Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

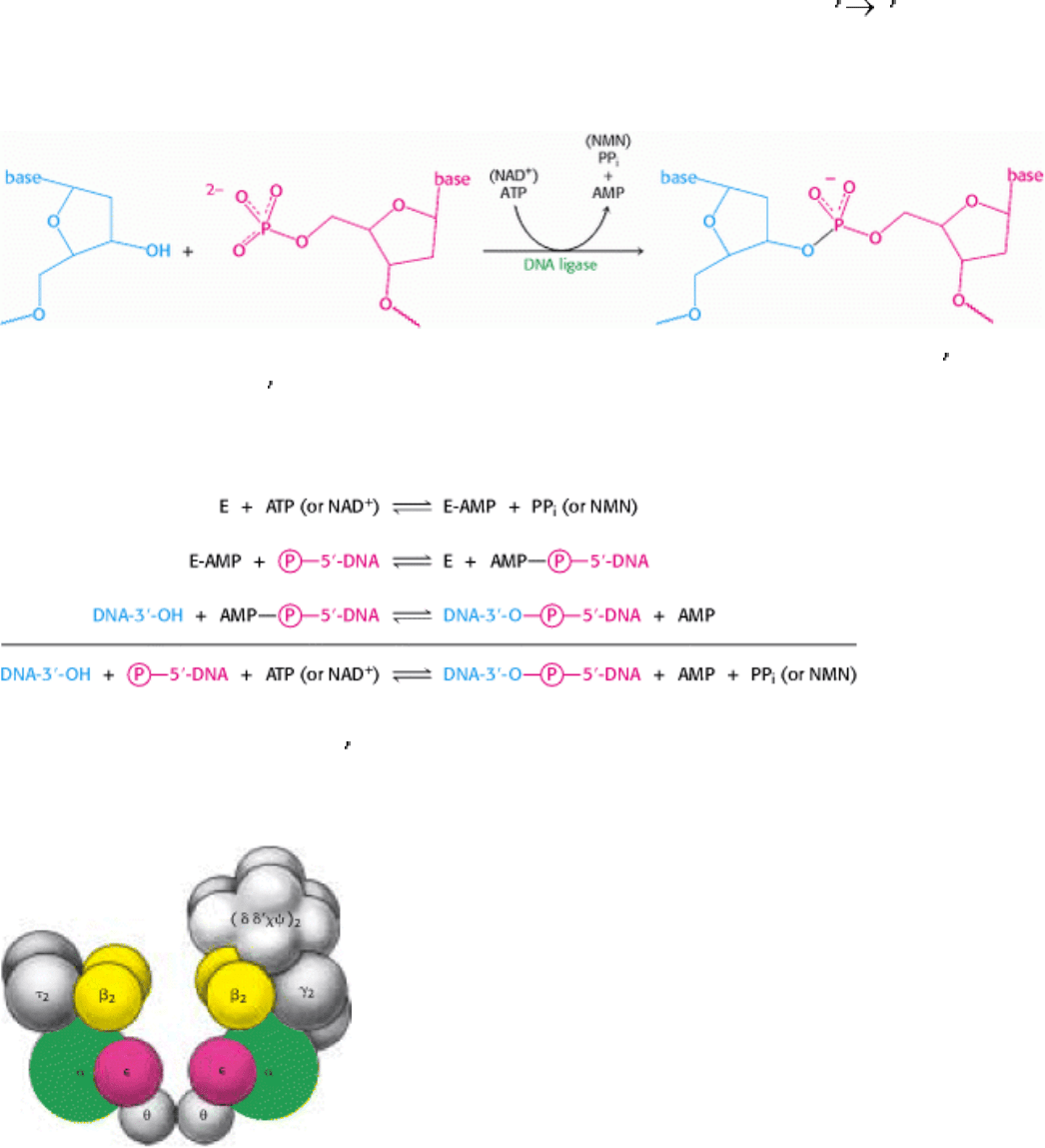
Figure 27.27. Okazaki Fragments. At a replication fork, both strands are synthesized in a 5 3 direction. The
leading strand is synthesized continuously, whereas the lagging strand is synthesized in short pieces termed Okazaki
fragments.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
Figure 27.28. DNA Ligase Reaction. DNA ligase catalyzes the joining of one DNA strand with a free 3
-hydroxyl
group to another with a free 5
-phosphate group. In eukaryotes and archaea, ATP is cleaved to AMP and PPi to drive this
reaction. In bacteria, NAD
+
is cleaved to AMP and nicotinamide mononucleotide (NMN).
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
Figure 27.29. DNA Ligase Mechanism. DNA ligation proceeds by the transfer of an AMP unit first to a lysine side
chain on DNA ligase and then to the 5
-phosphate group of the substrate. The AMP unit is released on formation of the
phosphodiester linkage in DNA.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
Figure 27.30. Proposed Architecture of DNA Polymerase III Holoenzyme. [After A. Kornberg and T. Baker, DNA
Replication, 2d ed. (W. H. Freeman and Company, 1992).]
