Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

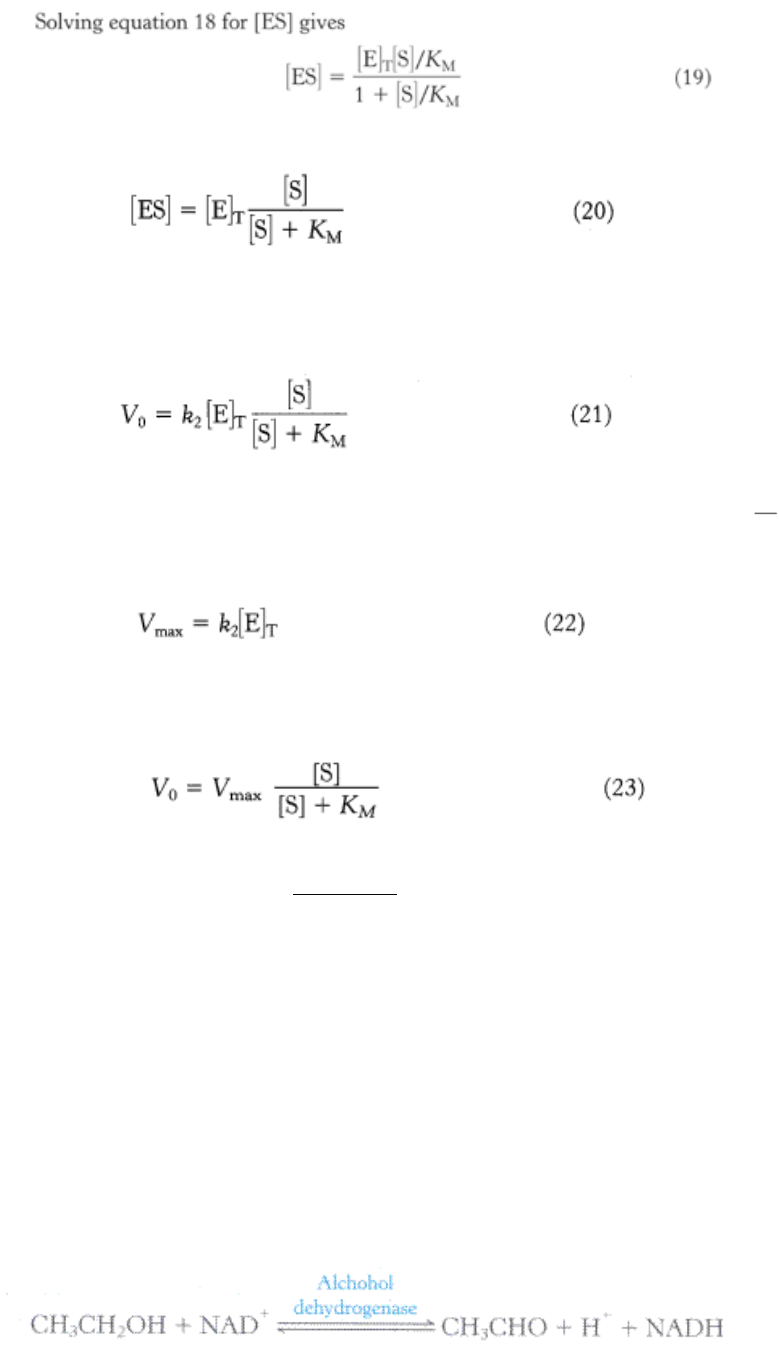
or
By substituting this expression for [ES] into equation 10, we obtain
The maximal rate, V
max
, is attained when the catalytic sites on the enzyme are saturated with substrate that is, when
[ES] = [E]
T
. Thus,
Substituting equation 22 into equation 21 yields the Michaelis-Menten equation:
This equation accounts for the kinetic data given in Figure 8.11. At very low substrate concentration, when [S] is much
less than K
M
, V
0
= (V
max
/K
M
)[S]; that is, the rate is directly proportional to the substrate concentration. At high
substrate concentration, when [S] is much greater than K
M
, V
0
= V
max
; that is, the rate is maximal, independent of
substrate concentration.
The meaning of K
M
is evident from equation 23. When [S] = K
M
, then V
0
= V
max
/2. Thus, K
M
is equal to the
substrate concentration at which the reaction rate is half its maximal value. K
M
is an important characteristic of an
enzyme-catalyzed reaction and is significant for its biological function.
