Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

only enzymes that exhibit Michaelis-Menten kinetics. Measurements of the rates of catalysis at different concentrations
of substrate and inhibitor serve to distinguish the two types of inhibition. In competitive inhibition, the inhibitor
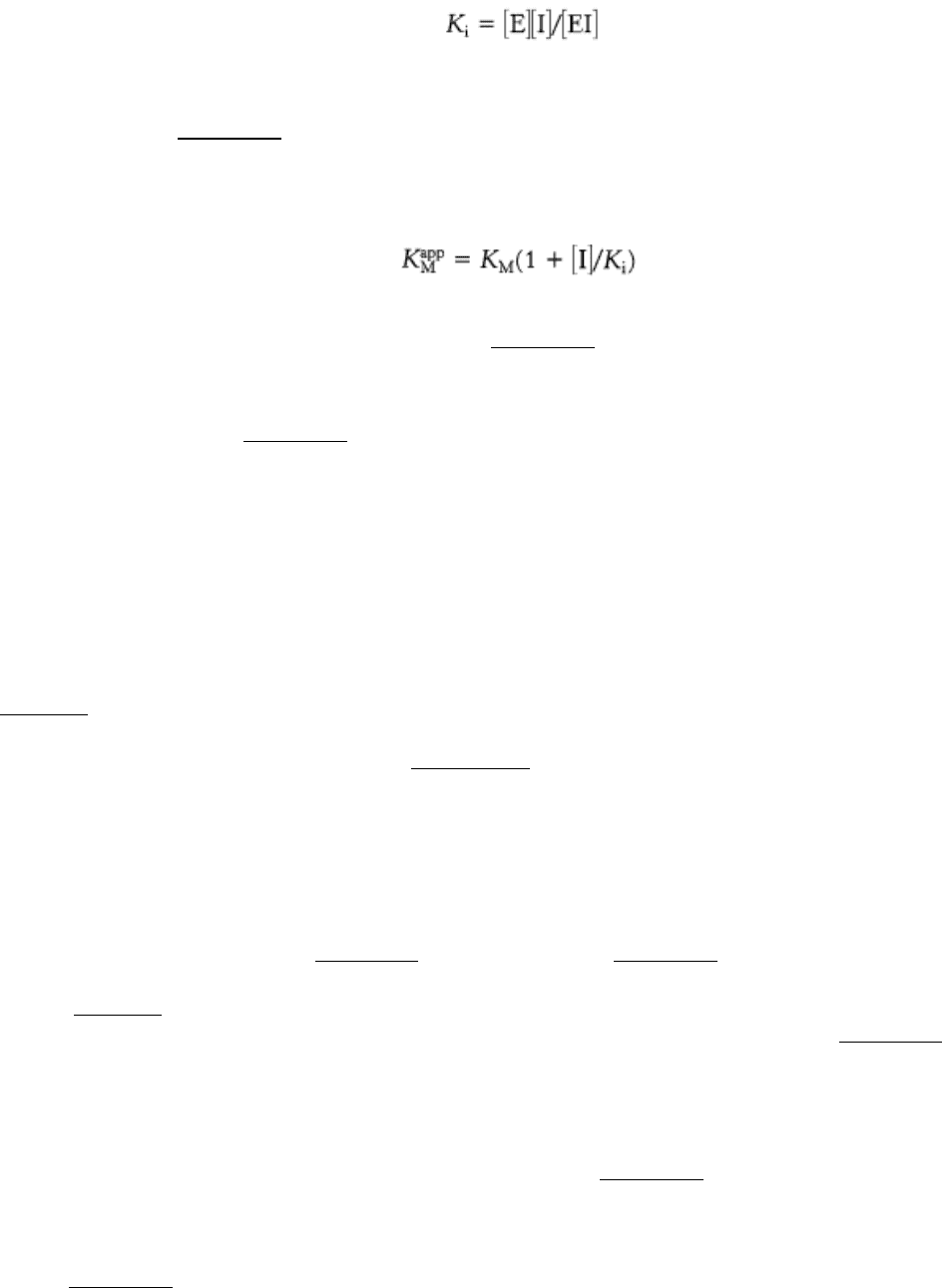
competes with the substrate for the active site. The dissociation constant for the inhibitor is given by
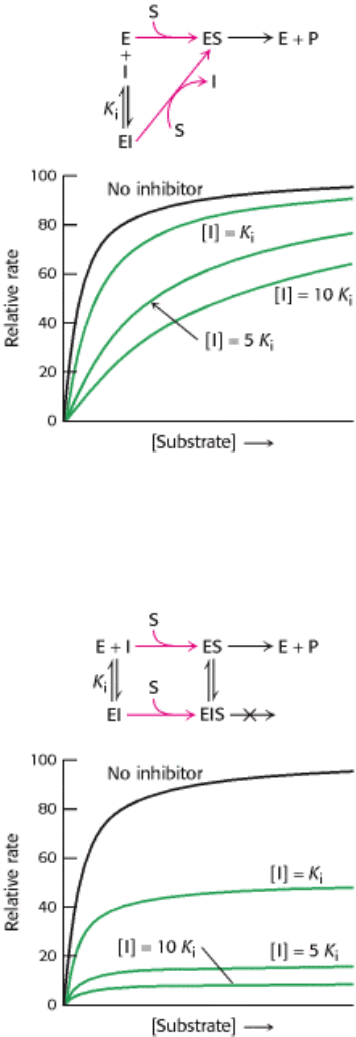
Because increasing the amount of substrate can overcome the inhibition, V
max
can be attained in the presence of a
competitive inhibitor (Figure 8.17). The hallmark of competitive inhibition is that it can be overcome by a sufficiently
high concentration of substrate. However, the apparent value of K
M
is altered; the effect of a competitive inhibitor is to
increase the apparent value of K
M
. This new value of K
M
, called K
app
M
, is numerically equal to
where [I] is the concentration of inhibitor and K
i
is the dissociation constant for the enzyme-inhibitor complex. As the
value of [I] increases, the value of K
app
M
increases (see Figure 8.17). In the presence of a competitive inhibitor, an
enzyme will have the same V
max
as in the absence of an inhibitor.
