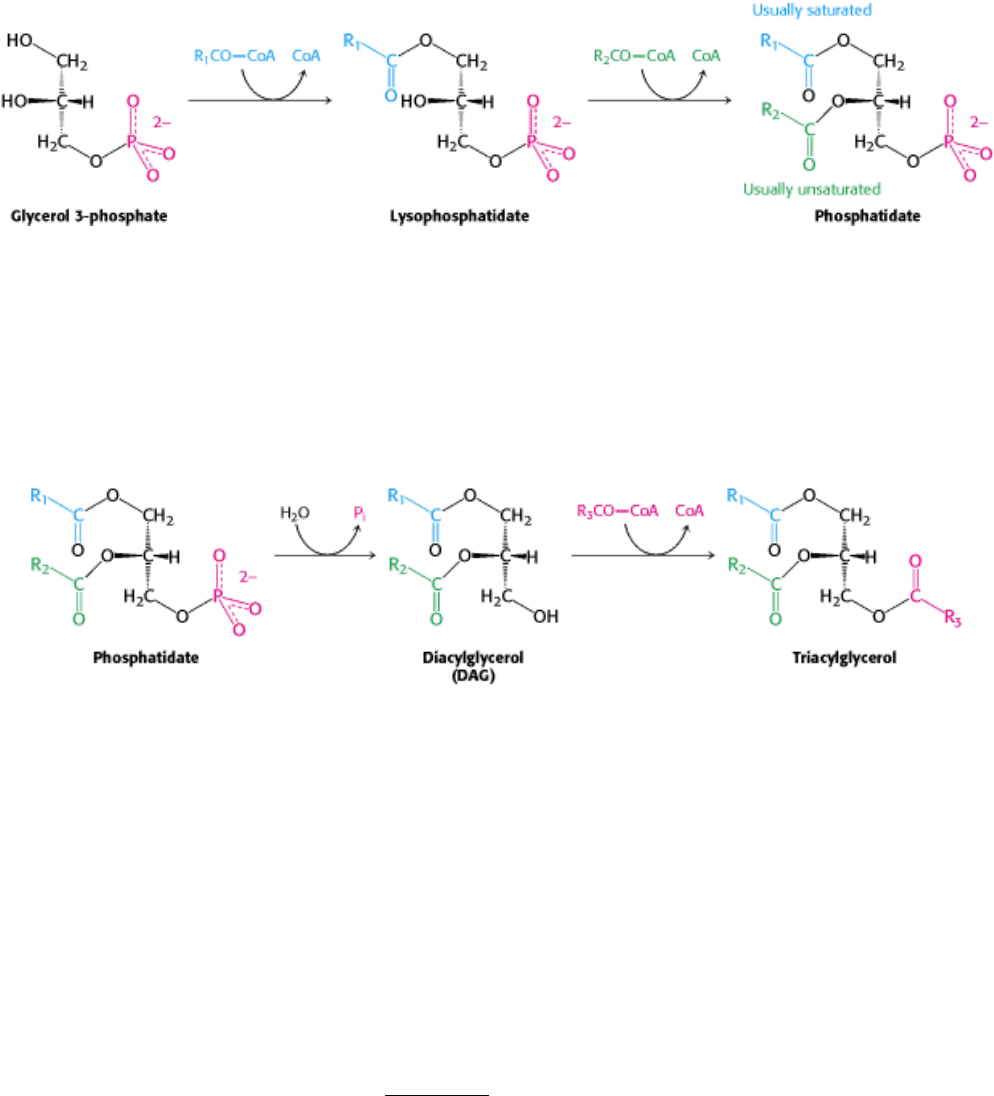
The first step in the synthesis of both phospholipids for membranes and triacylglycerols for energy storage is the
synthesis of phosphatidate (diacylglycerol 3-phosphate). In mammalian cells, phosphatidate is synthesized in the
endoplasmic reticulum and the outer mitochondrial membrane. It is formed by the addition of two fatty acids to glycerol
3-phosphate, which in turn is formed primarily by the reduction of dihydroxyacetone phosphate, a glycolytic
intermediate, and to a lesser extent by the phosphorylation of glycerol. Glycerol 3-phosphate is acylated by acyl CoA to
form lysophosphatidate, which is again acylated by acyl CoA to yield phosphatidate.
These acylations are catalyzed by glycerol phosphate acyltransferase. In most phosphatidates, the fatty acyl chain
attached to the C-1 atom is saturated, whereas the one attached to the C-2 atom is unsaturated.
The pathways diverge at phosphatidate. In the synthesis of triacylglycerols, phosphatidate is hydrolyzed by a specific
phosphatase to give a diacylglycerol (DAG). This intermediate is acylated to a triacylglycerol in a reaction that is
catalyzed by diglyceride acyltransferase. Both enzymes are associated in a triacylglycerol synthetase complex that is
bound to the endoplasmic reticulum membrane.