The physiological consequence of K
M
is illustrated by the sensitivity of some individuals to ethanol. Such persons
exhibit facial flushing and rapid heart rate (tachycardia) after ingesting even small amounts of alcohol. In the liver,
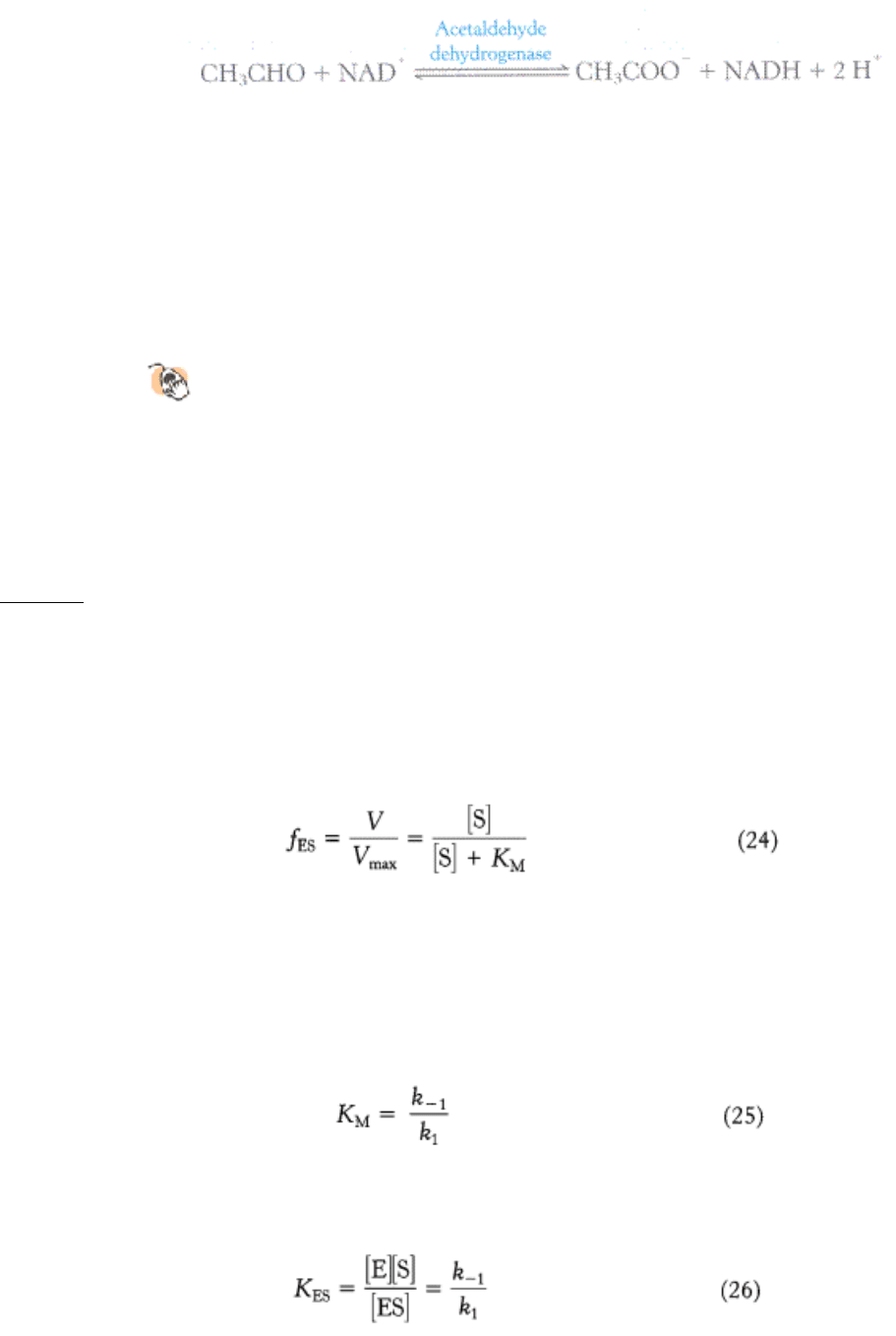
alcohol dehydrogenase converts ethanol into acetaldehyde.
Normally, the acetaldehyde, which is the cause of the symptoms when present at high concentrations, is processed to
acetate by acetaldehyde dehydrogenase.
Most people have two forms of the acetaldehyde dehydrogenase, a low K
M
mitochondrial form and a high K
M
cytosolic
form. In susceptible persons, the mitochondrial enzyme is less active due to the substitution of a single amino acid, and
acetaldehyde is processed only by the cytosolic enzyme. Because this enzyme has a high K
M
, less acetaldehyde is
converted into acetate; excess acetaldehyde escapes into the blood and accounts for the physiological effects.
8.4.1. The Significance of K
M
and V
max
Values
Conceptual Insights, Steady-State Enzyme Kinetics. Learn how the kinetic
parameters KMand Vmaxcan be determined experimentally using the enzyme
kinetics lab simulation in this media module.