In noncompetitive inhibition (Figure 8.18), substrate can still bind to the enzyme-inhibitor complex. However, the
enzyme-inhibitor-substrate complex does not proceed to form product. The value of V
max
is decreased to a new value
called V
app
max
while the value of K
M
is unchanged. Why is V
max
lowered while K
M
remains unchanged? In essence,
the inhibitor simply lowers the concentration of functional enzyme. The remaining enzyme behaves like a more dilute
solution of enzyme; V
max
is lower, but K
M
is the same. Noncompetitive inhibition cannot be overcome by increasing
the substrate concentration.
8.5.2. Irreversible Inhibitors Can Be Used to Map the Active Site
In Chapter 9, we will examine the chemical details of how enzymes function. The first step in obtaining the chemical
mechanism of an enzyme is to determine what functional groups are required for enzyme activity. How can we ascertain
these functional groups? X-ray crystallography (Section 4.5.2) of the enzyme bound to its substrate provides one
approach. Irreversible inhibitors that covalently bond to the enzyme provide an alternative and often complementary
means for elucidating functional groups at the enzyme active site because they modify the functional groups, which can
then be identified. Irreversible inhibitors can be divided into three categories: group-specific reagents, substrate analogs,
and suicide inhibitors.
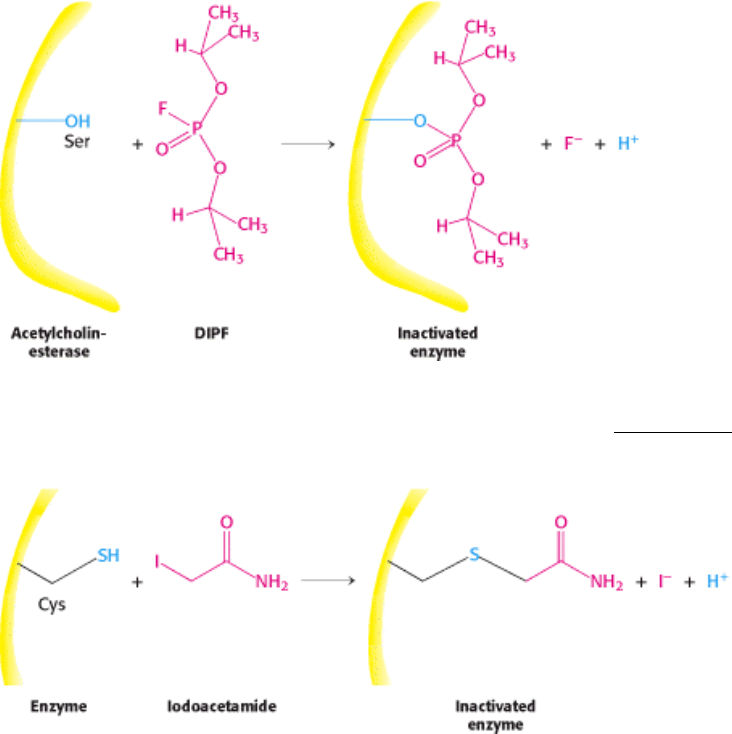
Group-specific reagents react with specific R groups of amino acids. Two examples of group-specific reagents are
diisopropylphosphofluoridate (DIPF; Figure 8.19) and iodoacetamide (Figure 8.20). DIPF modifies only 1 of the 28
serine residues in the proteolytic enzyme chymotrypsin, implying that this serine residue is especially reactive. As we
will see in Chapter 9, it is indeed the case that this serine residue is at the active site. DIPF also revealed a reactive serine
residue in acetylcholinesterase, an enzyme important in the transmission of nerve impulses (see Figure 8.19). Thus, DIPF
and similar compounds that bind and inactivate acetylcholinesterase are potent nerve gases.
