Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

11.
Evolutionary time machine. It has been suggested that ancestral protein sequences might be inferred from
evolutionary trees of sequences that exist today. The Conceptual Insights module on sequence analysis
allows you to try your hand at inferring ancestral sequences from model evolutionary trees. Based on this
experience, explain why you would not expect to be able to successfully infer dinosaur sequences.
I. The Molecular Design of Life 7. Exploring Evolution
Selected Readings
Book
Doolittle, R. F., 1987. Of UFS and ORFS . University Science Books.
Sequence alignment
S. Henikoff and J.G. Henikoff. 1992. Amino acid substitution matrices from protein blocks Proc. Natl. Acad. Sci. U.S.A.
89: 10915-10919. (PubMed) (Full Text in PMC)
M.S. Johnson and J.P. Overington. 1993. A structural basis for sequence comparisons: An evaluation of scoring
methodologies J. Mol. Biol. 233: 716-738. (PubMed)
L. Aravind and E.V. Koonin. 1999. Gleaning non-trivial structural, functional and evolutionary information about
proteins by iterative database searches J. Mol. Biol. 287: 1023-1040. (PubMed)
S.F. Altschul, T.L. Madden., A.A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D.J. Lipman. 1997. Gapped BLAST and
PSI-BLAST: A new generation of protein database search programs Nucleic Acids Res. 25: 3389-3402. (PubMed) (Full
Text in PMC)
Structure comparison
D. Bashford, C. Chothia, and A.M. Lesk. 1987. Determinants of a protein fold: Unique features of the globin amino acid
sequences J. Mol. Biol. 196: 199-216. (PubMed)
E.H. Harutyunyan, T.N. Safonova, I.P. Kuranova, A.N. Popov, A.V. Teplyakov, G.V. Obmolova, A.A. Rusakov, B.K.
Vainshtein, G.G. Dodson, and J.C. Wilson, et al. 1995. The structure of deoxy- and oxy-leghaemoglobin from lupin J.
Mol. Biol. 251: 104-115. (PubMed)
K.M. Flaherty, D.B. McKay, W. Kabsch, and K.C. Holmes. 1991. Similarity of the three-dimensional structures of actin
and the ATPase fragment of a 70-kDa heat shock cognate protein Proc. Natl. Acad. Sci. U. S. A. 88: 5041-5045.
(PubMed) (Full Text in PMC)
A.G. Murzin, S.E. Brenner, T. Hubbard, and C. Chothia. 1995. SCOP: A structural classification of proteins database for
the investigation of sequences and structures J. Mol. Biol. 247: 536-540. (PubMed)
C. Hadley and D.T. Jones. 1999. A systematic comparison of protein structure classification: SCOP, CATH and FSSP
Structure Fold. Des. 7: 1099-1112. (PubMed)
Domain detection
J.H. Ploegman, G. Drent, K.H. Kalk, and W.G. Hol. 1978. Structure of bovine liver rhodanese I: Structure determination
at 2.5 Å resolution and a comparison of the conformation and sequence of its two domains J. Mol. Biol. 123: 557-594.
(PubMed)
D.B. Nikolov, S.H. Hu, J. Lin, A. Gasch, A. Hoffmann, M. Horikoshi, N.H. Chua, R.G. Roeder, and S.K. Burley. 1992.
Crystal structure of TFIID TATA-box binding protein Nature 360: 40-46. (PubMed)
R.F. Doolittle. 1995. The multiplicity of domains in proteins Annu. Rev. Biochem. 64: 287-314. (PubMed)
A. Heger and L. Holm. 2000. Rapid automatic detection and alignment of repeats in protein sequences Proteins 41: 224-
237. (PubMed)
Evolutionary trees
R.F. Doolittle. 1992. Stein and Moore Award address Reconstructing history with amino acid sequences Protein Sci. 1:
191-200. (PubMed)
E. Zukerkandl and L. Pauling. 1965. Molecules as documents of evolutionary history J. Theor. Biol. 8: 357-366.
(PubMed)
Ancient DNA
M. Krings, A. Stone, R.W. Schmitz, H. Krainitzki, M. Stoneking, and S. Pääbo. 1997. Neandertal DNA sequences and
the origin of modern humans [see comments] Cell 90: 19-30. (PubMed)
M. Krings, H. Geisert, R.W. Schmitz, H. Krainitzki, and S. Pääbo. 1999. DNA sequence of the mitochondrial
hypervariable region II from the Neandertal type specimen Proc. Natl. Acad. Sci. U. S. A. 96: 5581-5585. (PubMed)
(Full Text in PMC)
Evolution in the laboratory
L. Gold, B. Polisky, O. Uhlenbeck, and M. Yarus. 1995. Diversity of oligonucleotide functions Annu. Rev. Biochem. 64:
763-797. (PubMed)
D.S. Wilson and J.W. Szostak. 1999. In vitro selection of functional nucleic acids Annu. Rev. Biochem. 68: 611-647.
(PubMed)
T. Hermann and D.J. Patel. 2000. Adaptive recognition by nucleic acid aptamers Science 287: 820-825. (PubMed)
Web sites
The Protein Databank (PDB) site is the repository for three-dimensional macromolecular structures. It currently contains
nearly 14,000 structures. (http://www.rcsb.org/pdb/)
National Center for Biotechnology Information (NCBI) contains molecular biological databases and software for
analysis. (http://www.ncbi.nlm.nih.gov/)
I. The Molecular Design of Life
8. Enzymes: Basic Concepts and Kinetics
Enzymes, the catalysts of biological systems, are remarkable molecular devices that determine the patterns of chemical
transformations. They also mediate the transformation of one form of energy into another. The most striking
characteristics of enzymes are their catalytic power and specificity. Catalysis takes place at a particular site on the
enzyme called the active site. Nearly all known enzymes are proteins. However, proteins do not have an absolute
monopoly on catalysis; the discovery of catalytically active RNA molecules provides compelling evidence that RNA was
an early biocatalyst (Section 2.2.2).