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
Figure 27.31. Structure of the Sliding Clamp.
The dimeric β2 subunit of DNA polymerase III forms a ring that
surrounds the DNA duplex. It allows the polymerase enzyme to move without falling off the DNA substrate.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
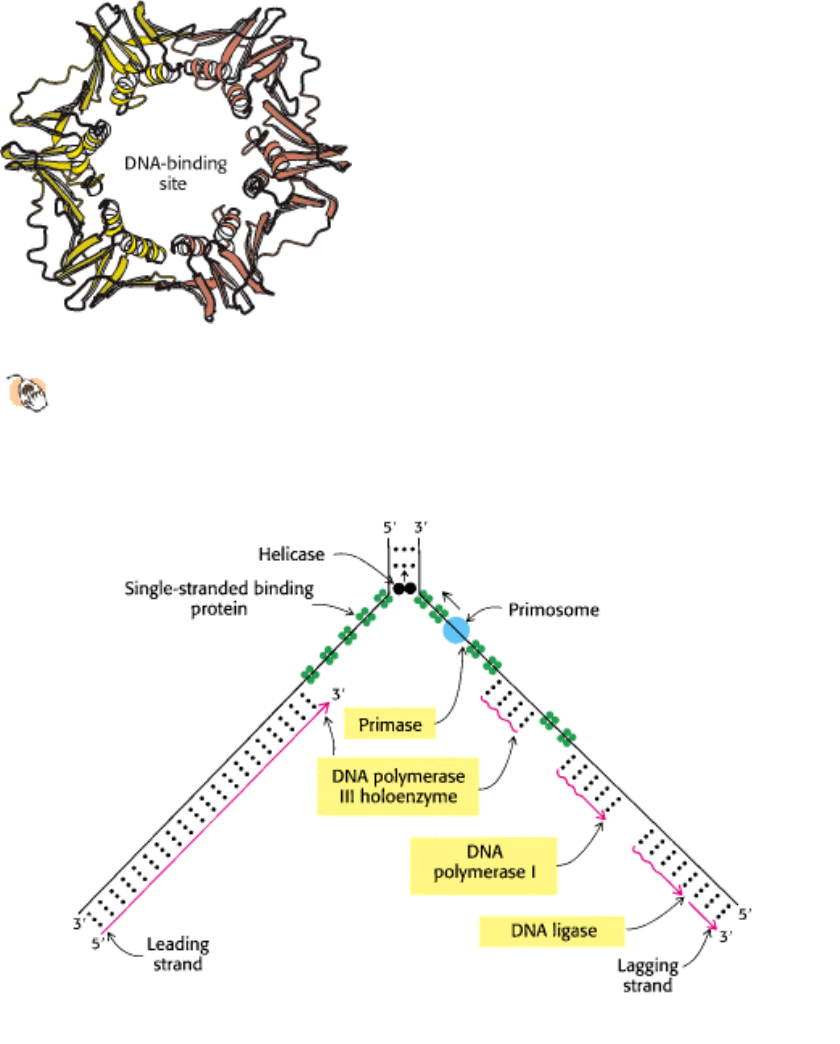
Figure 27.32. Replication Fork. Schematic representation of the enzymatic events at a replication fork in E. coli.
Enzymes shaded in yellow catalyze chain initiation, elongation, and ligation. The wavy lines on the lagging strand
denote RNA primers. [After A. Kornberg and T. Baker, DNA Replication, 2d ed. (W. H. Freeman and Company, 1992).]
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
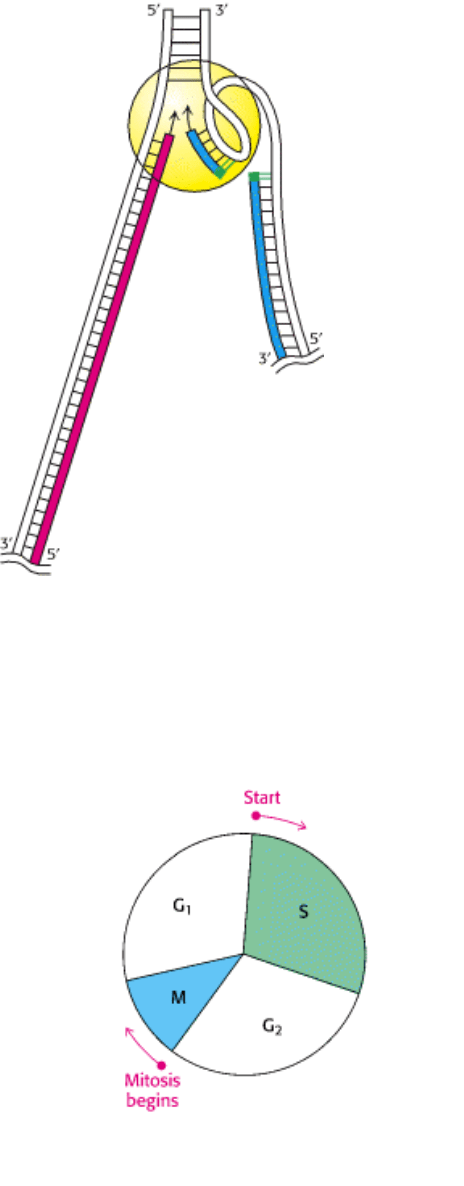
Figure 27.33. Coordination between the Leading and the Lagging Strands. The looping of the template for the
lagging strand enables a dimeric DNA polymerase III holoenzyme to synthesize both daughter strands. The leading
strand is shown in red, the lagging strand in blue, and the RNA primers in green. [Courtesy of Dr. Arthur Kornberg.]
