Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

the ATP that is cleaved by the aminoacyl-tRNA synthetase before the arrival of the tRNA at the ribosome.
With the peptide bond formed, the peptide chain is now attached to the tRNA in the A site on the 30S subunit while a
change in the interaction with the 50S subunit has placed that tRNA and its peptide in the P site of the large subunit. The
tRNA in the P site of the 30S subunit is now uncharged. For translation to proceed, the mRNA must be moved (or
translo-cated) so that the codon for the next amino acid to be added is in the A site. This translocation takes place
through the action of a protein enzyme called elongation factor G (Section 29.4.3), driven by the hydrolysis of GTP. On
completion of this step, the peptidyl-tRNA is now fully in the P site, and the uncharged initiator tRNA is in the E site
and has been disengaged from the mRNA. On dissociation of the initiator tRNA, the ribosome has returned to its initial
state except that the peptide chain is attached to a different tRNA, the one corresponding to the first codon past the
initiating AUG. Note that the peptide chain remains in the P site on the 50S subunit throughout this cycle, presumably
growing into the tunnel. This cycle is repeated as new aminoacyl-tRNAs move into the A site, allowing the polypeptide
to be elongated indefinitely.
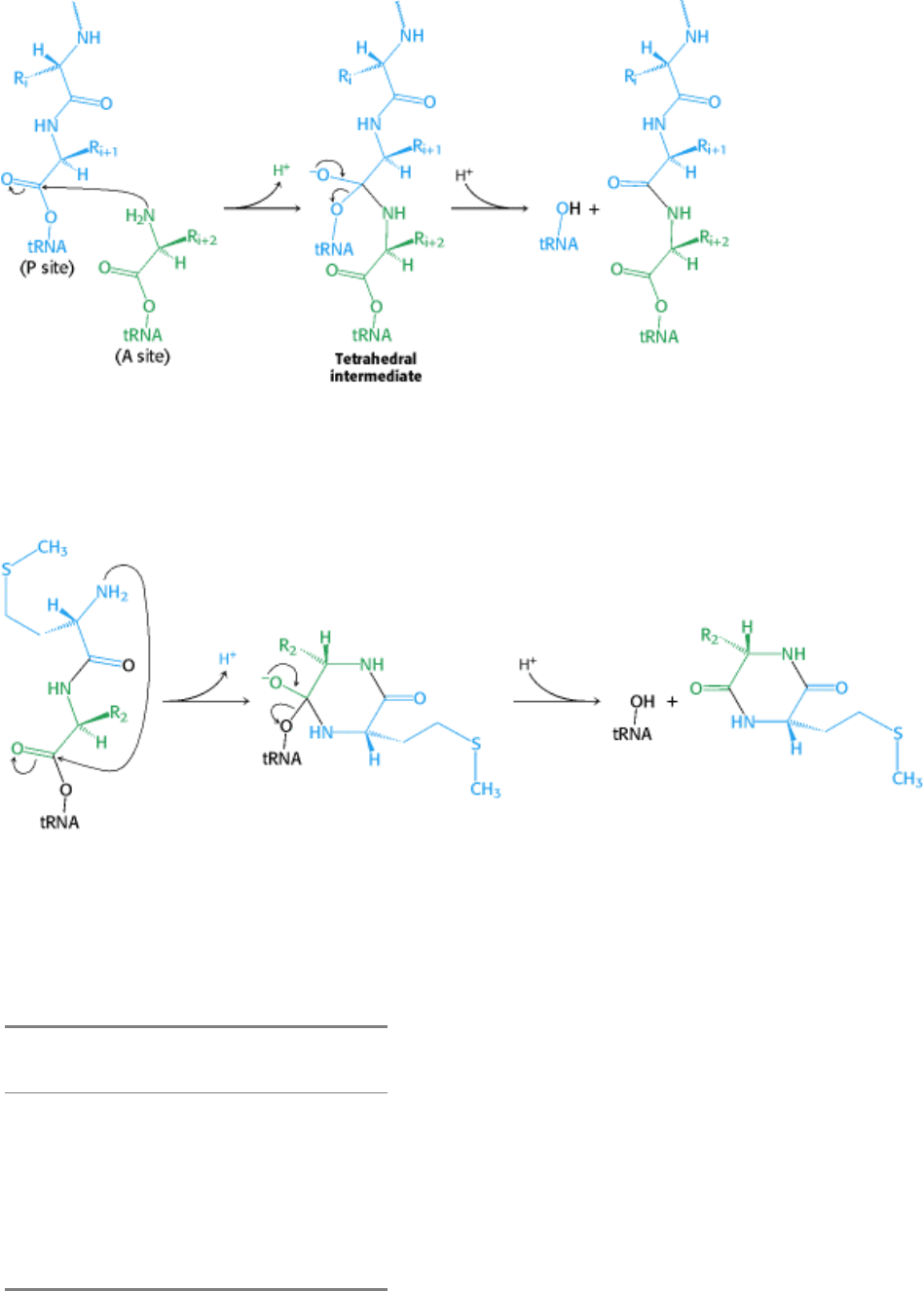
We can now understand why the amino terminus of the initial methionine molecule is modified by the attachment of a
formyl group. Chemical reactivity may have dictated this modification (Figure 29.26). Suppose that the amino-terminus
is not blocked. After the first peptidyl-transfer reaction, a dipeptide is linked to the tRNA in the P site. If a free amino
group is present in the terminal amino acid, this amino group can attack the carbonyl group of the ester linkage to the
tRNA, forming a very stable six-membered ring and terminating translation.