The Michaelis constant, K
M
, and the maximal rate, V
max
, can be readily derived from rates of catalysis measured at a
variety of substrate concentrations if an enzyme operates according to the simple scheme given in equation 23. The
derivation of K
M
and V
max
is most commonly achieved with the use of curve-fitting programs on a computer (see the
appendix to this chapter for alternative means of determining K
M
and V
max
). The K
M
values of enzymes range widely
(Table 8.5). For most enzymes, K
M
lies between 10
-1
and 10
-7
M. The K
M
value for an enzyme depends on the
particular substrate and on environmental conditions such as pH, temperature, and ionic strength. The Michaelis
constant, K
M
, has two meanings. First, K
M
is the concentration of substrate at which half the active sites are filled.
Thus, K
M
provides a measure of the substrate concentration required for significant catalysis to occur. In fact, for many
enzymes, experimental evidence suggests that K
M
provides an approximation of substrate concentration in vivo. When
the K
M
is known, the fraction of sites filled, f
ES
, at any substrate concentration can be calculated from
Second, K
M
is related to the rate constants of the individual steps in the catalytic scheme given in equation 9. In
equation 15, K
M
is defined as (k
-1
+ k
2
)/k
1
. Consider a limiting case in which k
-1
is much greater than k
2
. Under such
circumstances, the ES complex dissociates to E and S much more rapidly than product is formed. Under these conditions
(k
-1
>> k
2
),
The dissociation constant of the ES complex is given by
In other words, K
M
is equal to the dissociation constant of the ES complex if k
2
is much smaller than k
-
1
. When this
condition is met, K
M
is a measure of the strength of the ES complex: a high K
M
indicates weak binding; a low K
M
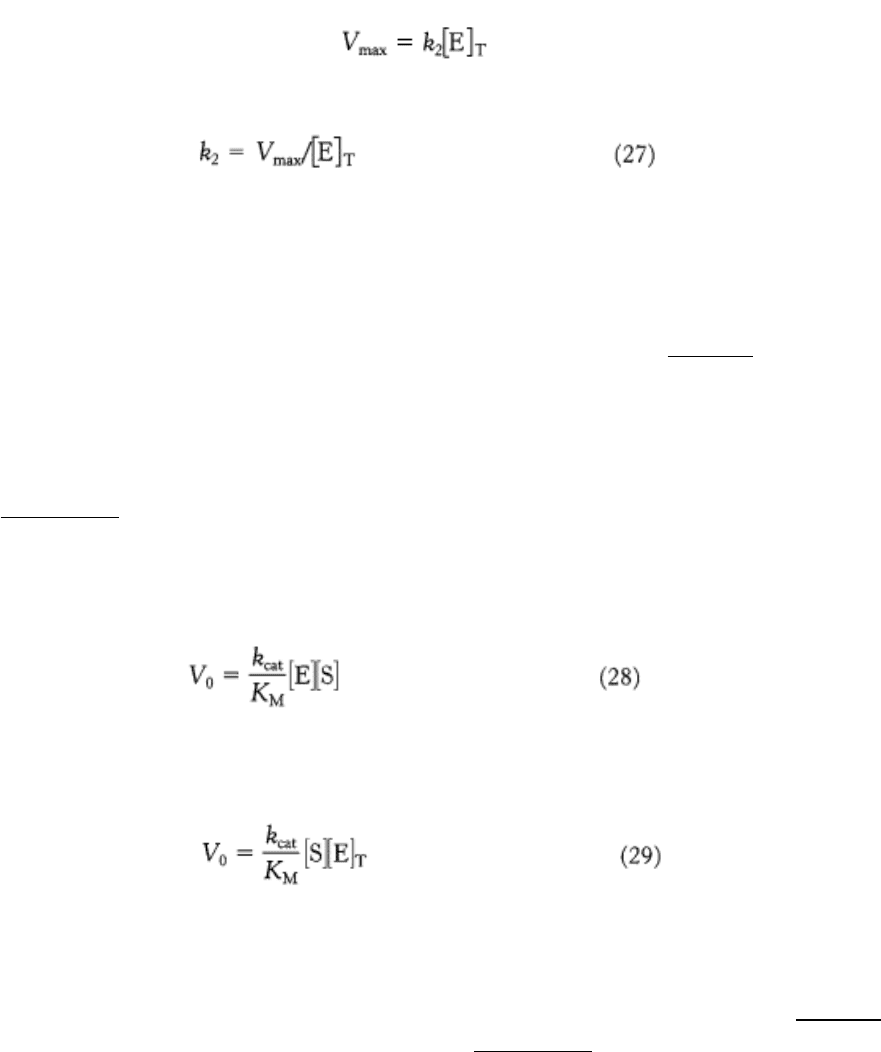
indicates strong binding. It must be stressed that K
M
indicates the affinity of the ES complex only when k
-1
is much
greater than k
2
.