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
Figure 27.34. Eukaryotic Cell Cycle. DNA replication and cell division must take place in a highly coordinated fashion
in eukaryotes. Mitosis (M) takes place only after DNA synthesis (S). Two gaps (G
1
and G
2
) in time separate the two
processes.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
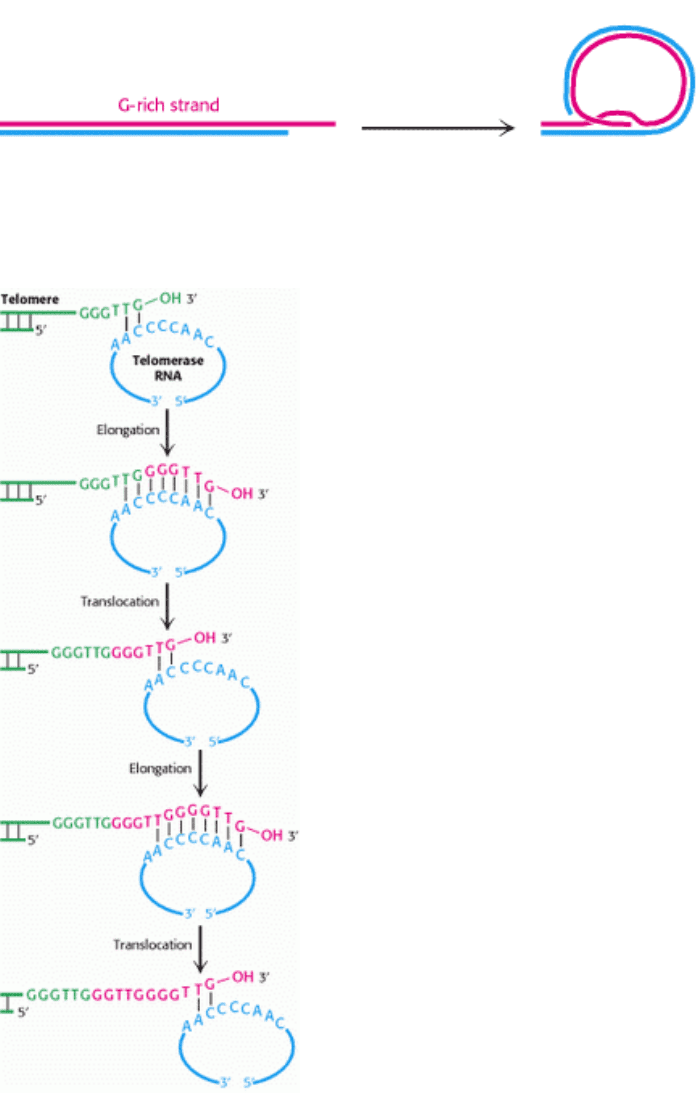
Figure 27.35. Proposed Model for Telomeres. A single-stranded segment of the G-rich strand extends from the end of
the telomere. In one model for telomeres, this single-stranded region invades the duplex to form a large duplex loop.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.4. DNA Replication of Both Strands Proceeds Rapidly from Specific Start Sites
Figure 27.36. Telomere Formation. Mechanism of synthesis of the G-rich strand of telomeric DNA. The RNA
template of telomerase is shown in blue and the nucleotides added to the G-rich strand of the primer are shown in red.
[After E. H. Blackburn. Nature 350(1991):569.]

III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair
27.5. Double-Stranded DNA Molecules with Similar Sequences Sometimes
Recombine
Most processes associated with DNA replication function to copy the genetic message as faithfully as possible.
However, several biochemical processes require the recombination of genetic material between two DNA molecules. In
genetic recombination, two daughter molecules are formed by the exchange of genetic material between two parent
molecules (Figure 27.37).
1. In meiosis, the limited exchange of genetic material between paired chromosomes provides a simple mechanism for
generating genetic diversity in a population.
2. As we shall see in Chapter 33, recombination plays a crucial role in generating molecular diversity for antibodies and
some other molecules in the immune system.
3. Some viruses utilize recombination pathways to integrate their genetic material into the DNA of the host cell.
4. Recombination is used to manipulate genes in, for example, the generation of "gene knockout" mice (Section 6.3.5).
Recombination is most efficient between DNA sequences that are similar in sequence. Such processes are often referred
to as homologous recombination reactions.