Proteins as a class of macromolecules are highly effective catalysts for an enormous diversity of chemical reactions
because of their capacity to specifically bind a very wide range of molecules. By utilizing the full repertoire of
intermolecular forces, enzymes bring substrates together in an optimal orientation, the prelude to making and breaking
chemical bonds. They catalyze reactions by stabilizing transition states, the highest-energy species in reaction pathways.
By selectively stabilizing a transition state, an enzyme determines which one of several potential chemical reactions
actually takes place.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
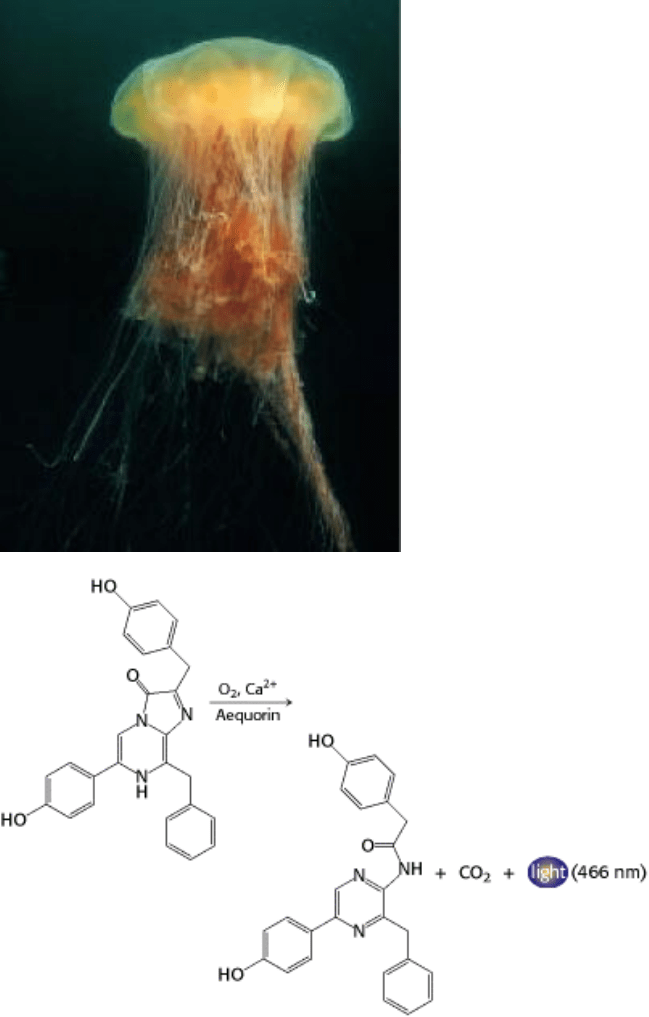
The activity of an enzyme is responsible for the glow of the luminescent jellyfish at left. The enzyme aequorin
catalyzes the oxidation of a compound by oxygen in the presence of calcium to release CO
2
and light. [(Left) Fred
Bavendam/Peter Arnold.]
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
8.1. Enzymes Are Powerful and Highly Specific Catalysts
Enzymes accelerate reactions by factors of as much as a million or more (Table 8.1). Indeed, most reactions in biological
systems do not take place at perceptible rates in the absence of enzymes. Even a reaction as simple as the hydration of
carbon dioxide is catalyzed by an enzyme namely, carbonic anhydrase (Section 9.2). The transfer of CO
2
from the
tissues into the blood and then to the alveolar air would be less complete in the absence of this enzyme. In fact, carbonic
anhydrase is one of the fastest enzymes known. Each enzyme molecule can hydrate 10
6
molecules of CO
2
per second.
This catalyzed reaction is 10
7
times as fast as the uncatalyzed one. We will consider the mechanism of carbonic
anhydrase catalysis in Chapter 9. Enzymes are highly specific both in the reactions that they catalyze and in their choice
of reactants, which are called substrates. An enzyme usually catalyzes a single chemical reaction or a set of closely
related reactions. Side reactions leading to the wasteful formation of by-products are rare in enzyme-catalyzed reactions,
in contrast with uncatalyzed ones.