The maximal rate, V
max
, reveals the turnover number of an enzyme, which is the number of substrate molecules
converted into product by an enzyme molecule in a unit time when the enzyme is fully saturated with substrate. It is equal
to the kinetic constant k
2
, which is also called k
cat
. The maximal rate, V
max
, reveals the turnover number of an enzyme
if the concentration of active sites [E]
T
is known, because
and thus
For example, a 10
-6
M solution of carbonic anhydrase catalyzes the formation of 0.6 M H
2
CO
3
per second when it is
fully saturated with substrate. Hence, k
2
is 6 × 10
5
s
-1
. This turnover number is one of the largest known. Each catalyzed
reaction takes place in a time equal to 1/k
2
, which is 1.7 µs for carbonic anhydrase. The turnover numbers of most
enzymes with their physiological substrates fall in the range from 1 to 10
4
per second (Table 8.6).
8.4.2. Kinetic Perfection in Enzymatic Catalysis: The k
cat
/K
M
Criterion
When the substrate concentration is much greater than K
M
, the rate of catalysis is equal to k
cat
, the turnover number, as
described in Section 8.4.1. However, most enzymes are not normally saturated with substrate. Under physiological
conditions, the [S]/K
M
ratio is typically between 0.01 and 1.0. When [S] << K
M
, the enzymatic rate is much less than k
cat
because most of the active sites are unoccupied. Is there a number that characterizes the kinetics of an enzyme under
these more typical cellular conditions? Indeed there is, as can be shown by combining equations 10 and 16 to give
When [S] << K
M
, the concentration of free enzyme, [E], is nearly equal to the total concentration of enzyme [E
T
], so
Thus, when [S] << K
M
, the enzymatic velocity depends on the values of k
cat
/K
M
, [S], and [E]
T
. Under these conditions,
k
cat
/K
M
is the rate constant for the interaction of S and E and can be used as a measure of catalytic efficiency. For
instance, by using k
cat
/K
M
values, one can compare an enzyme's preference for different substrates. Table 8.7 shows the
k
cat
/K
M
values for several different substrates of chymotrypsin (Section 9.1.1). Chymotrypsin clearly has a preference
for cleaving next to bulky, hydrophobic side chains.
How efficient can an enzyme be? We can approach this question by determining whether there are any physical limits on
the value of k
cat
/K
M
. Note that this ratio depends on k
1
, k
-1
, and k
cat
, as can be shown by substituting for K
M
.

Suppose that the rate of formation of product (k
cat
) is much faster than the rate of dissociation of the ES complex (k
-1
).
The value of k
cat
/K
M
then approaches k
1
. Thus, the ultimate limit on the value of k
cat
/K
M
is set by k
1
, the rate of
formation of the ES complex. This rate cannot be faster than the diffusion-controlled encounter of an enzyme and its
substrate. Diffusion limits the value of k
1
so that it cannot be higher than between 10
8
and 10
9
s
-1
M
-1
. Hence, the upper
limit on k
cat
/K
M
is between 10
8
and 10
9
s
-1
M
-1
.
The k
cat
/K
M
ratios of the enzymes superoxide dismutase, acetylcholinesterase, and triose phosphate isomerase are
between 10
8
and 10
9
s
-1
M
-1
. Enzymes such as these that have k
cat
/K
M
ratios at the upper limits have attained kinetic
perfection. Their catalytic velocity is restricted only by the rate at which they encounter substrate in the solution (Table
8.8). Any further gain in catalytic rate can come only by decreasing the time for diffusion. Remember that the active site
is only a small part of the total enzyme structure. Yet, for catalytically perfect enzymes, every encounter between
enzyme and substrate is productive. In these cases, there may be attractive electrostatic forces on the enzyme that entice
the substrate to the active site. These forces are sometimes referred to poetically as Circe effects.
Circe effect-
The utilization of attractive forces to lure a substrate into a site in
which it undergoes a transformation of structure, as defined by
William P. Jencks, an enzymologist, who coined the term.
A goddess of Greek mythology, Circe lured Odysseus's men to her
house and then transformed them into pigs.
The limit imposed by the rate of diffusion in solution can also be partly overcome by confining substrates and products
in the limited volume of a multienzyme complex. Indeed, some series of enzymes are associated into organized
assemblies (Section 17.1.9) so that the product of one enzyme is very rapidly found by the next enzyme. In effect,
products are channeled from one enzyme to the next, much as in an assembly line.