The liver is the primary site of triacylglycerol synthesis. From the liver, the triacylglycerols are transported to the
muscles for energy conversion or to the adipocytes for storage.
26.1.1. The Synthesis of Phospholipids Requires an Activated Intermediate
Phospholipid synthesis requires the combination of a diacylglyceride with an alcohol. As in most anabolic reactions, one
of the components must be activated. In this case, either of the two components may be activated, depending on the
source of the reactants.
Synthesis from an Activated Diacylglycerol.
The de novo pathway starts with the reaction of phosphatidate with cytidine triphosphate (CTP) to form cytidine
diphosphodiacylglycerol (CDP-diacylglycerol) (Figure 26.1). This reaction, like those of many biosyntheses, is driven
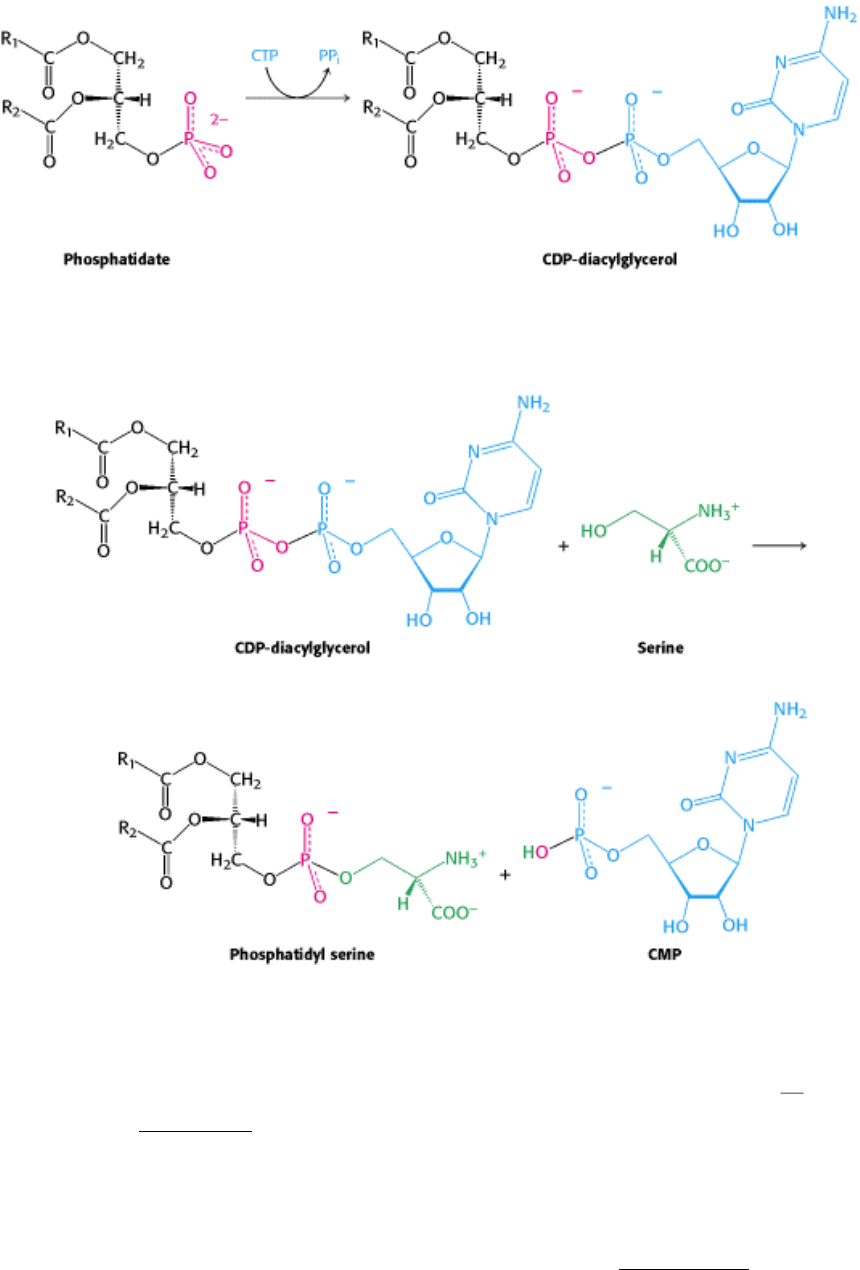
forward by the hydrolysis of pyrophosphate.
The activated phosphatidyl unit then reacts with the hydroxyl group of an alcohol to form a phosphodiester linkage. If
the alcohol is serine, the products are phosphatidyl serine and cytidine monophosphate (CMP).