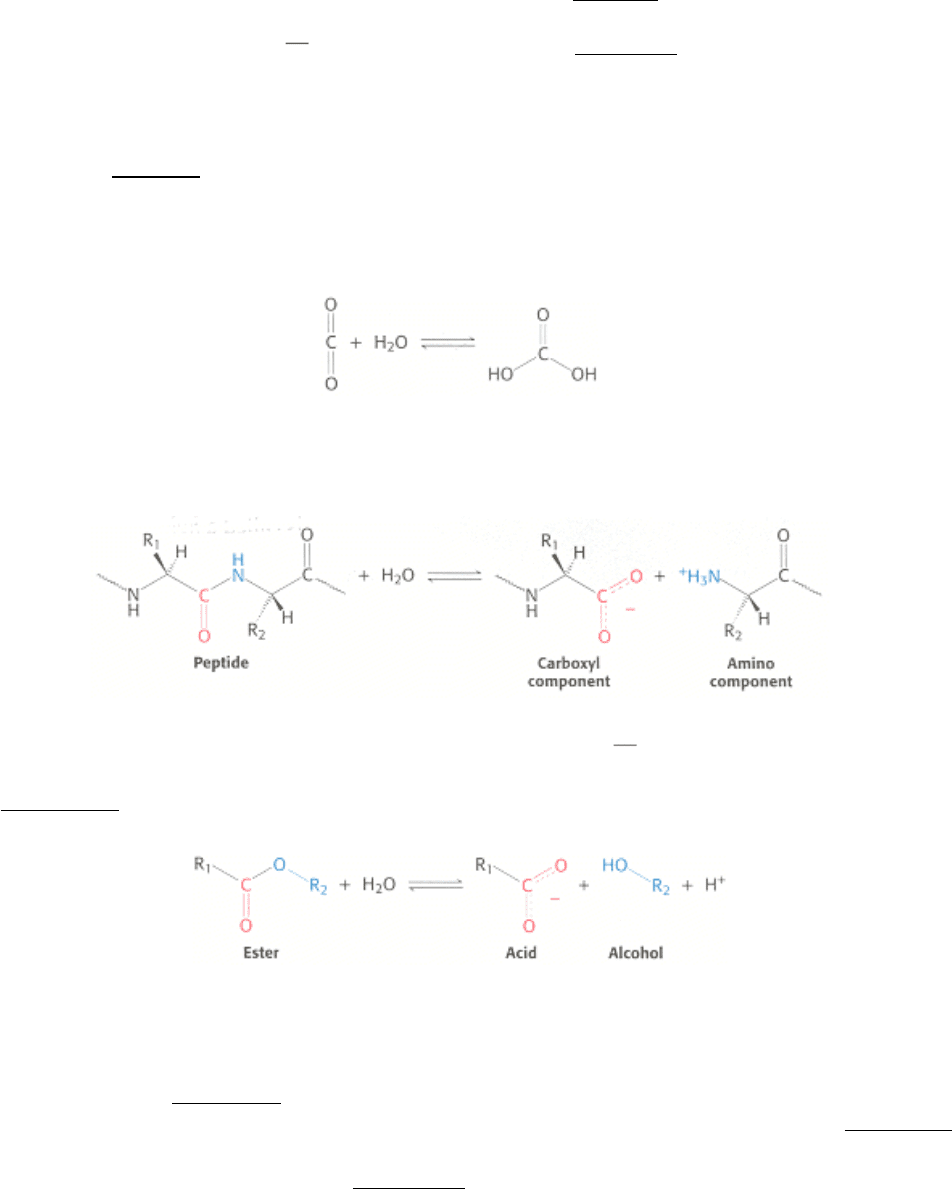
Let us consider proteolytic enzymes as an example. In vivo, these enzymes catalyze proteolysis, the hydrolysis of a
peptide bond.
Most proteolytic enzymes also catalyze a different but related reaction in vitro
namely, the hydrolysis of an ester bond.
Such reactions are more easily monitored than is proteolysis and are useful in experimental investigations of these
enzymes (Section 9.1.2).
Proteolytic enzymes differ markedly in their degree of substrate specificity. Subtilisin, which is found in certain bacteria,
is quite undiscriminating: it will cleave any peptide bond with little regard to the identity of the adjacent side chains.
Trypsin, a digestive enzyme, is quite specific and catalyzes the splitting of peptide bonds only on the carboxyl side of
lysine and arginine residues (Figure 8.1A). Thrombin, an enzyme that participates in blood clotting, is even more
specific than trypsin. It catalyzes the hydrolysis of Arg-Gly bonds in particular peptide sequences only (Figure 8.1B).
DNA polymerase I, a template-directed enzyme (Section 27.2), is another highly specific catalyst. It adds nucleotides to
a DNA strand that is being synthesized, in a sequence determined by the sequence of nucleotides in another DNA strand
that serves as a template. DNA polymerase I is remarkably precise in carrying out the instructions given by the template.
It inserts the wrong nucleotide into a new DNA strand less than one in a million times.
The specificity of an enzyme is due to the precise interaction of the substrate with the enzyme. This precision is a result
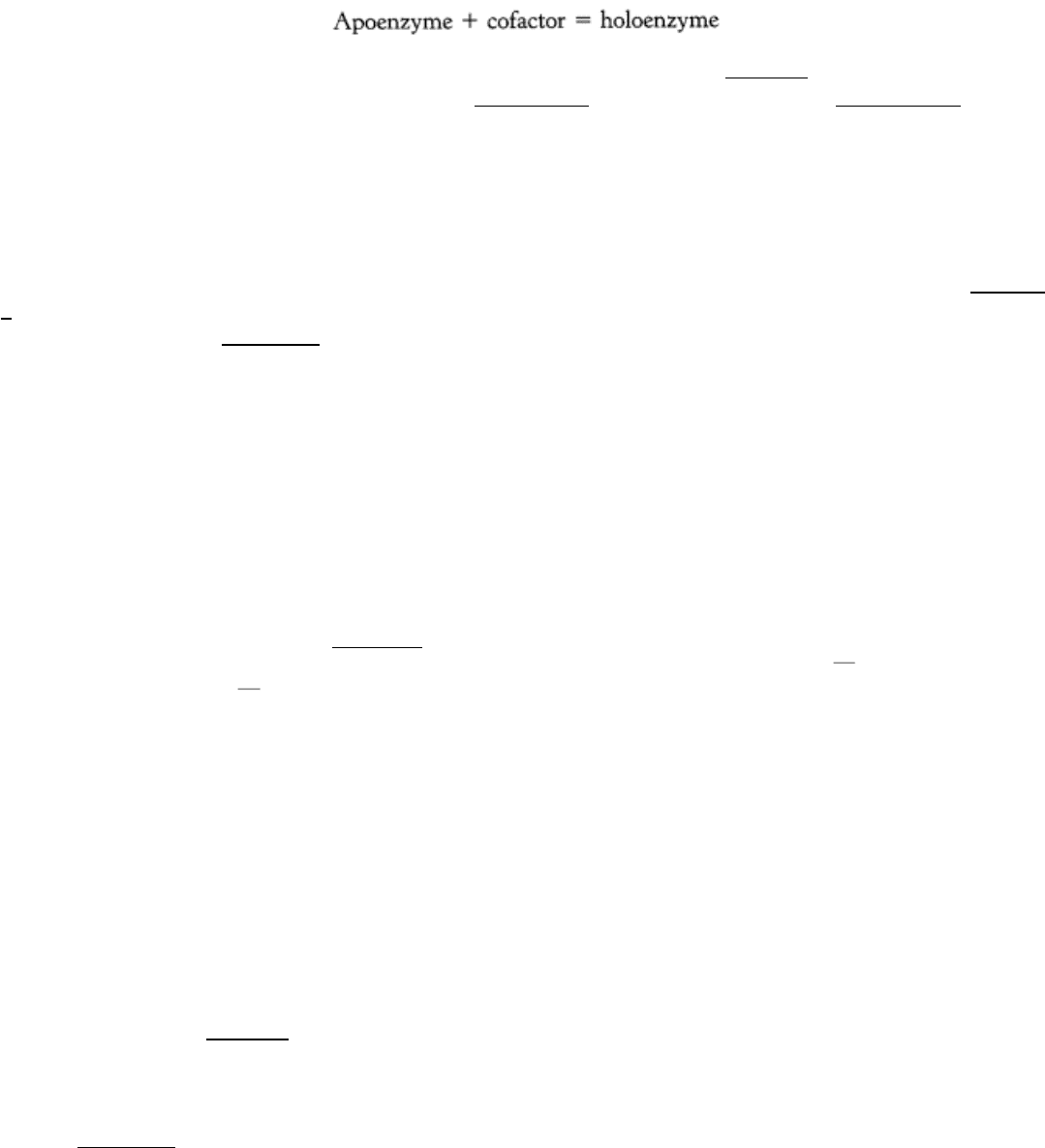
of the intricate three-dimensional structure of the enzyme protein.