8.4.3. Most Biochemical Reactions Include Multiple Substrates
Most reactions in biological systems usually include two substrates and two products and can be represented by the
bisubstrate reaction:
The majority of such reactions entail the transfer of a functional group, such as a phosphoryl or an ammonium group,
from one substrate to the other. In oxidation-reduction reactions, electrons are transferred between substrates. Multiple
substrate reactions can be divided into two classes: sequential displacement and double displacement.
Sequential Displacement.
In the sequential mechanism, all substrates must bind to the enzyme before any product is released. Consequently, in a
bisubstrate reaction, a ternary complex of the enzyme and both substrates forms. Sequential mechanisms are of two
types: ordered, in which the substrates bind the enzyme in a defined sequence, and random.
Many enzymes that have NAD
+
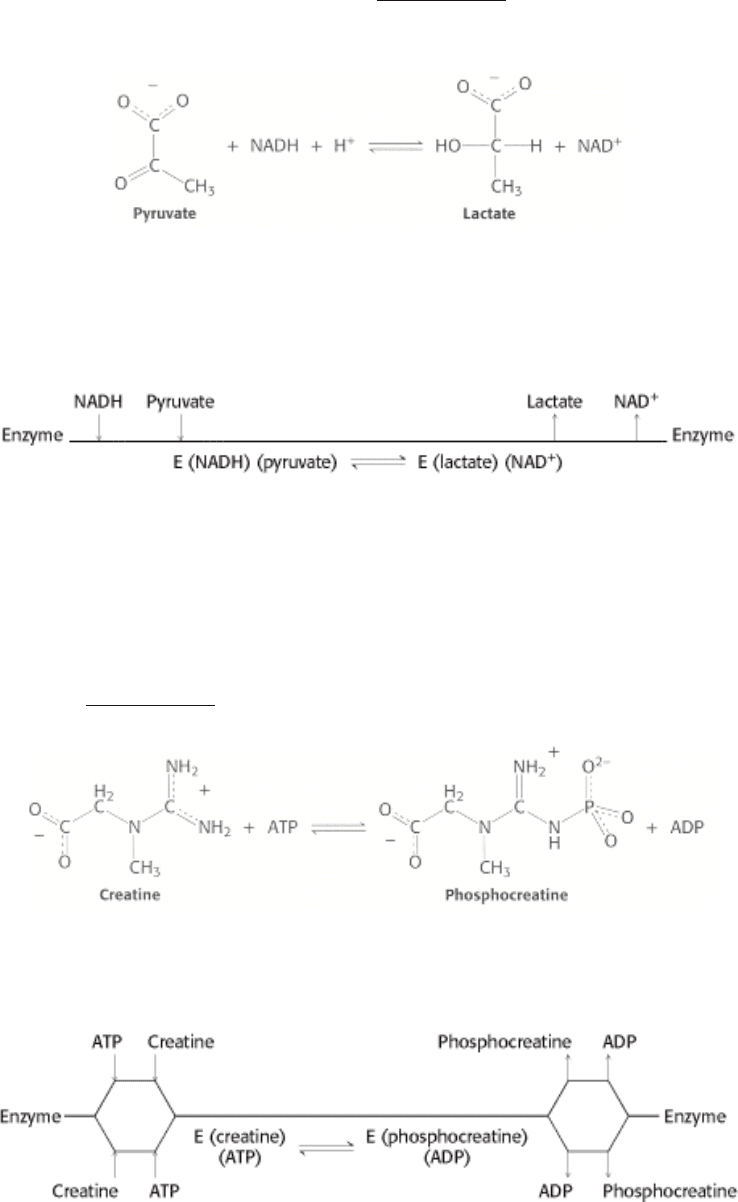
or NADH as a substrate exhibit the sequential ordered mechanism. Consider lactate
dehydrogenase, an important enzyme in glucose metabolism (Section 16.1.9). This enzyme reduces pyruvate to lactate
while oxidizing NADH to NAD
+
.
In the ordered sequential mechanism, the coenzyme always binds first and the lactate is always released first. This
sequence can be represented as follows in a notation developed by W. Wallace Cleland:
The enzyme exists as a ternary complex: first, consisting of the enzyme and substrates and, after catalysis, the enzyme
and products.
In the random sequential mechanism, the order of addition of substrates and release of products is random. Sequential
random reactions are illustrated by the formation of phosphocreatine and ADP from ATP and creatine, a reaction
catalyzed by creatine kinase (Section 14.1.5).
Phosphocreatine is an important energy source in muscle. Sequential random reactions can also be depicted in the
Cleland notation.
Although the order of certain events is random, the reaction still passes through the ternary complexes including, first,
substrates and, then, products.