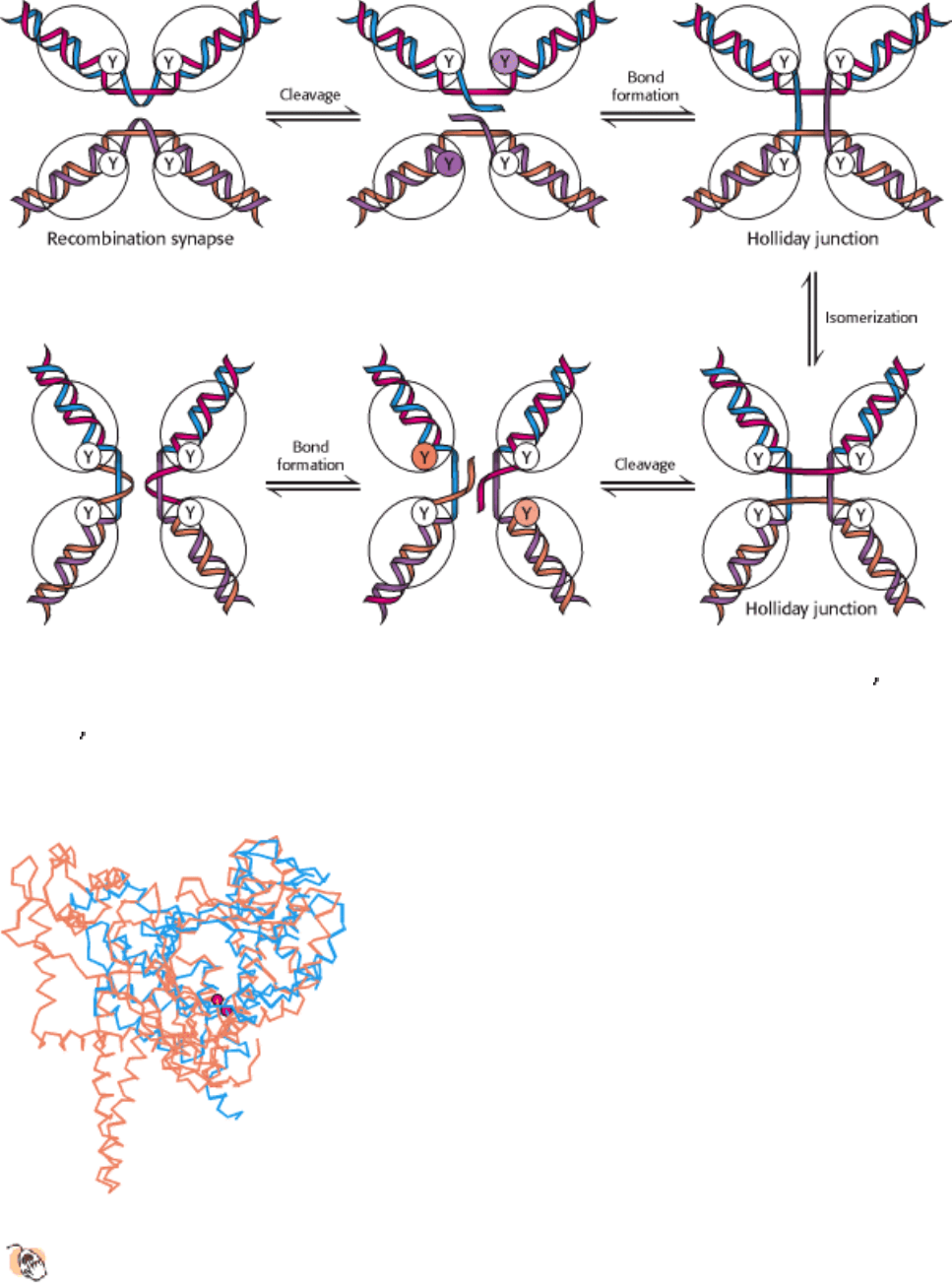
27.5.1. Recombination Reactions Proceed Through Holliday Junction Intermediates
The Structural Insights module for this chapter shows how a recombinase
forms a Holliday junction from two DNA duplexes and suggests how this
intermediate is resolved to produce recombinants.
Enzymes called recombinases catalyze the exchange of genetic material that takes place in recombination. By what
pathway do these enzymes catalyze this exchange? An appealing scheme was proposed by Robin Holliday in 1964. A
key intermediate in this mechanism is a crosslike structure, known as a Holliday junction, formed by four polynucleotide
chains. Such intermediates have been characterized by a wide range of techniques including x-ray crystallography
(Figure 27.38). Note that such intermediates can form only when the nucleotide sequences of the two parental duplexes
are very similar or identical in the region of recombination because specific base pairs must form between the bases of
the two parental duplexes.