Affinity labels are molecules that are structurally similar to the substrate for the enzyme that covalently modify active site
residues. They are thus more specific for the enzyme active site than are group-specific reagents. Tosyl-
l-phenylalanine
chloromethyl ketone (TPCK) is a substrate analog for chymotrypsin (Figure 8.21). TPCK binds at the active site; and
then reacts irreversibly with a histidine residue at that site, inhibiting the enzyme. The compound 3-bromoacetol is an
affinity label for the enzyme triose phosphate isomerase (TIM). It mimics the normal substrate, dihydroxyacetone
phosphate, by binding at the active site; then it covalently modifies the enzyme such that the enzyme is irreversibly
inhibited (Figure 8.22).
Suicide inhibitors, or mechanism-based inhibitors are modified substrates that provide the most specific means to modify
an enzyme active site. The inhibitor binds to the enzyme as a substrate and is initially processed by the normal catalytic
mechanism. The mechanism of catalysis then generates a chemically reactive intermediate that inactivates the enzyme
through covalent modification. The fact that the enzyme participates in its own irreversible inhibition strongly suggests
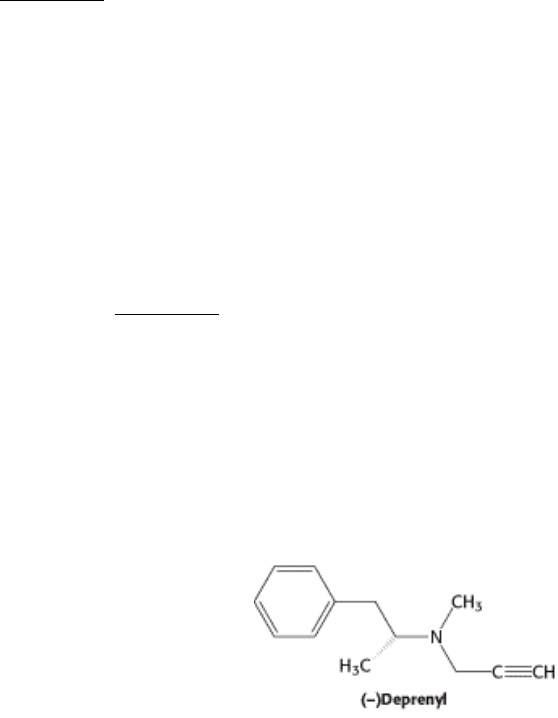
that the covalently modified group on the enzyme is catalytically vital. One example of such an inhibitor is N,N-
dimethylpropargylamine. A flavin prosthetic group of monoamine oxidase (MAO) oxidizes the N,N-
dimethylpropargylamine, which in turn inactivates the enzyme by covalently modifying the flavin prosthetic group by
alkylating N-5 (Figure 8.23). Monoamine oxidase deaminates neurotransmitters such as dopamine and serotonin,
lowering their levels in the brain. Parkinson disease is associated with low levels of dopamine, and depression is
associated with low levels of serotonin. The drug (-)deprenyl, which is used to treat Parkinson disease and depression, is
a suicide inhibitor of monoamine oxidase.
8.5.3. Transition-State Analogs Are Potent Inhibitors of Enzymes
We turn now to compounds that provide the most intimate views of the catalytic process itself. Linus Pauling proposed
in 1948 that compounds resembling the transition state of a catalyzed reaction should be very effective inhibitors of
enzymes. These mimics are called transition-state analogs. The inhibition of proline racemase is an instructive example.
The racemization of proline proceeds through a transition state in which the tetrahedral α- carbon atom has become
trigonal by loss of a proton (Figure 8.24). In the trigonal form, all three bonds are in the same plane; C
α
also carries a
net negative charge. This symmetric carbanion can be reprotonated on one side to give the
l isomer or on the other side
to give the d isomer. This picture is supported by the finding that the inhibitor pyrrole 2-carboxylate binds to the
racemase 160 times as tightly as does proline. The α-carbon atom of this inhibitor, like that of the transition state, is
trigonal. An analog that also carries a negative charge on C
α
would be expected to bind even more tightly. In general,
synthesizing compounds that more closely resemble the transition state than the substrate itself can produce highly
potent and specific inhibitors of enzymes. The inhibitory power of transition-state analogs underscores the essence of
catalysis: selective binding of the transition state.
8.5.4. Catalytic Antibodies Demonstrate the Importance of Selective Binding of the
Transition State to Enzymatic Activity
Antibodies that recognize transition states should function as catalysts, if our understanding of the importance of the
transition state to catalysis is correct. The preparation of an antibody that catalyzes the insertion of a metal ion into a
porphyrin nicely illustrates the validity of this approach. Ferrochelatase, the final enzyme in the biosynthetic pathway for
the production of heme, catalyzes the insertion of Fe
2+
into protoporphyrin IX. The nearly planar porphyrin must be bent
for iron to enter. The recently determined crystal structure of the ferrochelatase bound to a substrate analog confirms that
the enzyme does indeed bend one of the pyrole rings, distorting it 36 degrees to insert the iron.