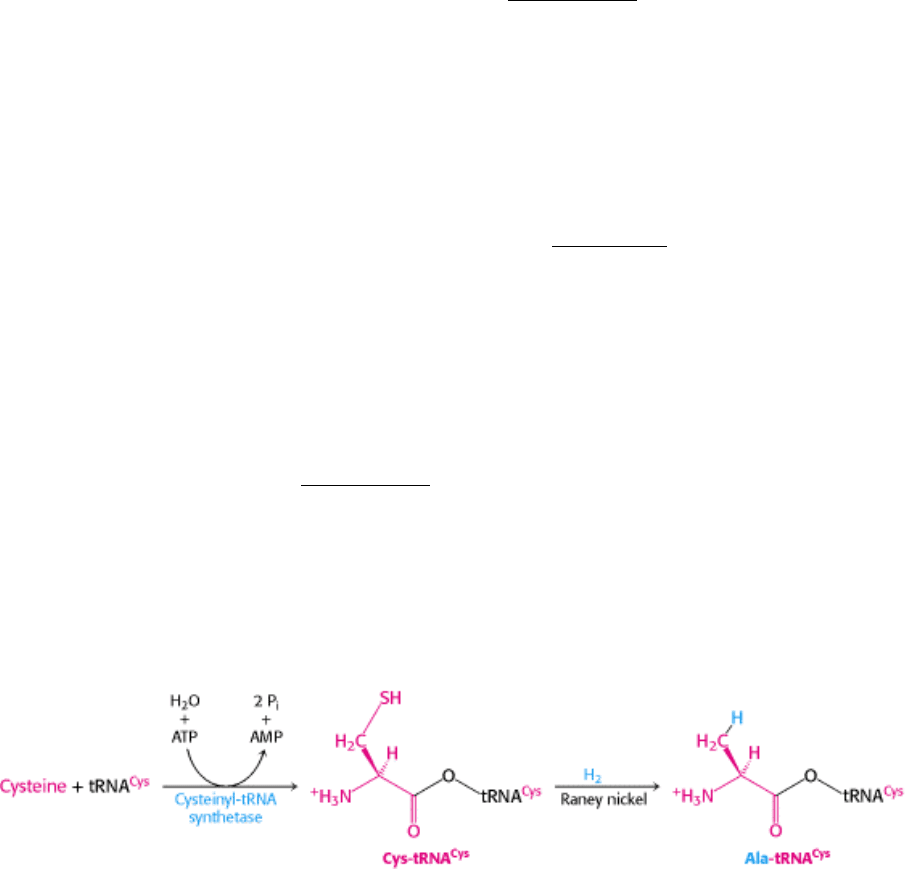
29.3.8. Only the Codon-Anticodon Interactions Determine the Amino Acid That Is
Incorporated
On the basis of the mechanism described in Section 29.3.7, the base-pairing interaction between the anticodon on the
incoming tRNA and the codon in the A site on mRNA determines which amino acid is added to the polypeptide chain.
Does the amino acid attached to the tRNA play any role in this process? This question was answered in the following
way. First, cysteine was attached to its cognate tRNA. The attached cysteine unit was then converted into alanine by
adding Raney nickel to Cys-tRNA
Cys
; the reaction removed the sulfur atom from the cysteine residue without affecting
its linkage to tRNA. Thus, a mischarged aminoacyl-tRNA was produced in which alanine was covalently attached to a
tRNA specific for cysteine.
Does this mischarged tRNA recognize the codon for cysteine or for alanine? The answer came when the tRNA was
added to a cell-free protein-synthesizing system. The template was a random copolymer of U and G in the ratio of 5:1,
which normally incorporates cysteine (encoded by UGU) but not alanine (encoded by GCN). However, alanine was
incorporated into a polypeptide when Ala-tRNA
Cys
was added to the incubation mixture. The same result was obtained
when mRNA for hemoglobin served as the template and [
14
C]alanyl-tRNA
Cys
was used as the mischarged aminoacyl-
tRNA. The only radioactive tryptic peptide produced was one that normally contained cysteine but not alanine. Thus, the
amino acid in aminoacyl-tRNA does not play a role in selecting a codon.