Likewise, phosphatidyl inositol is formed by the transfer of a diacylglycerol phosphate unit from CDP-diacylglycerol to
inositol. Subsequent phosphorylations catalyzed by specific kinases lead to the synthesis of phosphatidyl inositol 4,5-
bisphosphate, an important molecule in signal transduction. Recall that hormonal and sensory stimuli activate
phospholipase C, an enzyme that hydrolyzes this phospholipid to form two intracellular messengers
diacylglycerol and
inositol 1,4,5-trisphosphate (Section 15.2).
The fatty acid components of a phospholipid may vary, and thus phosphatidyl serine, as well as most other
phospholipids, represents a class of molecules rather than a single species. As a result, a single mammalian cell may
contain thousands of distinct phospholipids. Phosphatidyl inositol is unusual in that it has a nearly fixed fatty acid
composition. Stearic acid usually occupies the C-1 position and arachidonic acid (Section 22.6.2) the C-2 position.
In bacteria, the decarboxylation of phosphatidyl serine by a pyridoxal phosphate-dependent enzyme yields phosphatidyl
ethanolamine, another common phospholipid. The amino group of this phosphoglyceride is then methylated three times
to form phosphatidyl choline. S-Adenosylmethionine is the methyl donor.
In mammals, phosphatidyl ethanolamine can be formed from phosphatidyl serine by the enzyme-catalyzed exchange of
ethanolamine for the serine moiety of the phospholipid.
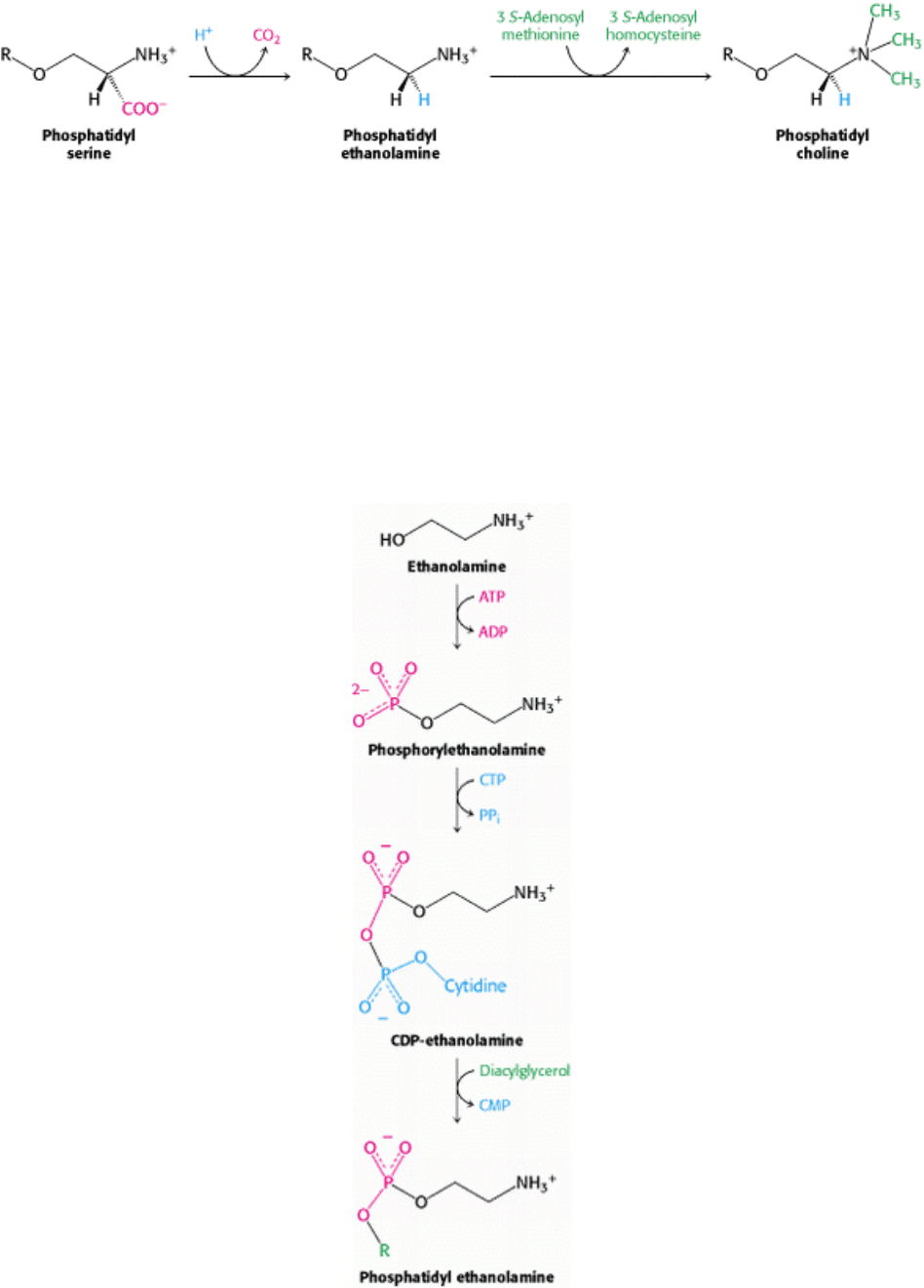
Synthesis from an Activated Alcohol.