The problem was to find a transition-state analog for this metallation reaction that could be used as an antigen
(immunogen) to generate an antibody. The solution came from the results of studies showing that an alkylated porphyrin,
N-methylprotoporphyrin, is a potent inhibitor of ferrochelatase. This compound resembles the transition state because N-
alkylation forces the porphyrin to be bent. Moreover, it was known that N-alkylporphyrins chelate metal ions 10
4
times
as fast as their unalkylated counterparts do. Bending increases the exposure of the pyrrole nitrogen lone pairs of electrons
to solvent, which facilitates metal in binding.
An antibody catalyst was produced with the use of an N-alkylporphyrin as the immunogen. The resulting antibody
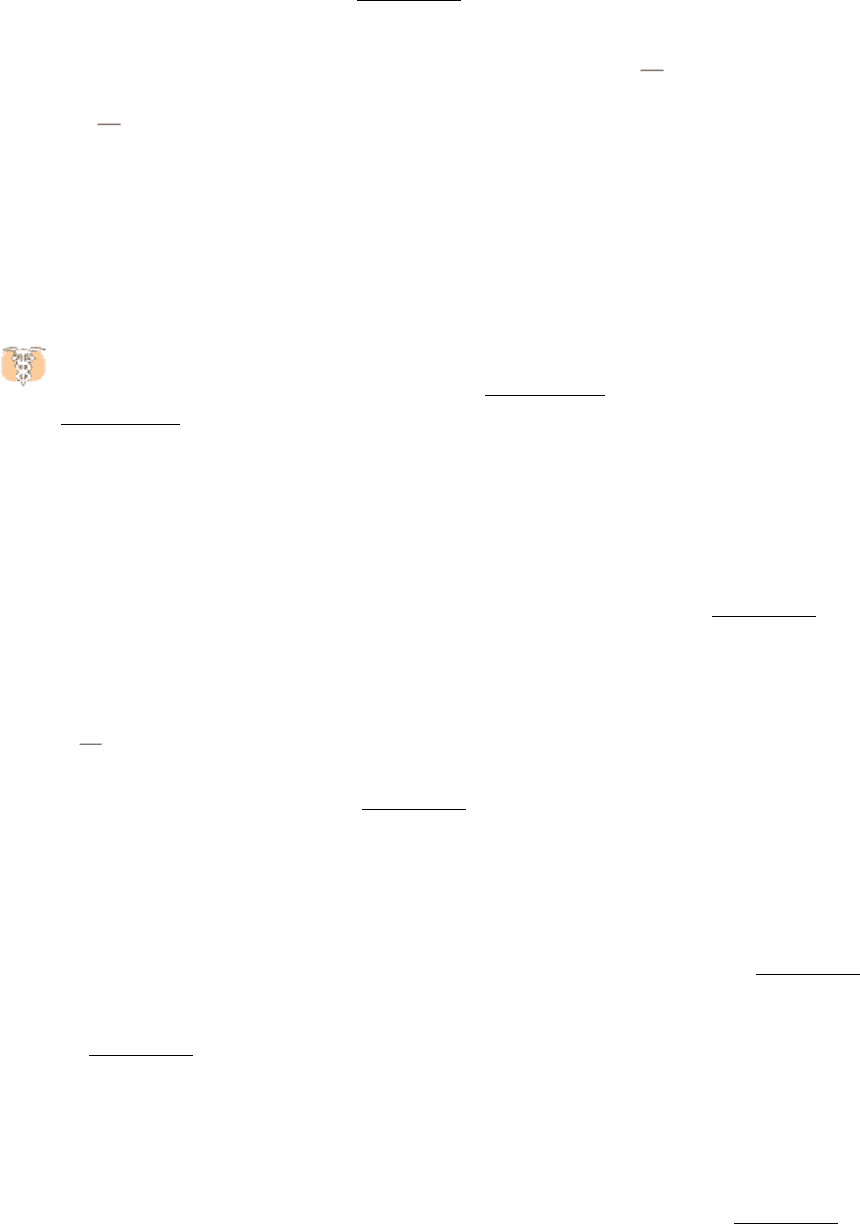
presumably distorts a planar porphyrin (Figure 8.25) to facilitate the entry of a metal. On average, an antibody molecule
metallated 80 porphyrin molecules per hour, a rate only 10-fold less than that of ferrochelatase and 2500-fold faster than
the uncatalyzed reaction. Catalytic antibodies (abzymes) can indeed be produced by using transition-state analogs as
antigens. Antibodies catalyzing many other kinds of chemical reactions exemplified by ester and amide hydrolysis,
amide-bond formation, transesterification, photoinduced cleavage, photoinduced dimerization, decarboxylation, and
oxidization have been produced with the use of similar strategies. The results of studies with transition-state analogs
provide strong evidence that enzymes can function complementary in structure to the transition state. The power of
transition-state analogs is now evident: (1) they are sources of insight into catalytic mechanisms, (2) they can serve as
potent and specific inhibitors of enzymes, and (3) they can be used as immunogens to generate a wide range of novel
catalysts.