In recent years, the ability of mischarged tRNAs to transfer their amino acid cargo to a growing polypeptide chain has
been used to synthesize peptides with amino acids not found in proteins incorporated into specific sites in a protein.
Aminoacyl-tRNAs are first linked to these unnatural amino acids by chemical methods. These mischarged aminoacyl-
tRNAs are added to a cell-free protein-synthesizing system along with specially engineered mRNA that contains codons
corresponding to the anticodons of the mischarged aminoacyl-tRNAs in the desired positions. The proteins produced
have unnatural amino acids in the expected positions. More than 100 different unnatural amino acids have been
incorporated in this way. However, only l-amino acids can be used; apparently this stereochemistry is required for
peptide-bond formation to take place.
29.3.9. Some Transfer RNA Molecules Recognize More Than One Codon Because of
Wobble in Base-Pairing
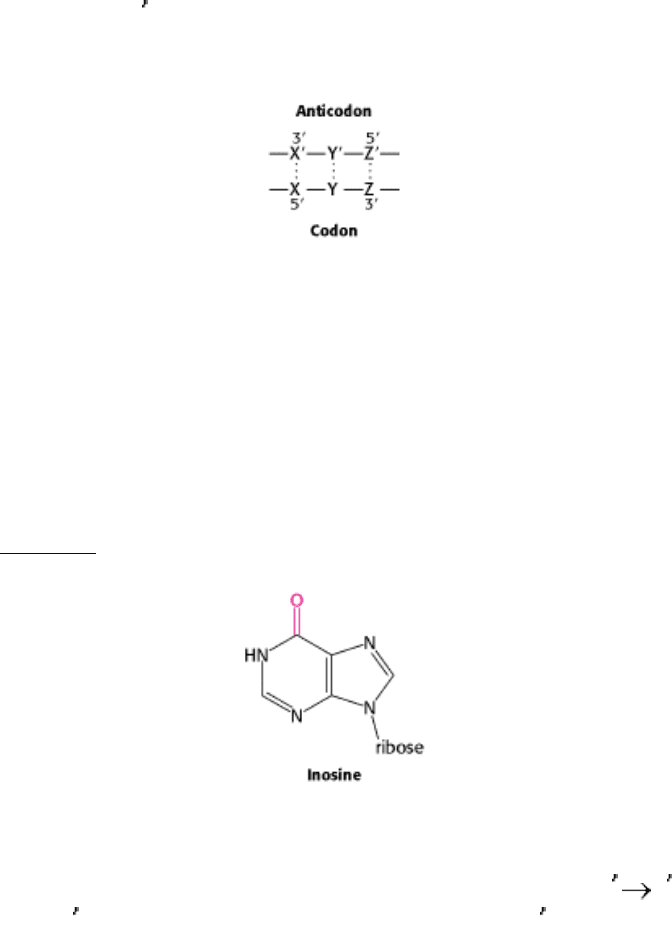
What are the rules that govern the recognition of a codon by the anticodon of a tRNA? A simple hypothesis is that each
of the bases of the codon forms a Watson-Crick type of base pair with a complementary base on the anticodon. The
codon and anticodon would then be lined up in an antiparallel fashion. In the diagram in the margin, the prime denotes
the complementary base. Thus X and X
would be either A and U (or U and A) or G and C (or C and G). According to
this model, a particular anticodon can recognize only one codon.