How are such intermediates formed from the parental duplexes and resolved to form products? Many details for this
process are now available, based largely on the results of studies of Cre recombinase from bacteriophage P1. This
mechanism begins with the recombinase binding to the DNA substrates (Figure 27.39). Four molecules of the enzyme
and their associated DNA molecules come together to form a recombination synapse. The reaction begins with the
cleavage of one strand from each duplex. The 5
-hydroxyl group of each cleaved strand remains free, whereas the 3 -
phosphoryl group becomes linked to a specific tyrosine residue in the recombinase. The free 5
ends invade the other
duplex in the synapse and attack the DNA-tyrosine units to form new phosphodiester-bonds and free the tyrosine
residues. These reactions result in the formation of a Holliday junction. This junction can then isomerize to form a
structure in which the polynucleotide chains in the center of the structure are reoriented. From this junction, the
processes of strand cleavage and phosphodiester-bond formation repeat. The result is a synapse containing the two
recombined duplexes. Dissociation of this complex generates the final recombined products.
27.5.2. Recombinases Are Evolutionarily Related to Topoisomerases
The intermediates that form in recombination reactions, with their tyrosine adducts possessing 3 -phosphoryl
groups, are reminiscent of the intermediates that form in the reactions catalyzed by topoisomerases. This
mechanistic similarity reflects deeper evolutionary relationships. Examination of the three-dimensional structures of
recombinases and type I topoisomerases reveals that these proteins are related by divergent evolution despite little amino
acid sequence similarity (Figure 27.40). From this perspective, the action of a recombinase can be viewed as an
intermolecular topoisomerase reaction. In each case, a tyrosine-DNA adduct is formed. In a topoisomerase reaction, this
adduct is resolved when the 5
-hydroxyl group of the same duplex attacks to reform the same phosphodiester bond that
was initially cleaved. In a recombinase reaction, the attacking 5
-hydroxyl group comes from a DNA chain that was not
initially linked to the phosphoryl group participating in the phosphodiester bond.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.5. Double-Stranded DNA Molecules with Similar Sequences Sometimes Recombine
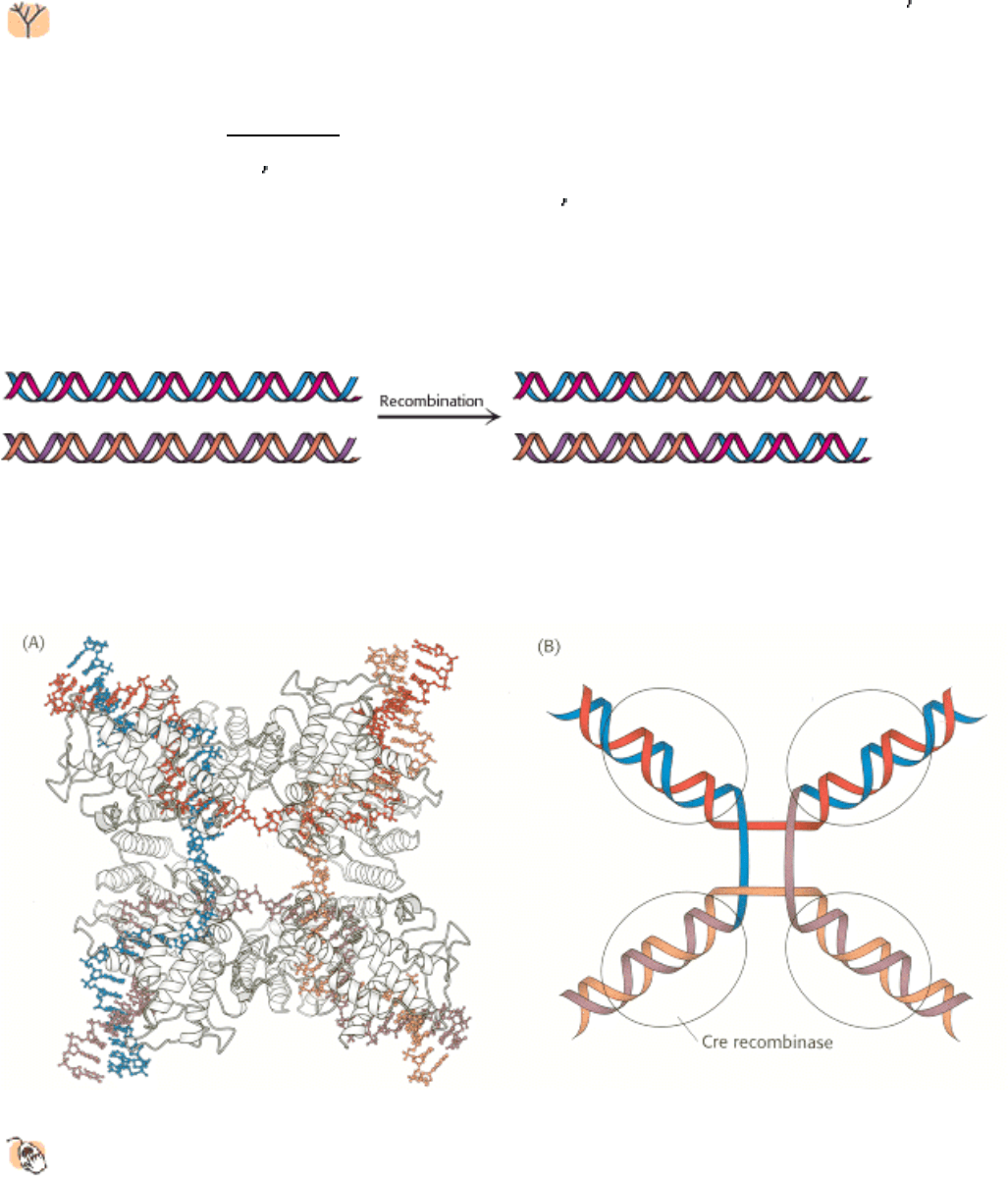
Figure 27.37. Recombination. Two DNA molecules can recombine with each other to form new DNA molecules that
have segments from both parental molecules.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.5. Double-Stranded DNA Molecules with Similar Sequences Sometimes Recombine
Figure 27.38. Holliday Junction.