Double-Displacement (Ping-Pong) Reactions.
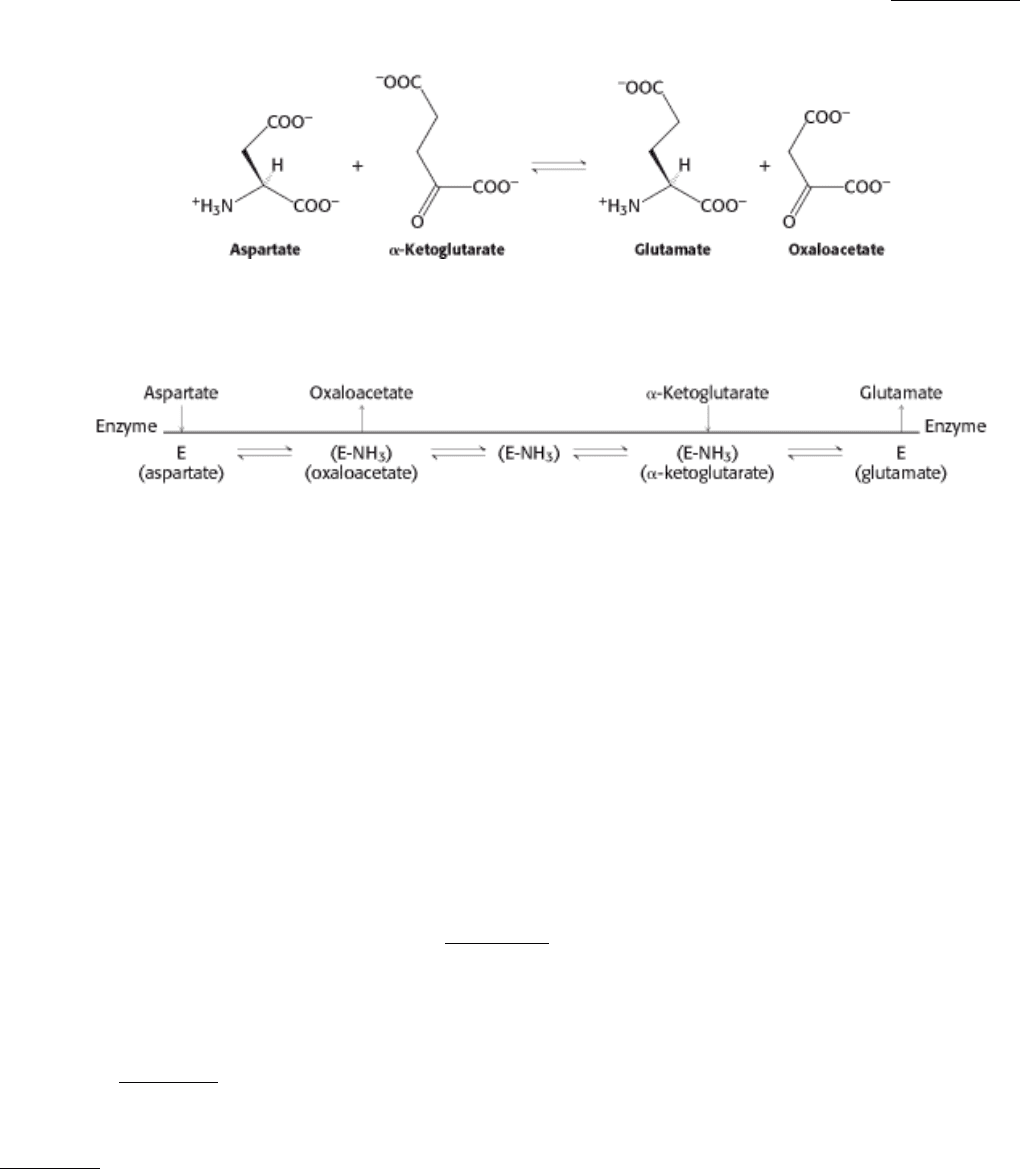
In double-displacement, or Ping-Pong, reactions, one or more products are released before all substrates bind the
enzyme. The defining feature of double-displacement reactions is the existence of a substituted enzyme intermediate, in
which the enzyme is temporarily modified. Reactions that shuttle amino groups between amino acids and α-keto acids
are classic examples of double-displacement mechanisms. The enzyme aspartate aminotransferase (Section 23.3.1)
catalyzes the transfer of an amino group from aspartate to α-ketoglutarate.
The sequence of events can be portrayed as the following diagram.