The facts are otherwise. As found experimentally, some pure tRNA molecules can recognize more than one codon. For
example, the yeast alanyl-tRNA binds to three codons: GCU, GCC, and GCA. The first two bases of these codons are
the same, whereas the third is different. Could it be that recognition of the third base of a codon is sometimes less
discriminating than recognition of the other two? The pattern of degeneracy of the genetic code indicates that this might
be so. XYU and XYC always encode the same amino acid; XYA and XYG usually do. Francis Crick surmised from
these data that the steric criteria might be less stringent for pairing of the third base than for the other two. Models of
various base pairs were built to determine which ones are similar to the standard A · U and G · C base pairs with regard
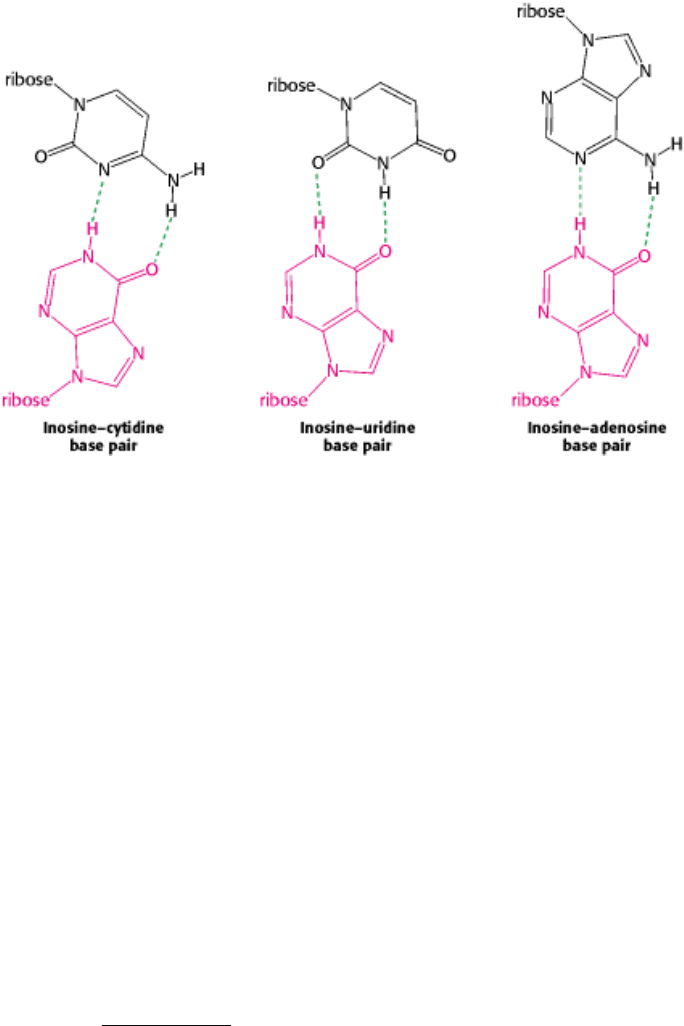
to the distance and angle between the glycosidic bonds. Inosine was included in this study because it appeared in several
anticodons. With the assumption of some steric freedom ("wobble") in the pairing of the third base of the codon, the
combinations shown in Table 29.3 seemed plausible.
The wobble hypothesis is now firmly established. The anticodons of tRNAs of known sequence bind to the codons
predicted by this hypothesis. For example, the anticodon of yeast alanyl-tRNA is IGC. This tRNA recognizes the codons
GCU, GCC, and GCA. Recall that, by convention, nucleotide sequences are written in the 5
3 direction unless
otherwise noted. Hence, I (the 5
base of this anticodon) pairs with U, C, or A (the 3 base of the codon), as predicted.
Two generalizations concerning the codon-anticodon interaction can be made:
1. The first two bases of a codon pair in the standard way. Recognition is precise. Hence, codons that differ in either of
their first two bases must be recognized by different tRNAs. For example, both UUA and CUA encode leucine but are
read by different tRNAs.
2. The first base of an anticodon determines whether a particular tRNA molecule reads one, two, or three kinds of