In mammals, phosphatidyl ethanolamine can also be synthesized from ethanolamine through the formation of CDP-
ethanolamine. In this case, the alcohol ethanolamine is phosphorylated by ATP to form the precursor,
phosphorylethanolamine. This precursor then reacts with CTP to form the activated alcohol, CDP-ethanolamine. The
phosphorylethanolamine unit of CDP-ethanolamine is then transferred to a diacylglycerol to form phosphatidyl
ethanolamine.
In mammals, a pathway that utilizes choline obtained from the diet ends in the synthesis of phosphatidyl choline, the
most common phospholipid in these organisms. In this case, choline is activated in a series of reactions analogous to
those in the activation of ethanolamine. Interestingly, the liver possesses an enzyme, phosphatidyl ethanolamine
methyltransferase, that synthesizes phosphatidyl choline from phosphatidyl ethanolamine, through the successive
methylation of ethanolamine. Thus, phosphatidyl choline can be produced by two distinct pathways, ensuring that this
phospholipid can be synthesized even if the components for one pathway are in limited supply.
Note that a cytidine nucleotide plays the same role in the synthesis of these phosphoglycerides as a uridine nucleotide
does in the formation of glycogen (Section 21.4.1). In all of these biosyntheses, an activated intermediate (UDP-glucose,
CDP-diacylglycerol, or CDP-alcohol) is formed from a phosphorylated substrate (glucose 1-phosphate, phosphatidate, or
a phosphorylalcohol) and a nucleoside triphosphate (UTP or CTP). The activated intermediate then reacts with a
hydroxyl group (the terminus of glycogen, the side chain of serine, or a diacylglycerol).
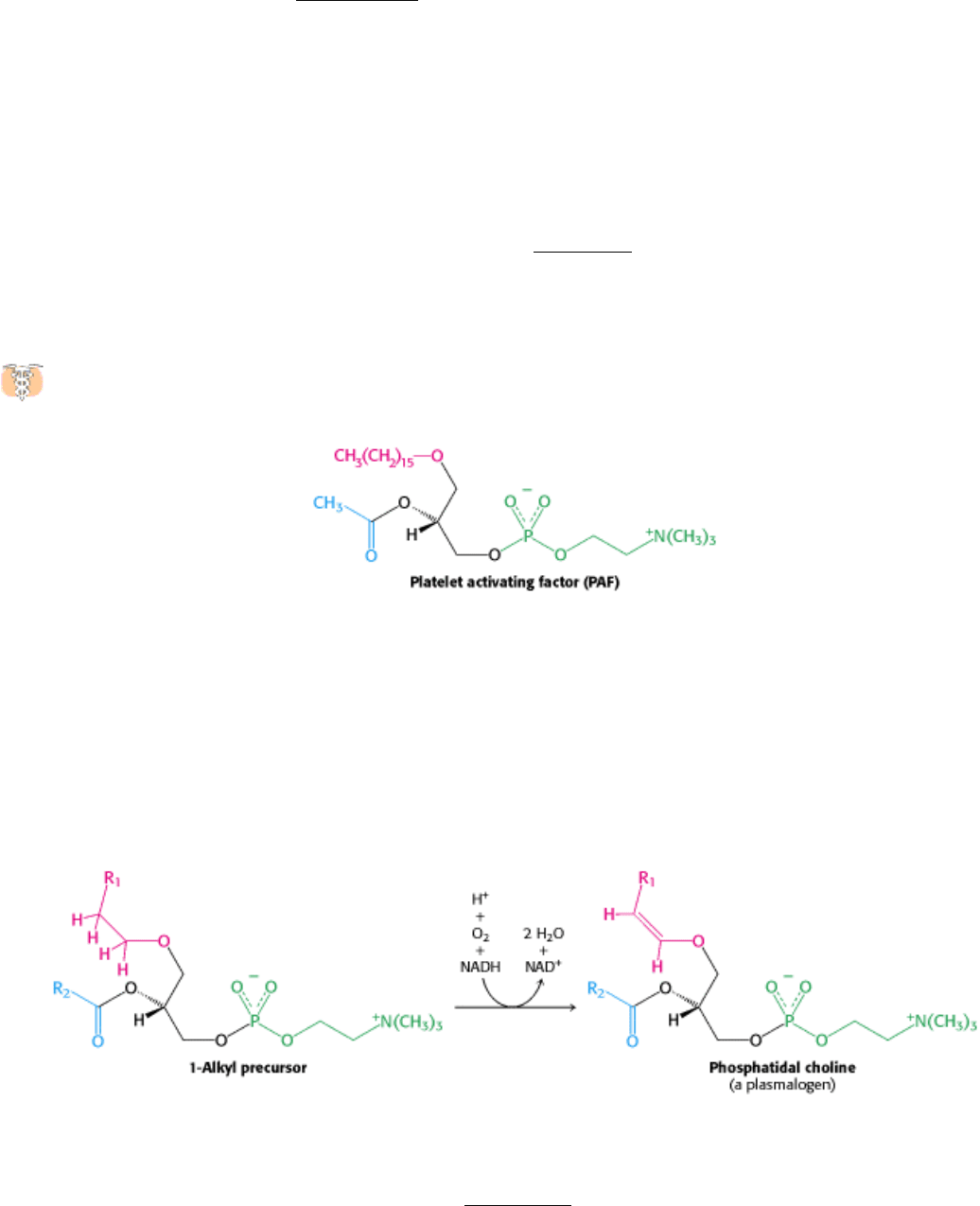
26.1.2. Plasmalogens and Other Ether Phospholipids Are Synthesized from
Dihydroxyacetone Phosphate
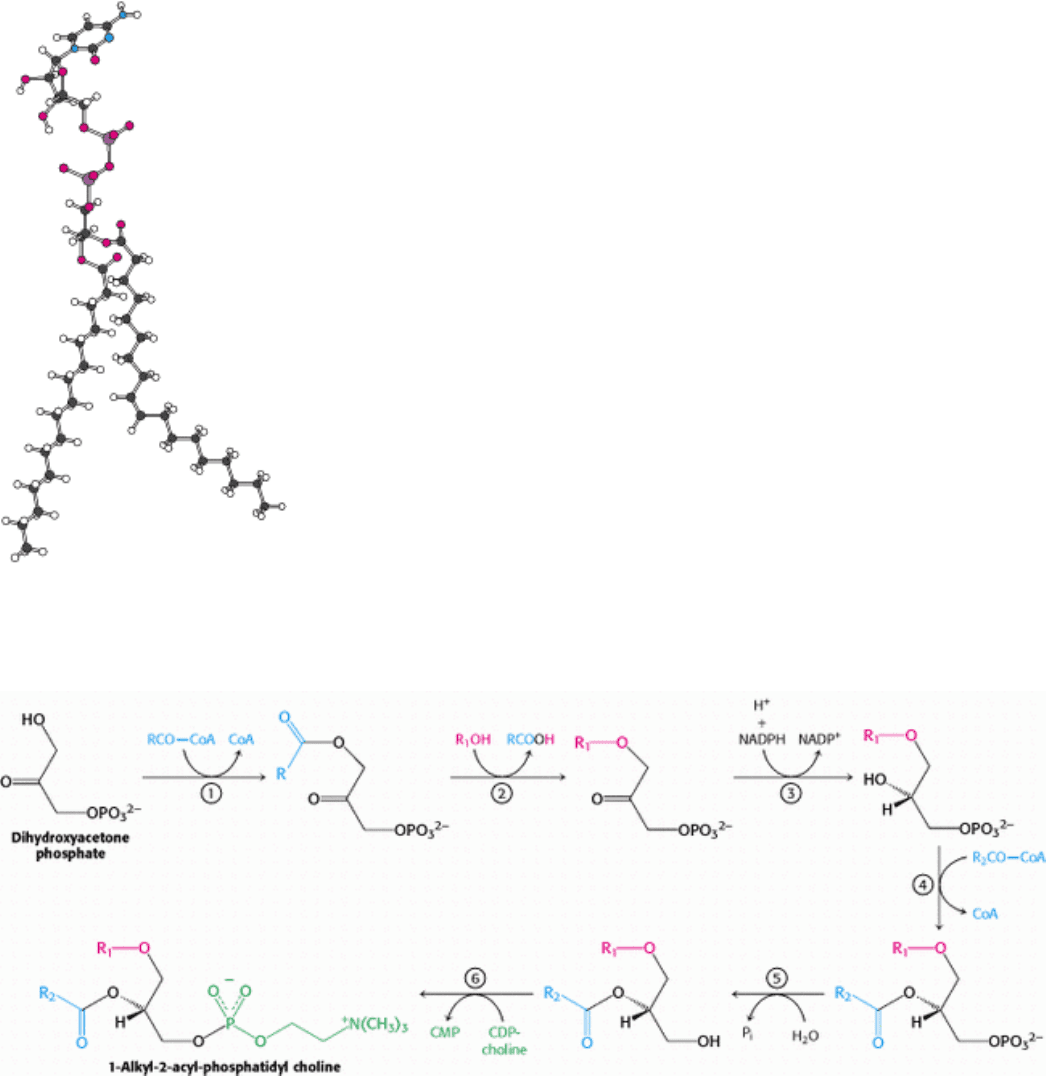
Glyceryl ether phospholipids contain an ether unit instead of an acyl unit at C-1 and are synthesized starting with
dihydroxyacetone phosphate rather than glycerol 3-phosphate (Figure 26.2). Acylation by a fatty acyl CoA yields a 1-
acyl derivative that exchanges with a long-chain alcohol to form an ether at C-1. NADPH reduces the keto group at C-2,
and the resulting alcohol is acylated by a long-chain acyl CoA. Removal of the 3-phosphate group yields 1-alkyl-2-
acylglycerol, which reacts with CDP-choline to form the ether analog of phosphatidyl choline.