After aspartate binds to the enzyme, the enzyme removes aspartate's amino group to form the substituted enzyme
intermediate. The first product, oxaloacetate, subsequently departs. The second substrate, α-ketoglutarate, binds to the
enzyme, accepts the amino group from the modified enzyme, and is then released as the final product, glutamate. In the
Cleland notation, the substrates appear to bounce on and off the enzyme analogously to a Ping-Pong ball bouncing on a
table.
8.4.4. Allosteric Enzymes Do Not Obey Michaelis-Menten Kinetics
The Michaelis-Menten model has greatly assisted the development of enzyme chemistry. Its virtues are simplicity and
broad applicability. However, the Michaelis-Menten model cannot account for the kinetic properties of many enzymes.
An important group of enzymes that do not obey Michaelis-Menten kinetics comprises the allosteric enzymes. These
enzymes consist of multiple subunits and multiple active sites.
Allosteric enzymes often display sigmoidal plots (Figure 8.14) of the reaction velocity V
0
versus substrate concentration
[S], rather than the hyperbolic plots predicted by the Michaelis-Menten equation (equation 23). In allosteric enzymes, the
binding of substrate to one active site can affect the properties of other active sites in the same enzyme molecule. A
possible outcome of this interaction between subunits is that the binding of substrate becomes cooperative; that is, the
binding of substrate to one active site of the enzyme facilitates substrate binding to the other active sites. As will be
considered in Chapter 10, such cooperativity results in a sigmoidal plot of V
0
versus [S]. In addition, the activity of an
allosteric enzyme may be altered by regulatory molecules that are reversibly bound to specific sites other than the
catalytic sites. The catalytic properties of allosteric enzymes can thus be adjusted to meet the immediate needs of a cell