8.5.5. Penicillin Irreversibly Inactivates a Key Enzyme in Bacterial Cell-Wall Synthesis
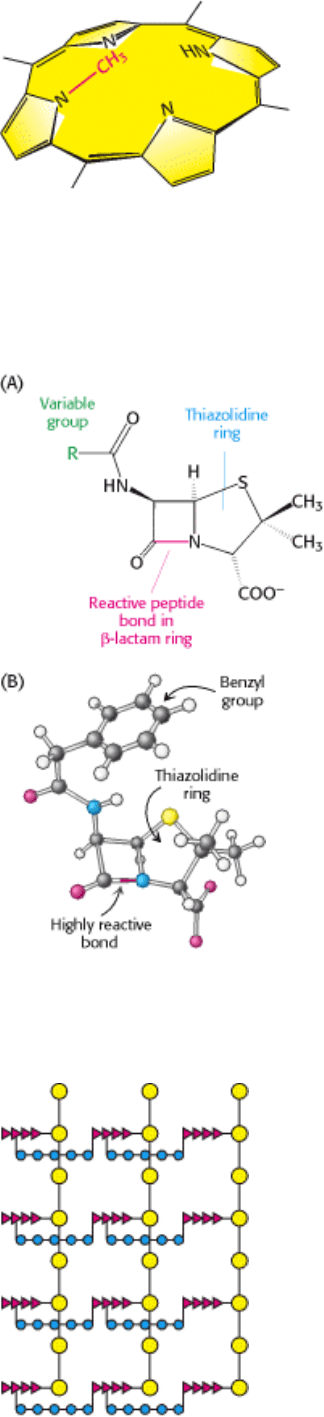
Penicillin, the first antibiotic discovered, consists of a thiazolidine ring fused to a β-lactam ring, to which a
variable R group is attached by a peptide bond (Figure 8.26A). In benzyl penicillin, for example, R is a benzyl
group (Figure 8.26B). This structure can undergo a variety of rearrangements, and, in particular, the β-lactam ring is very
labile. Indeed, this instability is closely tied to the antibiotic action of penicillin, as will be evident shortly.
How does penicillin inhibit bacterial growth? In 1957, Joshua Lederberg showed that bacteria ordinarily susceptible to
penicillin could be grown in its presence if a hypertonic medium were used. The organisms obtained in this way, called
protoplasts, are devoid of a cell wall and consequently lyse when transferred to a normal medium. Hence, penicillin was
inferred to interfere with the synthesis of the bacterial cell wall. The cell-wall macromolecule, called a peptidoglycan,
consists of linear polysaccharide chains that are cross-linked by short peptides (Figure 8.27). The enormous bag-shaped
peptidoglycan confers mechanical support and prevents bacteria from bursting in response to their high internal osmotic
pressure.
In 1965, James Park and Jack Strominger independently deduced that penicillin blocks the last step in cell-wall
synthesis
namely, the crosslinking of different peptidoglycan strands. In the formation of the cell wall of
Staphylococcus aureus, the amino group at one end of a pentaglycine chain attacks the peptide bond between two d-
alanine residues in another peptide unit (Figure 8.28). A peptide bond is formed between glycine and one of the d-alanine
residues; the other d-alanine residue is released. This cross-linking reaction is catalyzed by glycopeptide transpeptidase.
Bacterial cell walls are unique in containing d amino acids, which form cross-links by a mechanism different from that
used to synthesize proteins.
Penicillin inhibits the cross-linking transpeptidase by the Trojan horse stratagem. The transpeptidase normally forms an
acyl intermediate with the penultimate d-alanine residue of the d-Ala-d-Ala peptide (Figure 8.29). This covalent acyl-
enzyme intermediate then reacts with the amino group of the terminal glycine in another peptide to form the cross-link.
Penicillin is welcomed into the active site of the transpeptidase because it mimics the d-Ala-d-Ala moiety of the normal
substrate (Figure 8.30). Bound penicillin then forms a covalent bond with a serine residue at the active site of the
enzyme. This penicilloyl-enzyme does not react further. Hence, the transpeptidase is irreversibly inhibited and cell-wall
synthesis cannot take place.
Why is penicillin such an effective inhibitor of the transpeptidase? The highly strained, four-membered β-lactam ring of
penicillin makes it especially reactive. On binding to the transpeptidase, the serine residue at the active site attacks the
carbonyl carbon atom of the lactam ring to form the penicilloyl-serine derivative (Figure 8.31). Because the peptidase
participates in its own inactivation, penicillin acts as a suicide inhibitor.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.15. Distinction between a Competitive and a Noncompetitive Inhibitor. (Top) enzyme-substrate complex;
(middle) a competitive inhibitor binds at the active site and thus prevents the substrate from binding; (bottom) a
noncompetitive inhibitor does not prevent the substrate from binding.