8.1.1. Many Enzymes Require Cofactors for Activity
The catalytic activity of many enzymes depends on the presence of small molecules termed cofactors, although the
precise role varies with the cofactor and the enzyme. Such an enzyme without its cofactor is referred to as an apoenzyme;
the complete, catalytically active enzyme is called a holoenzyme.
Cofactors can be subdivided into two groups: metals and small organic molecules (Table 8.2). The enzyme carbonic
anhydrase, for example, requires Zn
2+
for its activity (Section 9.2.1). Glycogen phosphorylase (Section 21.1.5), which
mobilizes glycogen for energy, requires the small organic molecule pyridoxal phosphate (PLP).
Cofactors that are small organic molecules are called coenzymes. Often derived from vitamins, coenzymes can be either
tightly or loosely bound to the enzyme. If tightly bound, they are called prosthetic groups. Loosely associated
coenzymes are more like cosubstrates because they bind to and are released from the enzyme just as substrates and
products are. The use of the same coenzyme by a variety of enzymes and their source in vitamins sets coenzymes apart
from normal substrates, however. Enzymes that use the same coenzyme are usually mechanistically similar. In Chapter
9, we will examine the mechanistic importance of cofactors to enzyme activity. A more detailed discussion of coenzyme
vitamins can be found in Section 8.6.
8.1.2. Enzymes May Transform Energy from One Form into Another
In many biochemical reactions, the energy of the reactants is converted with high efficiency into a different form. For
example, in photosynthesis, light energy is converted into chemical-bond energy through an ion gradient. In
mitochondria, the free energy contained in small molecules derived from food is converted first into the free energy of an
ion gradient and then into a different currency, the free energy of adenosine triphosphate. Enzymes may then use the
chemical-bond energy of ATP in many ways. The enzyme myosin converts the energy of ATP into the mechanical
energy of contracting muscles. Pumps in the membranes of cells and organelles, which can be thought of as enzymes that
move substrates rather than chemically altering them, create chemical and electrical gradients by using the energy of
ATP to transport molecules and ions (Figure 8.2). The molecular mechanisms of these energy-transducing enzymes are
being unraveled. We will see in subsequent chapters how unidirectional cycles of discrete steps binding, chemical
transformation, and release lead to the conversion of one form of energy into another.
8.1.3. Enzymes Are Classified on the Basis of the Types of Reactions That They
Catalyze
Many enzymes have common names that provide little information about the reactions that they catalyze. For example, a
proteolytic enzyme secreted by the pancreas is called trypsin. Most other enzymes are named for their substrates and for
the reactions that they catalyze, with the suffix "ase" added. Thus, an ATPase is an enzyme that breaks down ATP,
whereas ATP synthase is an enzyme that synthesizes ATP.
To bring some consistency to the classification of enzymes, in 1964 the International Union of Biochemistry established
an Enzyme Commission to develop a nomenclature for enzymes. Reactions were divided into six major groups
numbered 1 through 6 (Table 8.3). These groups were subdivided and further subdivided, so that a four-digit number
preceded by the letters EC for Enzyme Commission could precisely identify all enzymes.