codons: C or A (one codon), U or G (two codons), or I (three codons). Thus, part of the degeneracy of the genetic code
arises from imprecision (wobble) in the pairing of the third base of the codon with the first base of the anticodon. We see
here a strong reason for the frequent appearance of inosine, one of the unusual nucleosides, in anticodons. Inosine
maximizes the number of codons that can be read by a particular tRNA molecule. The inosines in tRNA are formed by
deamination of adenosine after synthesis of the primary transcript.
Why is wobble tolerated in the third position of the codon but not in the first two? The 30S subunit has two adenine
bases (A1492 and A1493 in the 16S RNA) that form hydrogen bonds on the minor-groove side of the codon-anticodon
duplex. These interactions serve to check whether Watson-Crick base pairs are present in the first two positions of the
codon- anticodon duplex. No such inspection device is present for the third position so more-varied base pairs are
tolerated. This mechanism for ensuring fidelity is analogous to the minor-groove interactions utilized by DNA
polymerase for a similar purpose (Section 27.2.3). Thus, the ribosome plays an active role in decoding the codon-
anticodon interactions.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
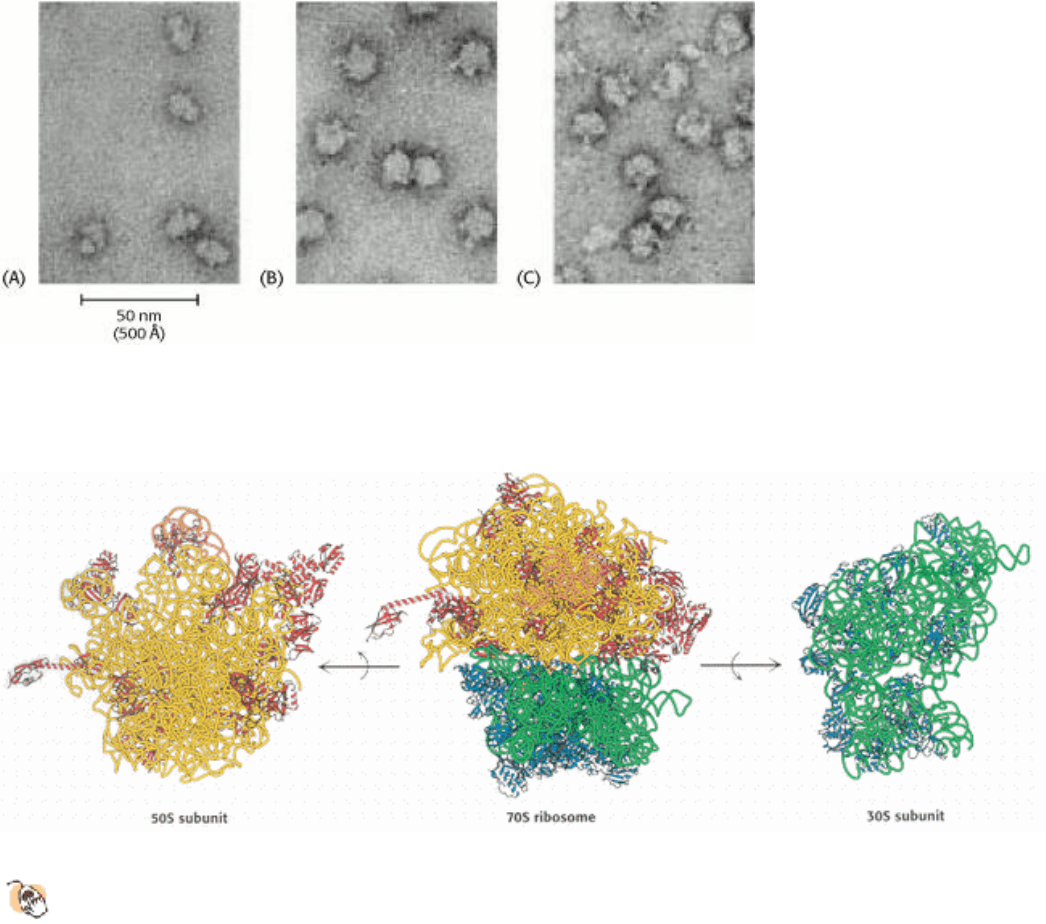
Figure 29.15. Ribosomes at Low Resolution. Electron micrographs of (A) 30S subunits, (B) 50S subunits, and (C) 70S
ribosomes. [Courtesy of Dr. James Lake.]
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.16. The Ribosome at High Resolution.
Detailed models of the ribosome based on the results of x-ray
crystallographic studies of the 70S ribosome and the 30S and 50S subunits. 23S RNA is shown in yellow, 5S RNA
in orange, 16S RNA in green, proteins of the 50S subunit in red, and proteins of the 30S subunit in blue.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
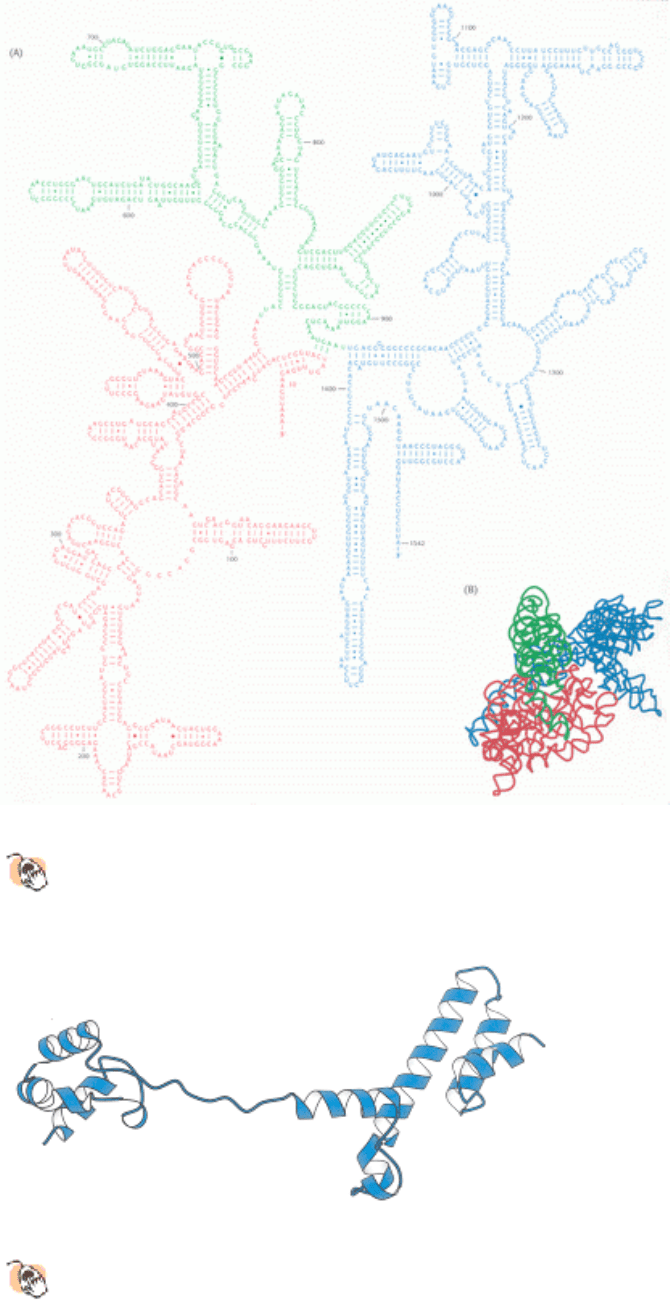
Figure 29.17. Ribosomal RNA Folding Pattern.