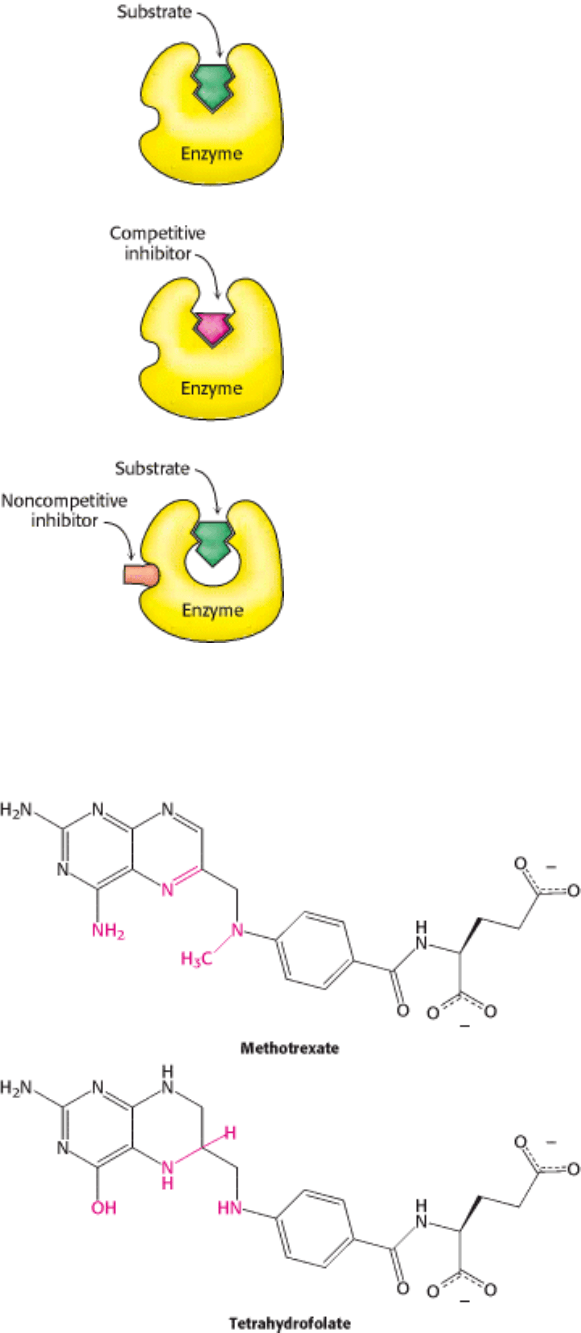
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.16. Enzyme Inhibitors. The cofactor tetrahydrofolate and its structural analog methotrexate. Regions with
structural differences are shown in red.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.17. Kinetics of a Competitive Inhibitor. As the concentration of a competitive inhibitor increases, higher
concentrations of substrate are required to attain a particular reaction velocity. The reaction pathway suggests how
sufficiently high concentrations of substrate can completely relieve competitive inhibition.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.18. Kinetics of a Noncompetitive Inhibitor. The reaction pathway shows that the inhibitor binds both to free
enzyme and to enzyme complex. Consequently, V
max
cannot be attained, even at high substrate concentrations.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.19. Enzyme Inhibition by Diisopropylphosphofluoridate (DIPF), a Group-Specific Reagent. DIPF can
inhibit an enzyme by covalently modifying a crucial serine residue (Section 9.1.1).
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.20. Enzyme Inactivation by Iodoacetamide, a Group-Specific Reagent. Iodoacetamide can inactivate an
enzyme by reacting with a critical cysteine residue.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
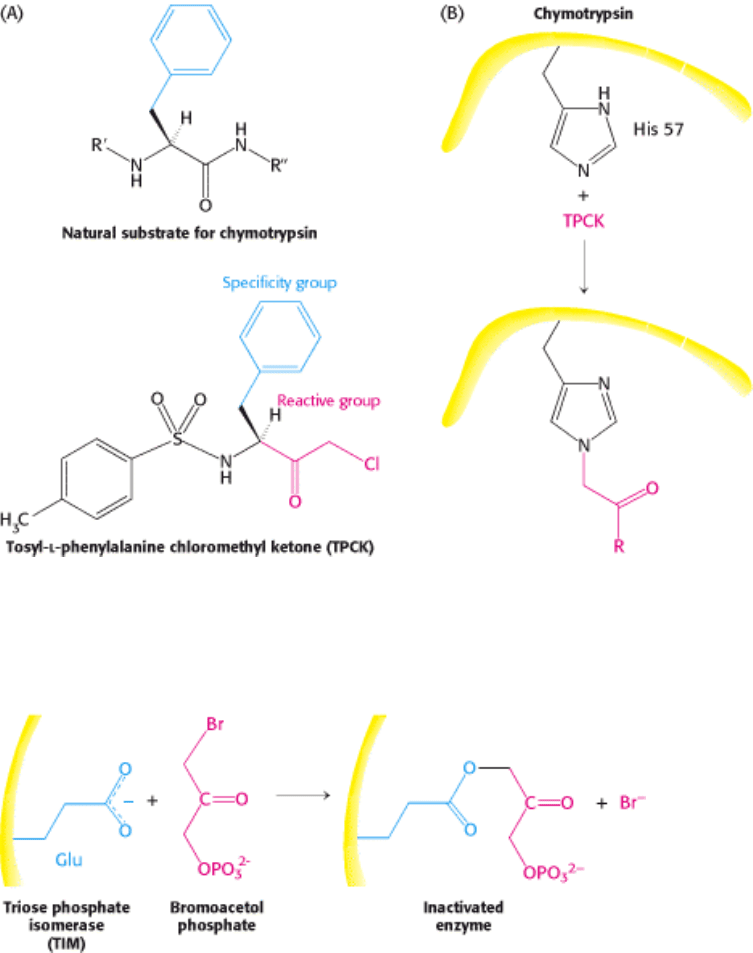
Figure 8.21. Affinity Labeling. (A) Tosyl-
l-phenylalanine chloromethyl ketone (TPCK) is a reactive analog of the
normal substrate for the enzyme chymotrypsin. (B) TPCK binds at the active site of chymotrypsin and modifies an