(A) The structure of a Holliday junction bound by Cre recombinase (gray), a
bacteriophage protein. (B) A schematic view of a Holliday junction.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.5. Double-Stranded DNA Molecules with Similar Sequences Sometimes Recombine
Figure 27.39. Recombination Mechanism. Recombination begins as two DNA molecules come together to form a
recombination synapse. One strand from each duplex is cleaved by the recombinase enzyme; the 3
end of each of the
cleaved strands is linked to a tyrosine (Y) residue on the recombinase enzyme. New phosphodiester bonds are formed
when a 5
end of the other cleaved strand in the complex attacks these tyrosine-DNA adducts. After isomerization, these
steps are repeated to form the recombined products.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair 27.5. Double-Stranded DNA Molecules with Similar Sequences Sometimes Recombine
Figure 27.40. Recombinases and Topoisomerase I.
A superposition of Cre recombinase (blue) and topoisomerase I
(orange) reveals that these two enzymes have a common structural core. The positions of the tyrosine residues that
participate in DNA cleavage reactions are shown as red spheres for both enzymes.
III. Synthesizing the Molecules of Life 27. DNA Replication, Recombination, and Repair
27.6. Mutations Involve Changes in the Base Sequence of DNA
We now turn from DNA replication to DNA mutations and repair. Several types of mutations are known: (1) the
substitution of one base pair for another, (2) the deletion of one or more base pairs, and (3) the insertion of one or more
base pairs. The spontaneous mutation rate of T4 phage is about 10
-7
per base per replication. E. coli and Drosophila
melanogaster have much lower mutation rates, of the order of 10
-10
.
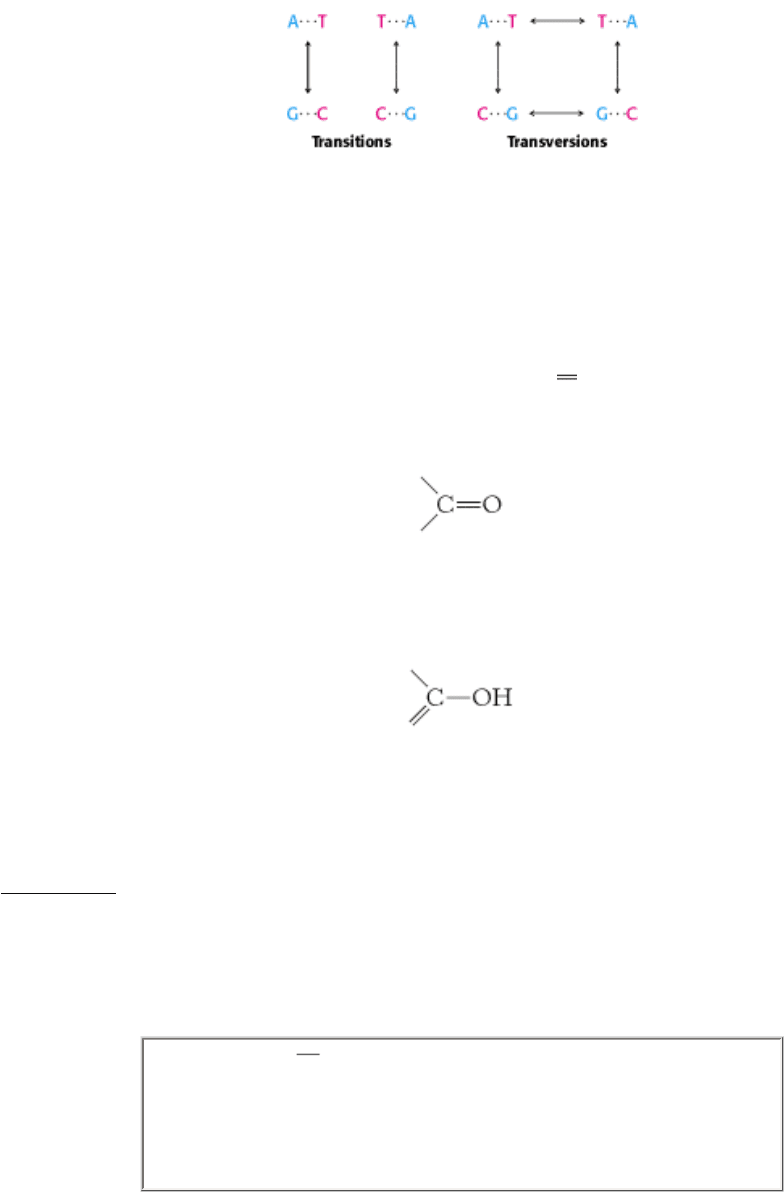
The substitution of one base pair for another is the a common type of mutation. Two types of substitutions are possible.