Platelet-activating factor (PAF) is an ether phospholipid implicated in a number of allergic and inflammatory
responses.
Subnanomolar concentrations of this 1-alkyl-2-acetyl ether analog of phosphatidyl choline induce the aggregation of
blood platelets, smooth muscle contraction, and the activation of cells of the immune system. It is also a mediator of
anaphylactic shock, a severe and often fatal allergic response. The presence of an acetyl group rather than a long-chain
acyl group at C-2 increases the water solubility of this lipid, enabling it to function in the aqueous environment of the
blood. PAF functions through a 7-TM receptor.
Plasmalogens are phospholipids containing an α,β-unsaturated ether at C-1. Phosphatidal choline, the plasmalogen
corresponding to phosphatidyl choline, is formed by desaturation of a 1-alkyl precursor.
The desaturase catalyzing this final step in the synthesis of a plasmalogen is an endoplasmic reticulum enzyme akin to
the one that introduces double bonds into long-chain fatty acyl CoA molecules. In both cases, O
2
and NADH are
reactants, and cytochrome b
5
participates in catalysis (Section 22.6).
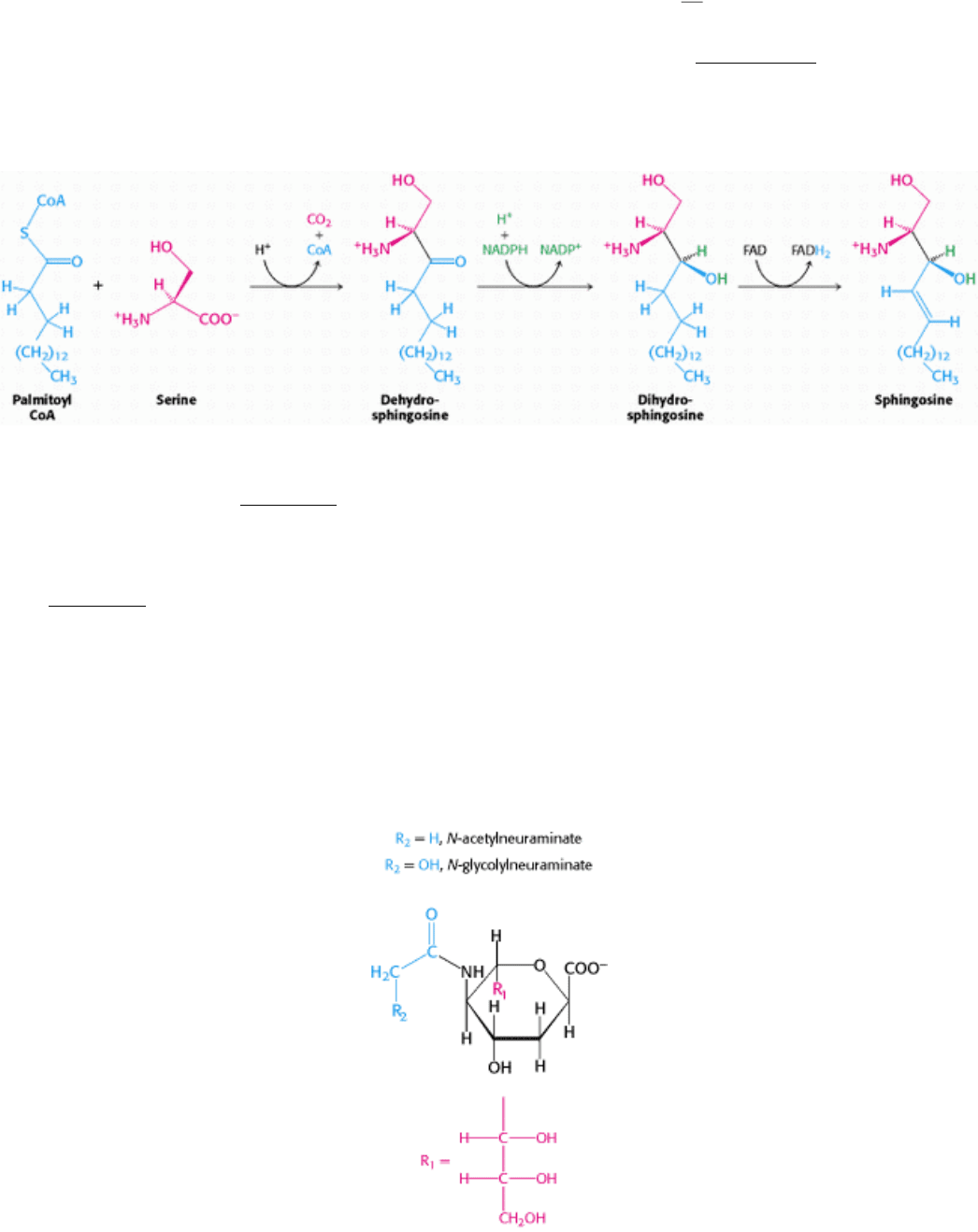
26.1.3. Sphingolipids Are Synthesized from Ceramide
We turn now from glycerol-based phospholipids to another class of membrane lipid
the sphingolipids. These lipids are
found in the plasma membranes of all eukaryotic cells, although the concentration is highest in the cells of the central
nervous system. The backbone of a sphingolipid is sphingosine, rather than glycerol (Section 12.3.1). Palmitoyl CoA and
serine condense to form dehydrosphingosine, which is then converted into sphingosine. The enzyme catalyzing this
reaction requires pyridoxal phosphate, revealing again the dominant role of this cofactor in transformations that include
amino acids.