essential histidine residue.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.22. Bromoacetol Phosphate, an Affinity Label for Triose Phosphate Isomerase (TIM). Bromoacetol
phosphate, an analog of dihydroxyacetone phosphate, binds at the active site of the enzyme and covalently modifies a
glutamic acid residue required for enzyme activity.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
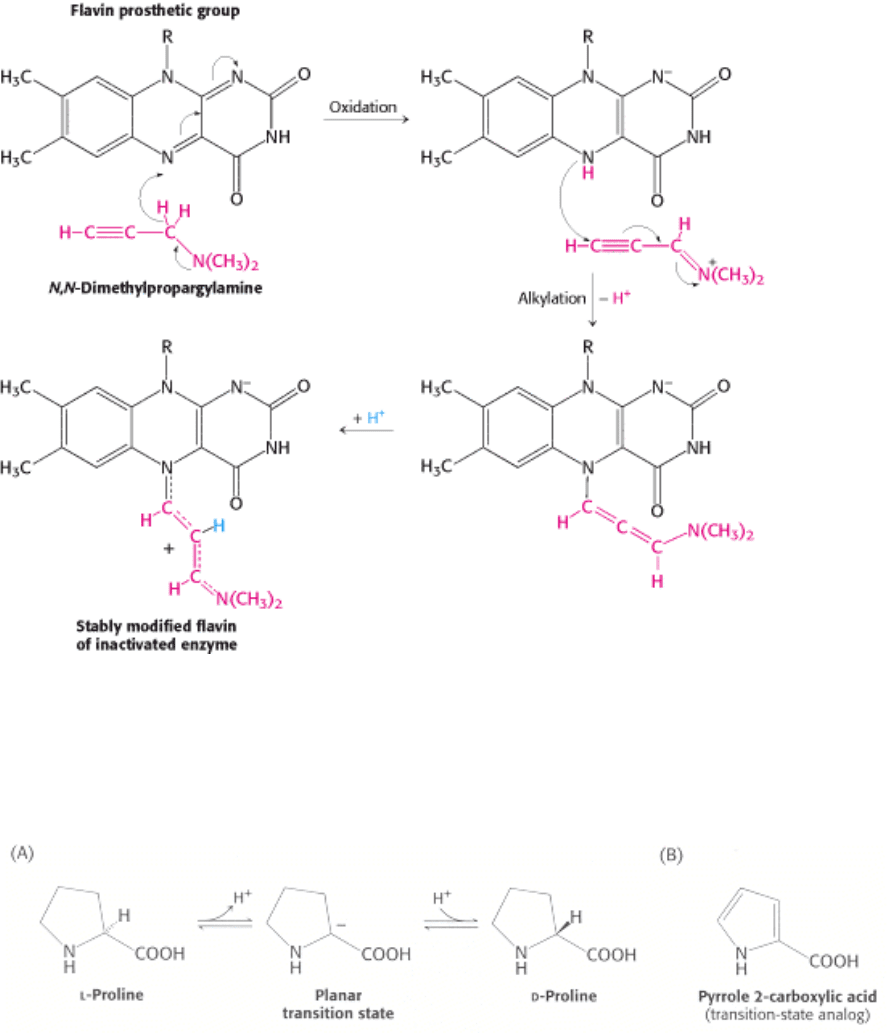
Figure 8.23. Mechanism-Based (Suicide) Inhibition. Monoamine oxidase, an enzyme important for neurotransmitter
synthesis, requires the cofactor FAD (flavin adenine dinucleotide). N,N-Dimethylpropargylamine inhibits monoamine
oxidase by covalently modifying the flavin prosthetic group only after the inhibitor is first oxidized. The N-5 flavin
adduct is stabilized by the addition of a proton.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.24. Inhibition by Transition State Analogs. (A) The isomerization of
l-proline to d-proline by proline
racemase, a bacterial enzyme, proceeds through a planar transition state in which the α carbon is trigonal rather than
tetrahedral. (B) Pyrrole 2-carboxylate, a transition state analog because of its trigonal geometry, is a potent inhibitor of
proline racemase.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.25. Use of Transition-State Analogs to Generate Catalytic Antibodies. The insertion of a metal ion into a
porphyrin by ferrochelatase proceeds through a transition state in which the porphyrin is bent. N-Methylmesoporphyrin,