Consider as an example nucleoside monophosphate (NMP) kinase, an enzyme that we will examine in detail in the next
chapter (Section 9.4). It catalyzes the following reaction:
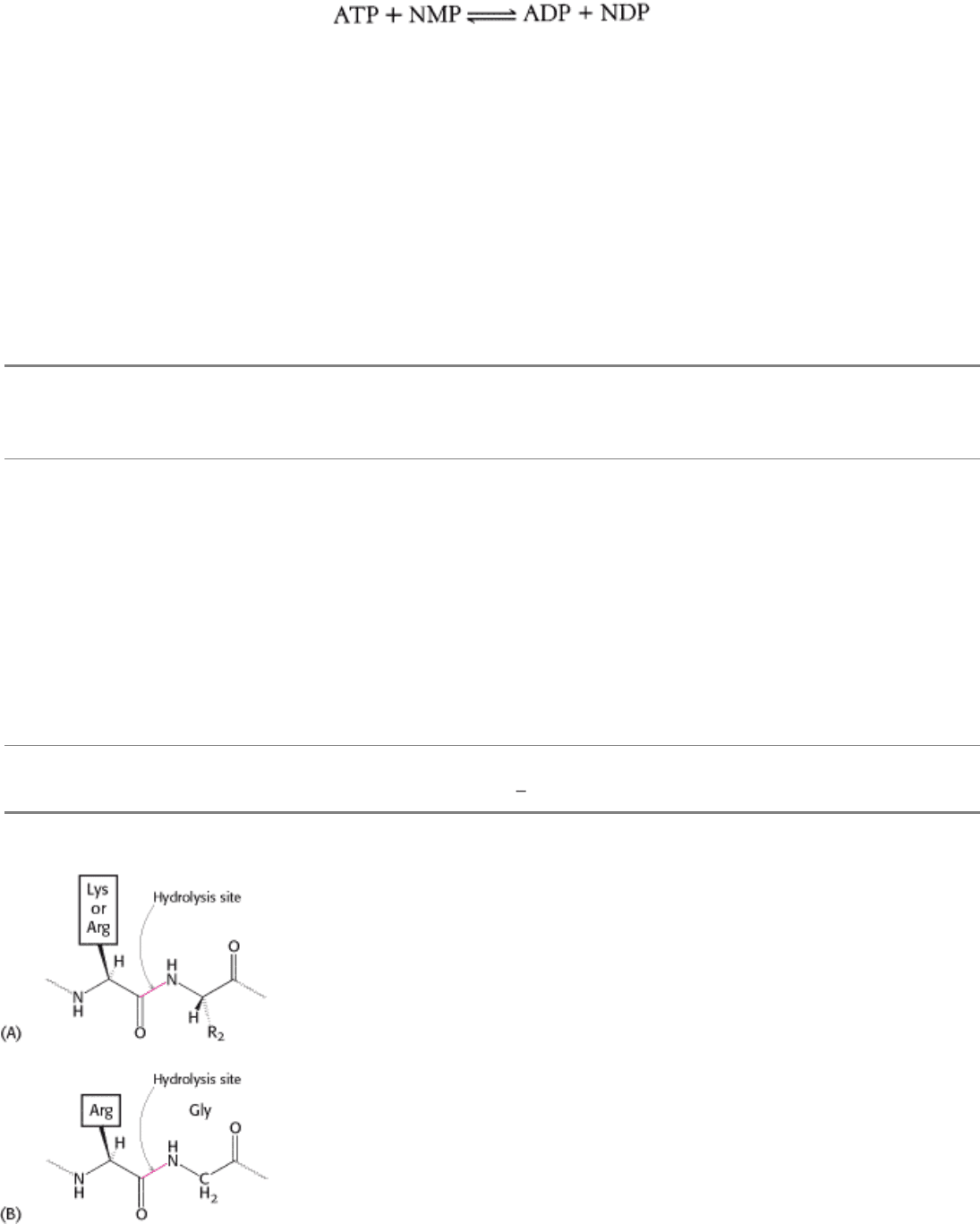
NMP kinase transfers a phosphoryl group from ATP to NMP to form a nucleoside diphosphate (NDP) and ADP.
Consequently, it is a transferase, or member of group 2. Many groups in addition to phosphoryl groups, such as sugars
and carbon units, can be transferred. Transferases that shift a phosphoryl group are designated 2.7. Various functional
groups can accept the phosphoryl group. If a phosphate is the acceptor, the transferase is designated 2.7.4. The final
number designates the acceptor more precisely. In regard to NMP kinase, a nucleoside monophosphate is the acceptor,
and the enzyme's designation is EC 2.7.4.4. Although the common names are used routinely, the classification number is
used when the precise identity of the enzyme might be ambiguous.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.1. Enzymes Are Powerful and Highly Specific Catalysts
Table 8.1. Rate enhancement by selected enzymes
Enzyme Nonenzymatic half-life Uncatalyzed rate (k
un
,
s
-1
)
Catalyzed rate (k
cat
, s
-1
)
Rate enhancement (k
cat
/
k
un
)
OMP decarboxylase 78,000,000 years
2.8 × 10
-16
39
1.4 × 10
17
Staphylococcal nuclease 130,000 years
1.7 × 10
-13
95
5.6 × 10
14
AMP nucleosidase 69,000 years
1.0 × 10
-11
60
6.0 × 10
12
Carboxypeptidase A 7.3 years
3.0 × 10
-9
578
1.9 × 10
11
Ketosteroid isomerase 7 weeks
1.7 × 10
-7
66,000
3.9 × 10
11
Triose phosphate isomerase 1.9 days
4.3 × 10
-6
4,300
1.0 × 10
9
Chorismate mutase 7.4 hours
2.6 × 10
-5
50
1.9 × 10
6
Carbonic anhydrase 5 seconds
1.3 × 10
-1
1 × 10
6
7.7 × 10
6
Abbreviations: OMP, orotidine monophosphate; AMP, adenosine monophosphate.
Source: After A. Radzicka and R. Wofenden. Science 267 (1995):90 93.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.1. Enzymes Are Powerful and Highly Specific Catalysts
Figure 8.1. Enzyme Specificity. (A) Trypsin cleaves on the carboxyl side of arginine and lysine residues, whereas (B)
thrombin cleaves Arg-Gly bonds in particular sequences specifically.