(A) The secondary structure of 16S ribosomal RNA deduced from
sequence comparison and the results of chemical studies. (B) The tertiary structure of 16S RNA determined by x-
ray crystallography. [Part A courtesy of Dr. Bryn Weiser and Dr. Harry Noller.]
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.18. Ribosomal Protein Structure.
The structure of ribosomal protein L19 of the 50S ribosomal subunit
reveals a long segment of extended structure that fits through some of the cavities within the 23S RNA molecule.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
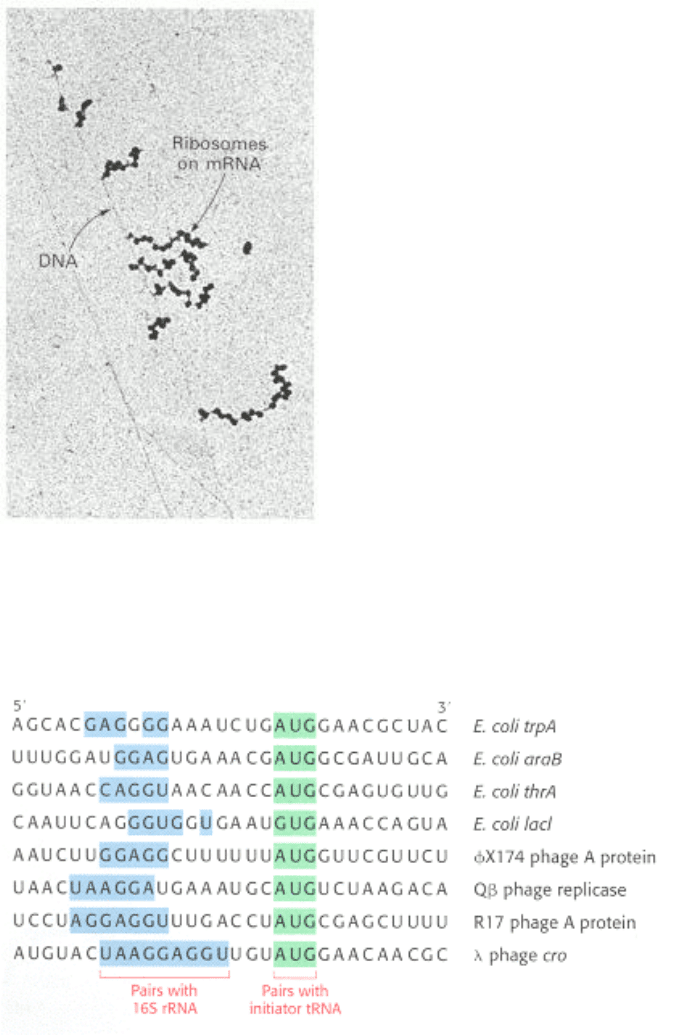
Figure 29.19. Polysomes. Transcription of a segment of DNA from E. coli generates mRNA molecules that are
immediately translated by multiple ribosomes. [From O. L. Miller, Jr., B. A. Hamkalo, and C. A. Thomas, Jr. Science 169
(1970):392.]