a bent porphyrin that resembles the transition state of the ferrochelatase-catalyzed reaction, was used to generate an
antibody that also catalyzes the insertion of a metal ion into a porphyrin ring.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.26. Structure of Penicillin. The reactive site of penicillin is the peptide bond of its β-lactam ring. (A)
Structural formula of penicillin. (B) Representation of benzyl penicillin.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
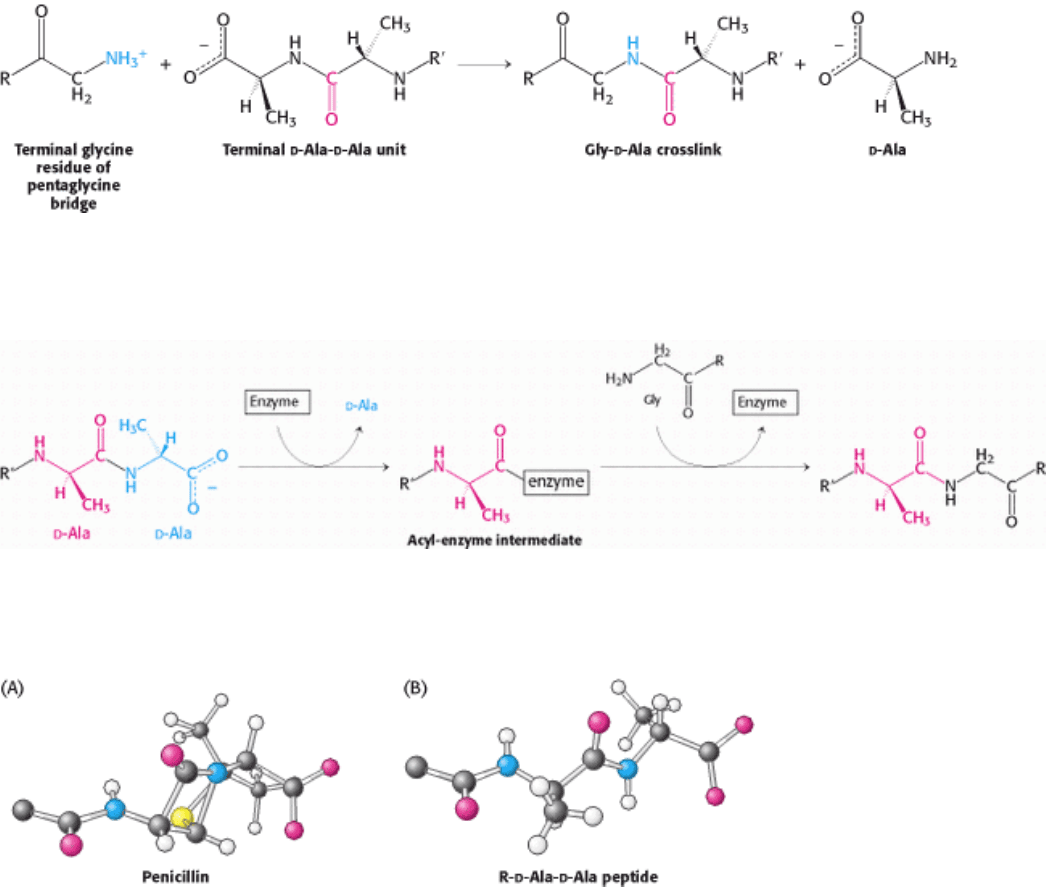
Figure 8.27. Schematic Representation of the Peptidoglycan in Staphylococcus aureus. The sugars are shown in
yellow, the tetrapeptides in red, and the pentaglycine bridges in blue. The cell wall is a single, enormous, bag-shaped
macromolecule because of extensive cross-linking.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.28. Formation of Cross-Links in S. aureus Peptidoglycan. The terminal amino group of the pentaglycine
bridge in the cell wall attacks the peptide bond between two
d-alanine residues to form a cross-link.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.29. Transpeptidation Reaction. An acyl-enzyme intermediate is formed in the transpeptidation reaction
leading to cross-link formation.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.5. Enzymes Can Be Inhibited by Specific Molecules
Figure 8.30. Conformations of Penicillin and a Normal Substrate. The conformation of penicillin in the vicinity of its
reactive peptide bond (A) resembles the postulated conformation of the transition state of R-
d-Ala-d-Ala (B) in the
transpeptidation reaction. [After B. Lee. J. Mol. Biol. 61(1971):464.]