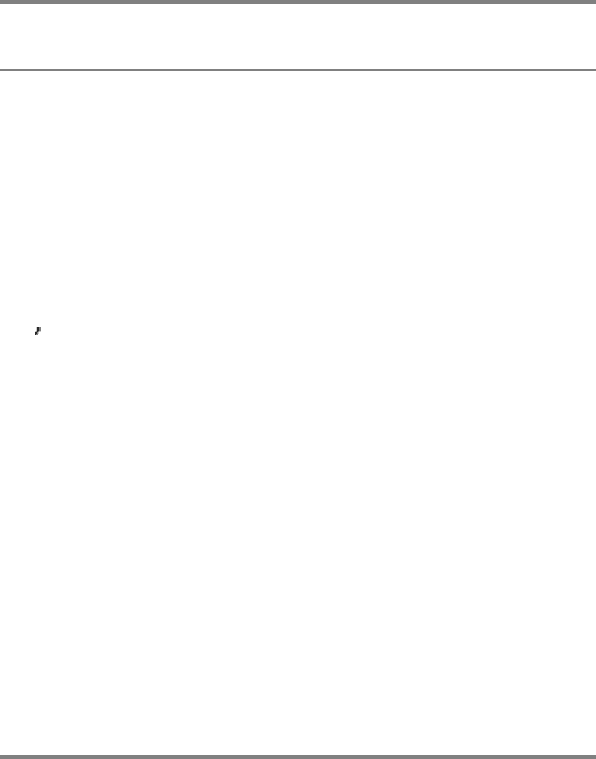
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.1. Enzymes Are Powerful and Highly Specific Catalysts
Table 8.2. Enzyme cofactors
Cofactor Enzyme
Coenzyme
Thiamine pyrophosphate Pyruvate dehydrogenase
Flavin adenine nucleotide Monoamine oxidase
Nicotinamide adenine dinucleotide Lactate dehydrogenase
Pyridoxal phosphate Glycogen phosphorylase
Coenzyme A (CoA) Acetyl CoA carboxylase
Biotin Pyruvate carboxylase
5
-Deoxyadenosyl cobalamin Methylmalonyl mutase
Tetrahydrofolate Thymidylate synthase
Metal
Zn
2+
Carbonic anhydrase
Zn
2+
Carboxypeptidase
Mg
2+
EcoRV
Mg
2+
Hexokinase
Ni
2+
Urease
Mo Nitrate reductase
Se Glutathione peroxidase
Mn
2+
Superoxide dismutase
K
+
Propionyl CoA carboxylase
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.1. Enzymes Are Powerful and Highly Specific Catalysts
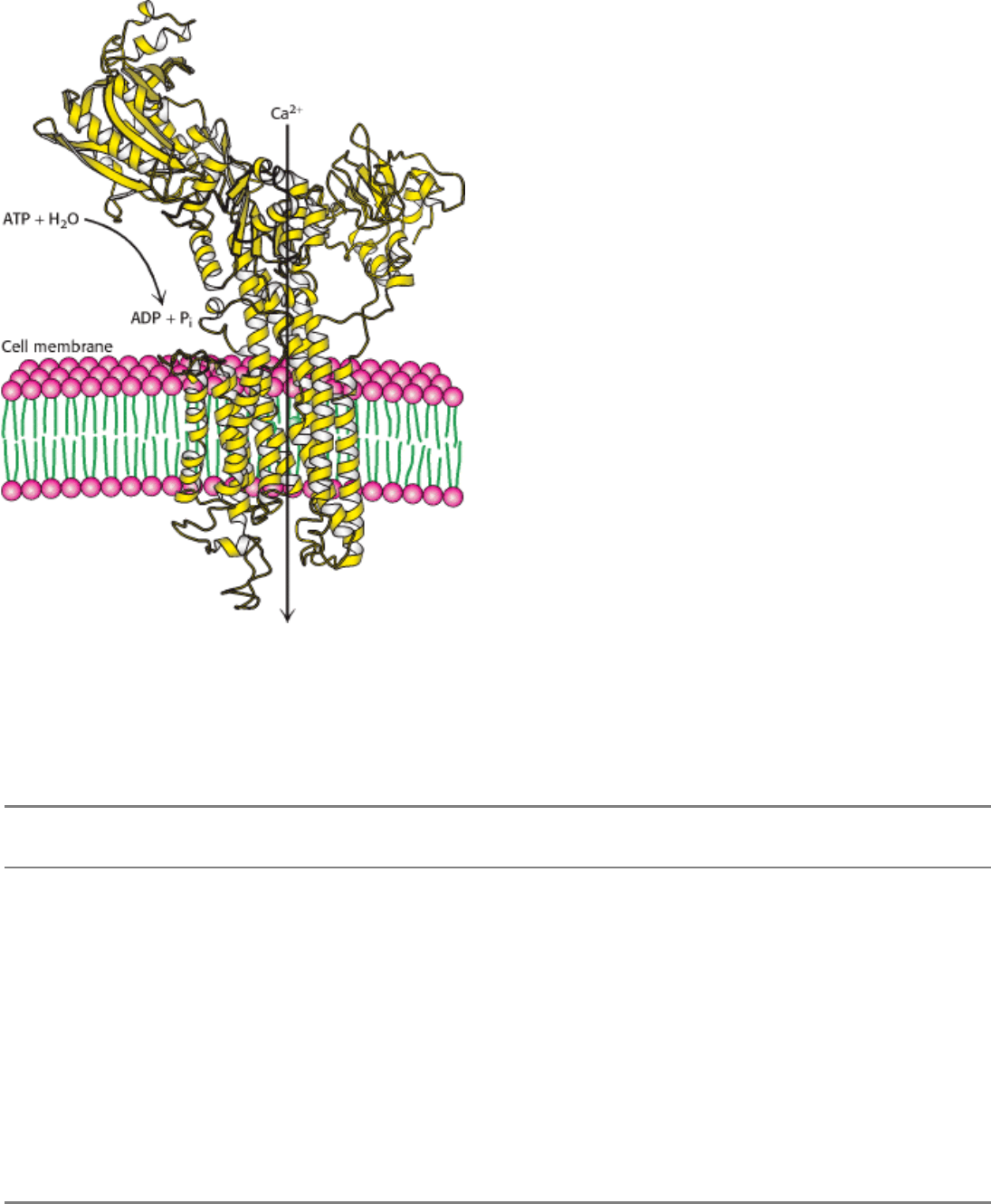
Figure 8.2. An Energy-Transforming Enzyme. Ca
2+
ATPase uses the energy of ATP hydrolysis to transport Ca
2+
across the membrane, generating a Ca
2+
gradient.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.1. Enzymes Are Powerful and Highly Specific Catalysts
Table 8.3. Six major classes of enzymes
Class Type of reaction Example Chapter
1. Oxidoreductases Oxidation-reduction Lactate dehydrogenase 16
2. Transferases Group transfer Nucleoside monophosphate kinase (NMP
kinase)
9
3. Hydrolases Hydrolysis reactions (transfer of functional
groups to water)
Chymotrypsin 9
4. Lyases Addition or removal of groups to form
double bonds
Fumarase 18
5. Isomerases Isomerization (intramolecular group
transfer)
Triose phosphate isomerase 16
6. Ligases Ligation of two substrates at the expense of
ATP hydrolysis
Aminoacyl-tRNA synthetase 29
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
8.2. Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes
Some of the principles of thermodynamics were introduced in Chapter 1 notably the idea of free energy (G). To fully
understand how enzymes operate, we need to consider two thermodynamic properties of the reaction: (1) the free-energy
difference (∆ G) between the products and reactants and (2) the energy required to initiate the conversion of reactants to
products. The former determines whether the reaction will be spontaneous, whereas the later determines the rate of the
reaction. Enzymes affect only the latter. First, we will consider the thermodynamics of reactions and then, in Section 8.3,
the rates of reactions.