A transition is the replacement of one purine by the other or that of one pyrimidine by the other. In contrast, a
transversion is the replacement of a purine by a pyrimidine or that of a pyrimidine by a purine.
Watson and Crick suggested a mechanism for the spontaneous occurrence of transitions in a classic paper on the DNA
double helix. They noted that some of the hydrogen atoms on each of the four bases can change their location to produce
a tautomer. An amino group (-NH
2
) can tautomerize to an imino form ( NH). Likewise, a keto group (
can tautomerizeto an enol form
. The fraction of each type of base in the formof these imino and enol tautomers is about10
-4
. These transient tautomers
can form nonstandard base pairs that fit into a double helix. For example, the imino tautomer of adenine can pair with
cytosine (Figure 27.41). This A*-C pairing (the asterisk denotes the imino tautomer) would allow C to become
incorporated into a growing DNA strand where T was expected, and it would lead to a mutation if left uncorrected. In the
next round of replication, A* will probably retautomerize to the standard form, which pairs as usual with thymine, but
the cytosine residue will pair with guanine. Hence, one of the daughter DNA molecules will contain a G-C base pair in
place of the normal A-T base pair.
Tautomerization
The interconversion of two isomers that differ only in the position of
protons (and, often, double bonds).
27.6.1. Some Chemical Mutagens Are Quite Specific
Base analogs such as 5-bromouracil and 2-aminopurine can be incorporated into DNA and are even more likely than
normal nucleic acid bases to form transient tautomers that lead to transition mutations. 5-Bromouracil, an analog of
thymine, normally pairs with adenine. However, the proportion of 5-bromouracil in the enol tautomer is higher than that
of thymine because the bromine atom is more electronegative than is a methyl group on the C-5 atom. Thus, the
incorporation of 5-bromouracil is especially likely to cause altered base-pairing in a subsequent round of DNA
replication (Figure 27.42).
Other mutagens act by chemically modifying the bases of DNA. For example, nitrous acid (HNO
2
) reacts with bases that
contain amino groups. Adenine is oxidatively deaminated to hypoxanthine, cytosine to uracil, and guanine to xanthine.
Hypoxanthine pairs with cytosine rather than with thymine (Figure 27.43). Uracil pairs with adenine rather than with
guanine. Xanthine, like guanine, pairs with cytosine. Consequently, nitrous acid causes A-T G-C transitions.
A different kind of mutation is produced by flat aromatic molecules such as the acridines (Figure 27.44). These
compounds intercalate in DNA that is, they slip in between adjacent base pairs in the DNA double helix.
Consequently, they lead to the insertion or deletion of one or more base pairs. The effect of such mutations is to alter the
reading frame in translation, unless an integral multiple of three base pairs is inserted or deleted. In fact, the analysis of
such mutants contributed greatly to the revelation of the triplet nature of the genetic code.
Some compounds are converted into highly active mutagens through the action of enzymes that normally play a role in
detoxification. A striking example is aflatoxin B
1
, a compound produced by molds that grows on peanuts and other
foods. A cytochrome P450 enzyme (Section 26.4.3) converts this compound into a highly reactive epoxide (Figure
27.45). This agent reacts with the N-7 atom of guanosine to form an adduct that frequently leads to a G-C-to-T-A
transversion.
27.6.2. Ultraviolet Light Produces Pyrimidine Dimers
The ultraviolet component of sunlight is a ubiquitous DNA-damaging agent. Its major effect is to covalently link
adjacent pyrimidine residues along a DNA strand (Figure 27.46). Such a pyrimidine dimer cannot fit into a double helix,
and so replication and gene expression are blocked until the lesion is removed.
27.6.3. A Variety of DNA-Repair Pathways Are Utilized
The maintenance of the integrity of the genetic message is key to life. Consequently, all cells possess mechanisms to
repair damaged DNA. Three types of repair pathways are direct repair, base-excision repair, and nucleotide-excision
repair (Figure 27.47).
An example of direct repair is the photochemical cleavage of pyrimidine dimers. Nearly all cells contain a
photoreactivating enzyme called DNA photolyase. The E. coli enzyme, a 35-kd protein that contains bound N
5
,N
10
-
methenyltetrahydrofolate and flavin adenine dinucleotide cofactors, binds to the distorted region of DNA. The enzyme
uses light energy
specifically, the absorption of a photon by the N
5
,N
10
-methenyltetrahydrofolate coenzyme to
form an excited state that cleaves the dimer into its original bases.