In all sphingolipids, the amino group of sphingosine is acylated: a long-chain acyl CoA reacts with sphingosine to form
ceramide (N-acyl sphingosine) Figure 26.3). The terminal hydroxyl group also is substituted. In sphingomyelin, a
component of the myelin sheath covering many nerve fibers, the substituent is phosphorylcholine, which comes from
phosphatidyl choline. In a cerebroside, the substituent is glucose or galactose. UDP-glucose or UDP-galactose is the
sugar donor. In a ganglioside, an oligosaccharide is linked to the terminal hydroxyl group of ceramide by a glucose
residue (Figure 26.4).
26.1.4. Gangliosides Are Carbohydrate-Rich Sphingolipids That Contain Acidic Sugars
In gangliosides, the most complex sphingolipids, an oligosaccharide chain attached to the ceramide contains at least one
acidic sugar. The acidic sugar is N-acetylneuraminate or N-glycolylneuraminate. These acidic sugars are called sialic
acids. Their nine-carbon backbones are synthesized from phosphoenolpyruvate (a three-carbon unit) and N-
acetylmannosamine 6-phosphate (a six-carbon unit).
Gangliosides are synthesized by the ordered, step-by-step addition of sugar residues to ceramide. The synthesis of these
complex lipids requires the activated sugars UDP-glucose, UDP-galactose, and UDP-N-acetylgalactosamine, as well as
the CMP derivative of N-acetylneuraminate. CMP-N-acetylneuraminate is synthesized from CTP and N-acetylneur-
aminate. The structure of the resulting ganglioside is determined by the specificity of the glycosyltransferases in the cell.
More than 60 different gangliosides have been characterized (see Figure 26.4 for the structure of ganglioside G
M
1
).
26.1.5. Sphingolipids Confer Diversity on Lipid Structure and Function
The structures of sphingolipids and the more abundant glycerophospholipids are very similar. Given the structural
similarity of these two types of lipids, why are sphingolipids required at all? Indeed, the prefix "sphingo" was applied to
capture the "sphinxlike" properties of this enigmatic class of lipids. Although the precise role of sphingolipids is not
firmly established, progress toward solving the riddle of their function is being made. The most notable function
attributed to sphingolipids is their role as a source of second messengers. For instance, ceramide derived from a
sphingolipid may initiate programmed cell death in some cell types.
26.1.6. Respiratory Distress Syndrome and Tay-Sachs Disease Result from the
Disruption of Lipid Metabolism
Respiratory distress syndrome is a pathological condition resulting from a failure in the biosynthetic pathway for
dipalmitoyl phosphatidyl choline. This phospholipid, in conjunction with specific proteins and other
phospholipids, is found in the extracellular fluid that surrounds the alveoli of the lung, where it decreases the surface
tension of the fluid to prevent lung collapse at the end of the expiration phase of breathing. Premature infants may suffer
from respiratory distress syndrome because their immature lungs do not synthesize enough dipalmitoyl phosphatidyl
choline.