8.2.1. The Free-Energy Change Provides Information About the Spontaneity but Not
the Rate of a Reaction
As stated in Section 1.3.3, the free-energy change of a reaction (∆ G) tells us if the reaction can occur spontaneously:
1. A reaction can occur spontaneously only if ∆ G is negative. Such reactions are said to be exergonic.
2. A system is at equilibrium and no net change can take place if ∆ G is zero.
3. A reaction cannot occur spontaneously if ∆ G is positive. An input of free energy is required to drive such a reaction.
These reactions are termed endergonic.
Two additional points need to be emphasized. The ∆ G of a reaction depends only on the free energy of the products (the
final state) minus the free energy of the reactants (the initial state). The ∆G of a reaction is independent of the path (or
molecular mechanism) of the transformation. The mechanism of a reaction has no effect on ∆ G. For example, the ∆ G
for the oxidation of glucose to CO
2
and H
2
O is the same whether it occurs by combustion in vitro or by a series of
enzyme-catalyzed steps in a cell. The ∆ G provides no information about the rate of a reaction. A negative ∆ G indicates
that a reaction can occur spontaneously, but it does not signify whether it will proceed at a perceptible rate. As will be
discussed shortly (Section 8.3), the rate of a reaction depends on the free energy of activation (∆ G
), which is largely
unrelated to the ∆ G of the reaction.
8.2.2. The Standard Free-Energy Change of a Reaction Is Related to the Equilibrium
Constant
As for any reaction, we need to be able to determine ∆ G for an enzymecatalyzed reaction in order to know whether the
reaction is spontaneous or an input of energy is required. To determine this important thermodynamic parameter, we
need to take into account the nature of both the reactants and the products as well as their concentrations.
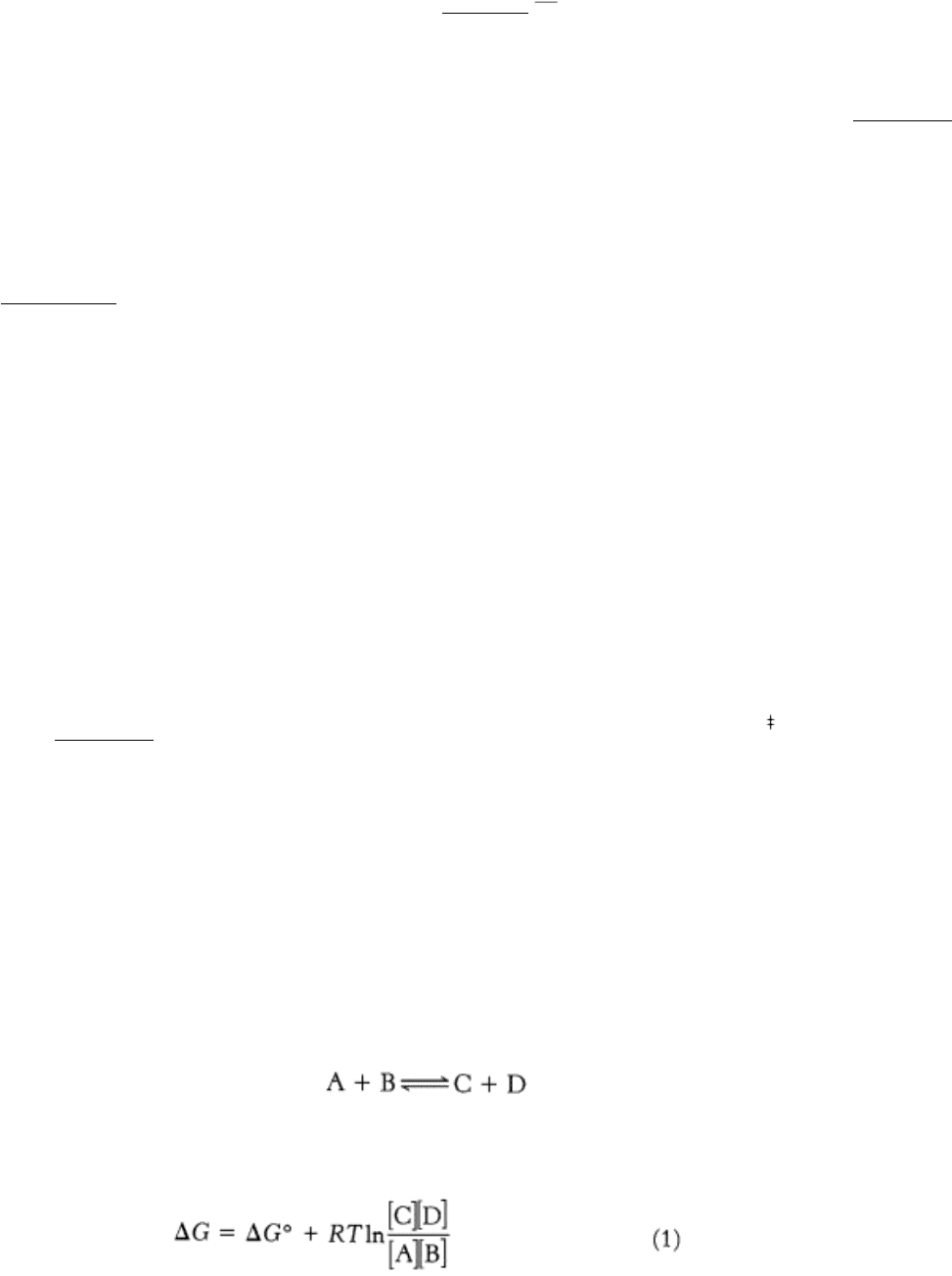
Consider the reaction
The ∆ G of this reaction is given by
in which ∆ G° is the standard free-energy change, R is the gas constant, T is the absolute temperature, and [A], [B], [C],
and [D] are the molar concentrations (more precisely, the activities) of the reactants. ∆ G° is the freeenergy change for
this reaction under standard conditions that is, when each of the reactants A, B, C, and D is present at a concentration
of 1.0 M (for a gas, the standard state is usually chosen to be 1 atmosphere). Thus, the ∆ G of a reaction depends on the
nature of the reactants (expressed in the ∆ G° term of equation 1) and on their concentrations (expressed in the
logarithmic term of equation 1).