The excision of modified bases such as 3-methyladenine by the E. coli enzyme AlkA is an example of base-excision
repair. The binding of this enzyme to damaged DNA flips the affected base out of the DNA double helix and into the
active site of the enzyme (Figure 27.48). Base flipping also occurs in the enzymatic addition of methyl groups to DNA
bases (Section 24.2.7). The enzyme then acts as a glycosylase, cleaving the glycosidic bond to release the damaged base.
At this stage, the DNA backbone is intact, but a base is missing. This hole is called an AP site because it is apurinic
(devoid of A or G) or apyrimidinic (devoid of C or T). An AP endonuclease recognizes this defect and nicks the
backbone adjacent to the missing base. Deoxyribose phosphodiesterase excises the residual deoxyribose phosphate unit,
and DNA polymerase I inserts an undamaged nucleotide, as dictated by the base on the undamaged complementary
strand. Finally, the repaired strand is sealed by DNA ligase.
One of the best-understood examples of nucleotide-excision repair is the excision of a pyrimidine dimer. Three
enzymatic activities are essential for this repair process in E. coli (Figure 27.49). First, an enzyme complex consisting of
the proteins encoded by the uvrABC genes detects the distortion produced by the pyrimidine dimer. A specific uvrABC
enzyme then cuts the damaged DNA strand at two sites, 8 nucleotides away from the dimer on the 5
side and 4
nucleotides away on the 3
side. The 12-residue oligonucleotide excised by this highly specific excinuclease (from the
Latin exci,"to cut out") then diffuses away. DNA polymerase I enters the gap to carry out repair synthesis. The 3
end of
the nicked strand is the primer, and the intact complementary strand is the template. Finally, the 3
end of the newly
synthesized stretch of DNA and the original part of the DNA chain are joined by DNA ligase.
27.6.4. The Presence of Thymine Instead of Uracil in DNA Permits the Repair of
Deaminated Cytosine
The presence in DNA of thymine rather than uracil was an enigma for many years. Both bases pair with adenine. The
only difference between them is a methyl group in thymine in place of the C-5 hydrogen atom in uracil. Why is a
methylated base employed in DNA and not in RNA? The existence of an active repair system to correct the deamination
of cytosine provides a convincing solution to this puzzle.
Cytosine in DNA spontaneously deaminates at a perceptible rate to form uracil. The deamination of cytosine is
potentially mutagenic because uracil pairs with adenine, and so one of the daughter strands will contain an U-A base pair
rather than the original C-G base pair (Figure 27.50). This mutation is prevented by a repair system that recognizes uracil
to be foreign to DNA. This enzyme, uracil DNA glycosylase, is homologous to AlkA. The enzyme hydrolyzes the
glycosidic bond between the uracil and deoxyribose moieties but does not attack thymine-containing nucleotides. The
AP site generated is repaired to reinsert cytosine. Thus, the methyl group on thymine is a tag that distinguishes thymine
from deaminated cytosine. If thymine were not used in DNA, uracil correctly in place would be indistinguishable from
uracil formed by deamination. The defect would persist unnoticed, and so a C-G base pair would necessarily be mutated
to U-A in one of the daughter DNA molecules. This mutation is prevented by a repair system that searches for uracil and
leaves thymine alone. Thymine is used instead of uracil in DNA to enhance the fidelity of the genetic message. In
contrast, RNA is not repaired, and so uracil is used in RNA because it is a less-expensive building block.
27.6.5. Many Cancers Are Caused by Defective Repair of DNA
As discussed in Chapter 15, cancers are caused by mutations in genes associated with growth control. Defects in
DNA-repair systems are expected to increase the overall frequency of mutations and, hence, the likelihood of a
cancer-causing mutation. Xeroderma pigmentosum, a rare human skin disease, is genetically transmitted as an autosomal
recessive trait. The skin in an affected homozygote is extremely sensitive to sunlight or ultraviolet light. In infancy,
severe changes in the skin become evident and worsen with time. The skin becomes dry, and there is a marked atrophy
of the dermis. Keratoses appear, the eyelids become scarred, and the cornea ulcerates. Skin cancer usually develops at
several sites. Many patients die before age 30 from metastases of these malignant skin tumors.
Ultraviolet light produces pyrimidine dimers in human DNA, as it does in E. coli DNA. Furthermore, the repair
mechanisms are similar. Studies of skin fibroblasts from patients with xeroderma pigmentosum have revealed a
biochemical defect in one form of this disease. In normal fibro-blasts, half the pyrimidine dimers produced by ultraviolet
radiation are excised in less than 24 hours. In contrast, almost no dimers are excised in this time interval in fibroblasts
derived from patients with xeroderma pigmentosum. The results of these studies show that xeroderma pigmentosum can
be produced by a defect in the excinuclease that hydrolyzes the DNA backbone near a pyrimidine dimer. The drastic
clinical consequences of this enzymatic defect emphasize the critical importance of DNA-repair processes. The disease
can also be caused by mutations in eight other genes for DNA repair, which attests to the complexity of repair processes.