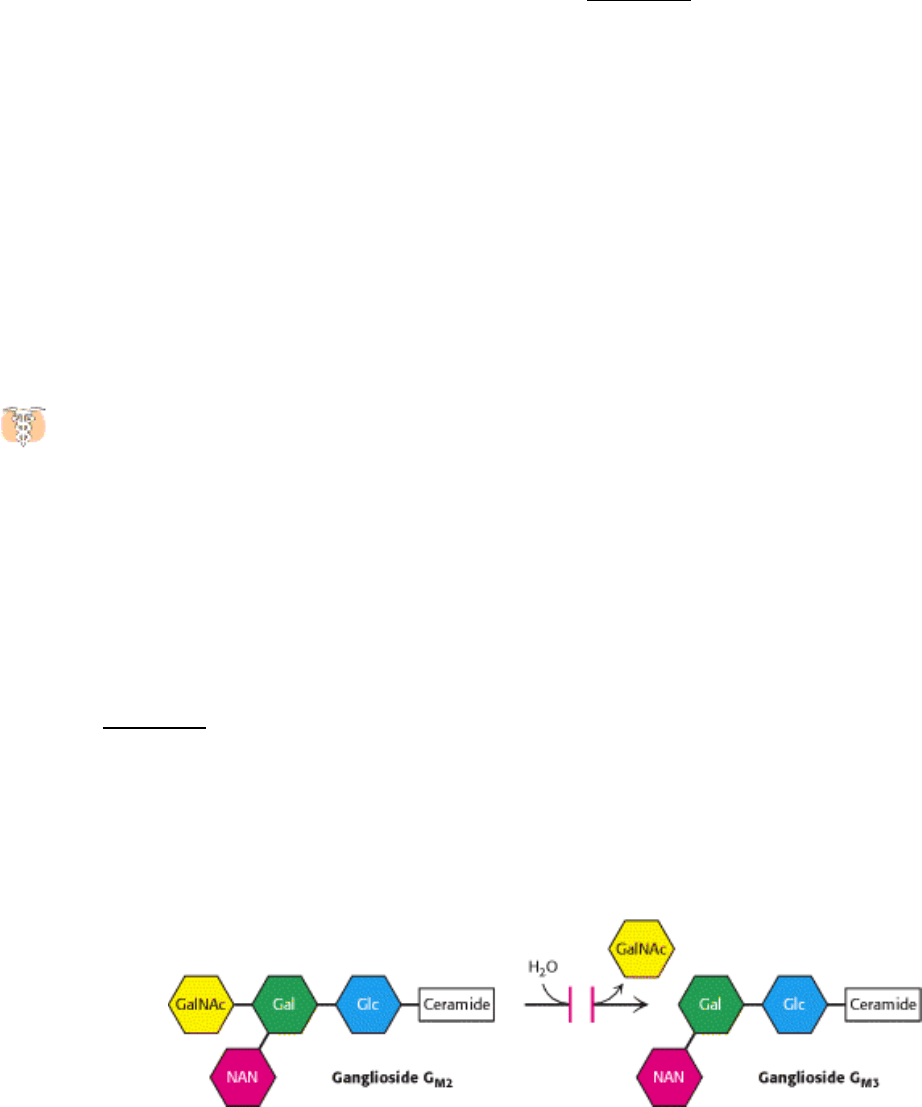
Tay-Sachs disease is caused by a failure of lipid degradation: an inability to degrade gangliosides. Gangliosides are
found in highest concentration in the nervous system, particularly in gray matter, where they constitute 6% of the lipids.
Gangliosides are normally degraded inside lysosomes by the sequential removal of their terminal sugars but, in Tay-
Sachs disease, this degradation does not occur. As a consequence, neurons become enormously swollen with lipid-filled
lysosomes (Figure 26.5). An affected infant displays weakness and retarded psychomotor skills before 1 year of age. The
child is demented and blind by age 2 and usually dead before age 3.
The ganglioside content of the brain of an infant with Tay-Sachs disease is greatly elevated. The concentration of
ganglioside G
M
2
is many times as high as normal because its terminal N-acetylgalactosamine residue is removed very
slowly or not at all. The missing or deficient enzyme is a specific β -N-acetylhexosaminidase.
Tay-Sachs disease can be diagnosed in the course of fetal development. Amniotic fluid is obtained by amniocentesis and
assayed for β-N-acetylhexosaminidase activity.
III. Synthesizing the Molecules of Life 26. The Biosynthesis of Membrane Lipids and Steroids 26.1. Phosphatidate Is a Common Intermediate in the Synthesis of Phospholipids and Triacylglycerols
Figure 26.1. Structure of CDP-Diacylglycerol. A key intermediate in the synthesis of phospholipids consists of
phosphatidate and CMP joined by a pyrophosphate linkage.
III. Synthesizing the Molecules of Life 26. The Biosynthesis of Membrane Lipids and Steroids 26.1. Phosphatidate Is a Common Intermediate in the Synthesis of Phospholipids and Triacylglycerols
Figure 26.2. Synthesis of an Ether Phospholipid. Steps in the synthesis include (1) acylation of dihydroxyacetone
phosphate by acyl CoA, (2) exchange of an alcohol for the carboxylic acid, (3) reduction by NADPH, (4) acylation by a
second acyl CoA, (5) hydrolysis of the phosphate ester, and (6) transfer of a phosphocholine moiety.