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.20. Initiation Sites. Sequences of mRNA initiation sites for protein synthesis in some bacterial and viral
mRNA molecules. Comparison of these sequences reveals some recurring features.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
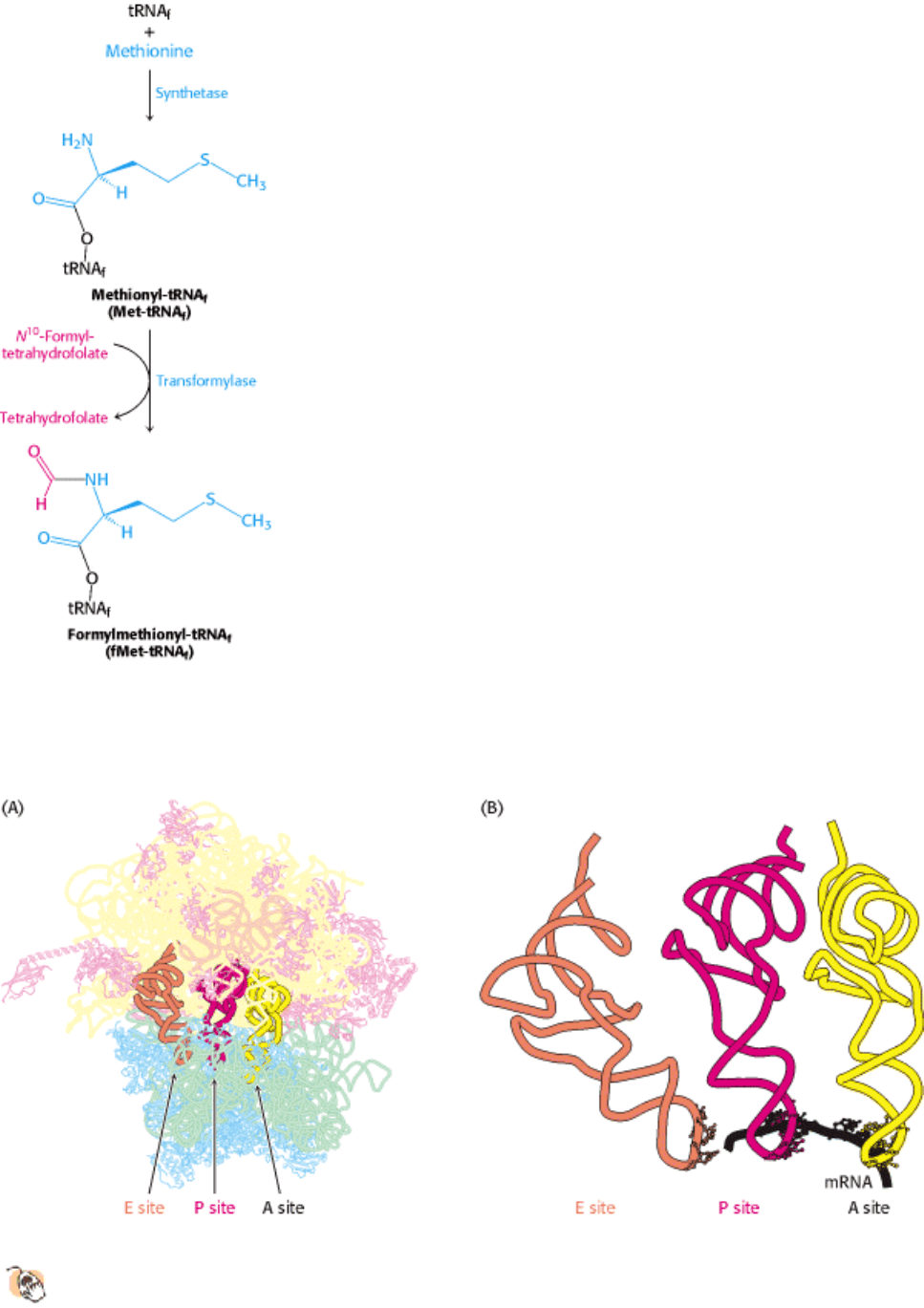
Figure 29.21. Formylation of Methionyl-tRNA. Initiator tRNA (tRNA
f
) is first charged with methionine, and then a
formyl group is transferred to the methionyl- tRNA
f
from N
10
-formyltetrahydrofolate.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.22. Transfer RNA-Binding Sites.
(A) Three tRNA-binding sites are present on the 70S ribosome. They are
called the A (for aminoacyl), P (for peptidyl), and E (for exit) sites. Each tRNA molecule contacts both the 30S
and the 50S subunit. (B) The tRNA molecules in sites A and P are base paired with mRNA.

III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.23. Polypeptide Escape Path.
A tunnel passes through the 50S subunit beginning at the site of peptide-bond
formation (shown in blue). The growing polypeptide chain passes through this tunnel.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.24. Mechanism of Protein Synthesis. The cycle begins with peptidyl-tRNA in the P site. An aminoacyl-
tRNA binds in the A site. With both sites occupied, a new peptide bond is formed. The tRNAs and the mRNA are
translocated through the action of elongation factor G, which moves the deacylated tRNA to the E site. Once there, it is
free to dissociate to complete the cycle.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.25. Peptide-Bond Formation. The amino group of the aminoacyl-tRNA attacks the carbonyl group of the
ester linkage of the peptidyl-tRNA to form a tetrahedral intermediate. This intermediate collapses to form the peptide
bond and release the deacylated tRNA.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Figure 29.26. A Role for Formylation. With a free terminal amino group, dipeptidyl-tRNA can cyclize to cleave itself
from tRNA. Formylation of the amino terminus blocks this reaction.