Units of energy-
A calorie (cal) is equivalent to the amount of heat required to raise
the temperature of 1 gram of water from 14.5°C to 15.5°C.
A kilocalorie (kcal) is equal to 1000 cal.
A joule (J) is the amount of energy needed to apply a 1-newton force
over a distance of 1 meter.
A kilojoule (kJ) is equal to 1000 J.
1 kcal = 4.184 kJ
A convention has been adopted to simplify free-energy calculations for biochemical reactions. The standard state is
defined as having a pH of 7. Consequently, when H
+
is a reactant, its activity has the value 1 (corresponding to a pH of
7) in equations 1 and 4 (below). The activity of water also is taken to be 1 in these equations. The standard free-energy
change at pH 7, denoted by the symbol ∆ G°
will be used throughout this book. The kilocalorie (abbreviated kcal) and
the kilojoule (kJ) will be used as the units of energy. One kilocalorie is equivalent to 4.184 kilojoules.
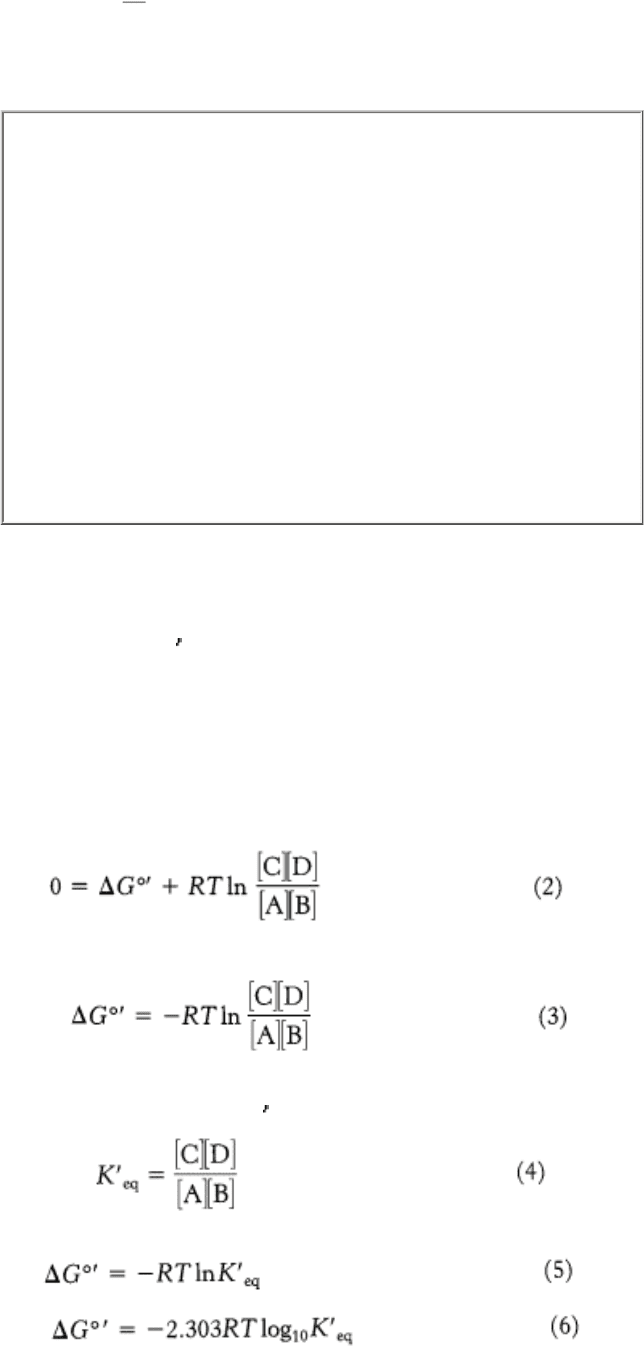
The relation between the standard free energy and the equilibrium constant of a reaction can be readily derived. This
equation is important because it displays the energetic relation between products and reactants in terms of their
concentrations. At equilibrium, ∆ G = 0. Equation 1 then becomes
and so
The equilibrium constant under standard conditions, K
eq
, is defined as
Substituting equation 4 into equation 3 gives
which can be rearranged to give