III. Synthesizing the Molecules of Life 29. Protein Synthesis 29.3. A Ribosome Is a Ribonucleoprotein Particle (70S) Made of a Small (30S) and a Large (50S) Subunit
Table 29.3. Allowed pairings at the third base of the codon according to the wobble hypothesis
First base of anticodon Third base of codon
C G
A U
U A or G
G U or C
I U, C, or A

III. Synthesizing the Molecules of Life 29. Protein Synthesis
29.4. Protein Factors Play Key Roles in Protein Synthesis
Although rRNA is paramount in the process of translation, protein factors also are required for the efficient synthesis of
a protein. Protein factors participate in the initiation, elongation, and termination of protein synthesis. P-loop NTPases of
the G-protein family play particularly important roles. Recall that these proteins serve as molecular switches as they
cycle between a GTP-bound form and a GDP-bound form (Section 15.1.2).
29.4.1. Formylmethionyl-tRNA
f
Is Placed in the P Site of the Ribosome During
Formation of the 70S Initiation Complex
Messenger RNA and formylmethionyl-tRNA
f
must be brought to the ribosome for protein synthesis to begin. How is
this accomplished? Three protein initiation factors (IF1, IF2, and IF3) are essential. The 30S ribosomal subunit first
forms a complex with IF1 and IF3 (Figure 29.27). The binding of these factors to the 30S subunit prevents it from
prematurely joining the 50S subunit to form a dead-end 70S complex, devoid of mRNA and fMet-tRNA
f
. Initiation
factor 2, a member of the G-protein family, binds GTP, and the concomitant conformational change enables IF
2
to
associate with formylmethionyl-tRNA
f
. The IF2-GTP-initiator tRNA complex binds with mRNA (correctly positioned
by the Shine-Dalgarno sequence interaction with the 16S rRNA) and the 30S subunit to form the 30S initiation complex.
The hydrolysis of GTP bound to IF2 on entry of the 50S subunit leads to the release of the initiation factors. The result is
a 70S initiation complex.
When the 70S initiation complex has been formed, the ribosome is ready for the elongation phase of protein synthesis.
The fMet-tRNA
f
molecule occupies the P site on the ribosome. The other two sites for tRNA molecules, the A site and
the E site, are empty. Formylmethionyl-tRNA
f
is positioned so that its anticodon pairs with the initiating AUG (or GUG)
codon on mRNA. This interaction sets the reading frame for the translation of the entire mRNA.
29.4.2. Elongation Factors Deliver Aminoacyl-tRNA to the Ribosome
The second phase of protein synthesis is the elongation cycle. This phase begins with the insertion of an aminoacyl-
tRNA into the empty A site on the ribosome. The particular species inserted depends on the mRNA codon in the A site.
The cognate aminoacyl-tRNA does not simply leave the synthetase and diffuse to the A site. Rather, it is delivered to the
A site in association with a 43-kd protein called elongation factor Tu (EF-Tu). Elongation factor Tu, another member of
the G-protein family, binds aminoacyl-tRNA only in the GTP form (Figure 29.28). The binding of EF-Tu to aminoacyl-
tRNA serves two functions. First, EF-Tu protects the delicate ester linkage in aminoacyl-tRNA from hydrolysis. Second,
the GTP in EF-Tu is hydrolyzed to GDP when an appropriate complex between the EF-Tu-aminoacyl-tRNA complex
and the ribosome has formed. If the anticodon is not properly paired with the codon, hydrolysis does not take place and
the aminoacyl-tRNA is not transferred to the ribosome. This mechanism allows the free energy of GTP hydrolysis to
contribute to the fidelity of protein synthesis.
How is EF-Tu in the GDP form reset to bind another aminoacyl-tRNA? Elongation Factor Ts, a second elongation
factor, joins the EF-Tu complex and induces the dissociation of GDP. Finally, GTP binds to EF-Tu, and EF-Ts is
concomitantly released. It is noteworthy that EF-Tu does not interact with fMet-tRNA
f
. Hence, this initiator tRNA is not
delivered to the A site. In contrast, Met-tRNA
m
, like all other aminoacyl-tRNAs, does bind to EF-Tu. These findings
account for the fact that internal AUG codons are not read by the initiator tRNA. Conversely, initiation factor 2
recognizes fMet-tRNA
f
but no other tRNA.
This GTP-GDP cycle of EF-Tu is reminiscent of those of the heterotrimeric G proteins in signal transduction (Section
15.1.2) and the Ras proteins in growth control (Section 15.4.2). This similarity is due to their evolutionary heritage,
inasmuch as the amino-terminal domain of EF-Tu is homologous to the P-loop NTPase domains in the other G proteins.