(Chapter 10). For this reason, allosteric enzymes are key regulators of metabolic pathways in the cell.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
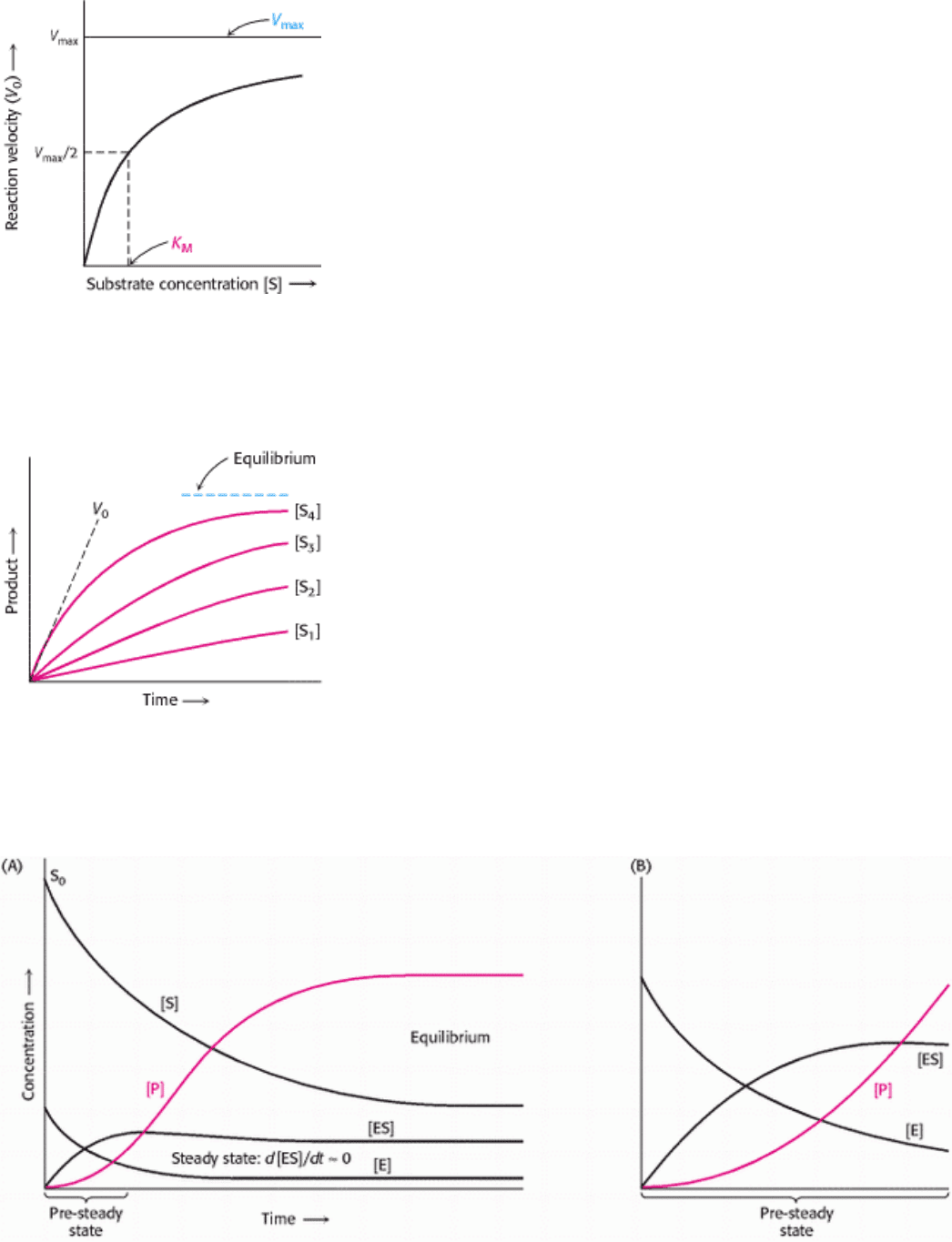
Figure 8.11. Michaelis-Menten Kinetics. A plot of the reaction velocity (V
0
) as a function of the substrate
concentration [S] for an enzyme that obeys Michaelis-Menten kinetics shows that the maximal velocity (V
max
) is
approached asymptotically. The Michaelis constant (K
M
) is the substrate concentration yielding a velocity of V
max
/2.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
Figure 8.12. Determining Initial Velocity. The amount of product formed at different substrate concentrations is
plotted as a function of time. The initial velocity (V
0
) for each substrate concentration is determined from the slope of
the curve at the beginning of a reaction, when the reverse reaction is insignificant.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
Figure 8.13. Changes in the Concentration of Reaction Participants of an Enzyme-Catalyzed Reaction with Time.
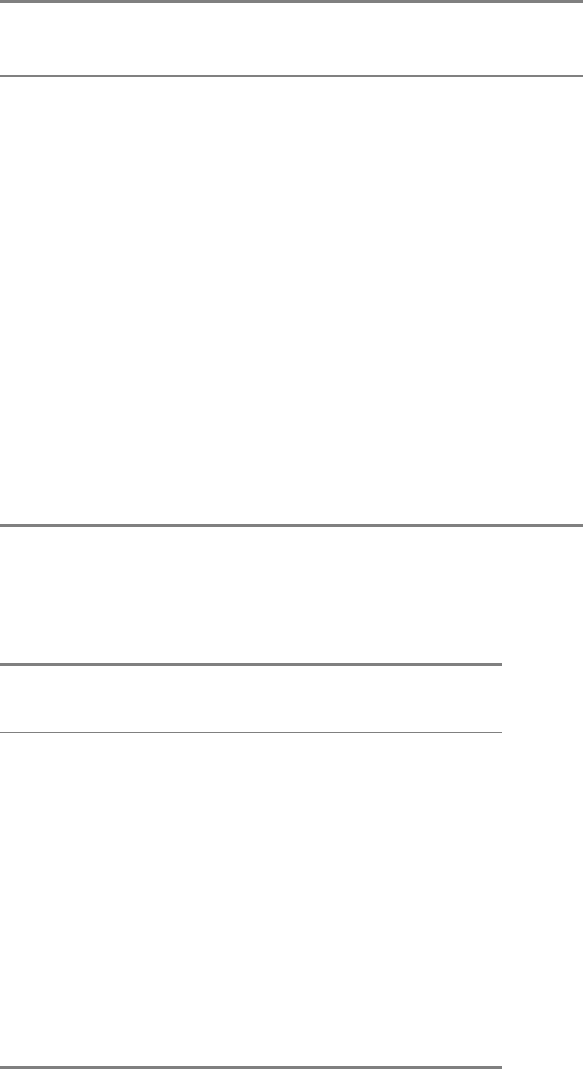
Concentration changes under (A) steady-state conditions, and (B) the pre-steady-state conditions.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
Table 8.5. K
M
values of some enzymes
Enzyme Substrate
K
M
(µM)
Chymotrypsin Acetyl-l-tryptophanamide 5000
Lysozyme Hexa-N-acetylglucosamine 6
β-Galactosidase
Lactose 4000
Threonine deaminase Threonine 5000
Carbonic anhydrase CO
2
8000
Penicillinase Benzylpenicillin 50
Pyruvate carboxylase Pyruvate 400
HCO
3
-
1000
ATP 60
Arginine-tRNA synthetase Arginine 3
tRNA 0.4
ATP 300
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
Table 8.6. Maximum turnover numbers of some enzymes
Enzyme Turnover number (per second)
Carbonic anhydrase 600,000
3-Ketosteroid isomerase 280,000
Acetylcholinesterase 25,000
Penicillinase 2,000
Lactate dehydrogenase 1,000
Chymotrypsin 100
DNA polymerase I 15
Tryptophan synthetase 2
Lysozyme 0.5
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
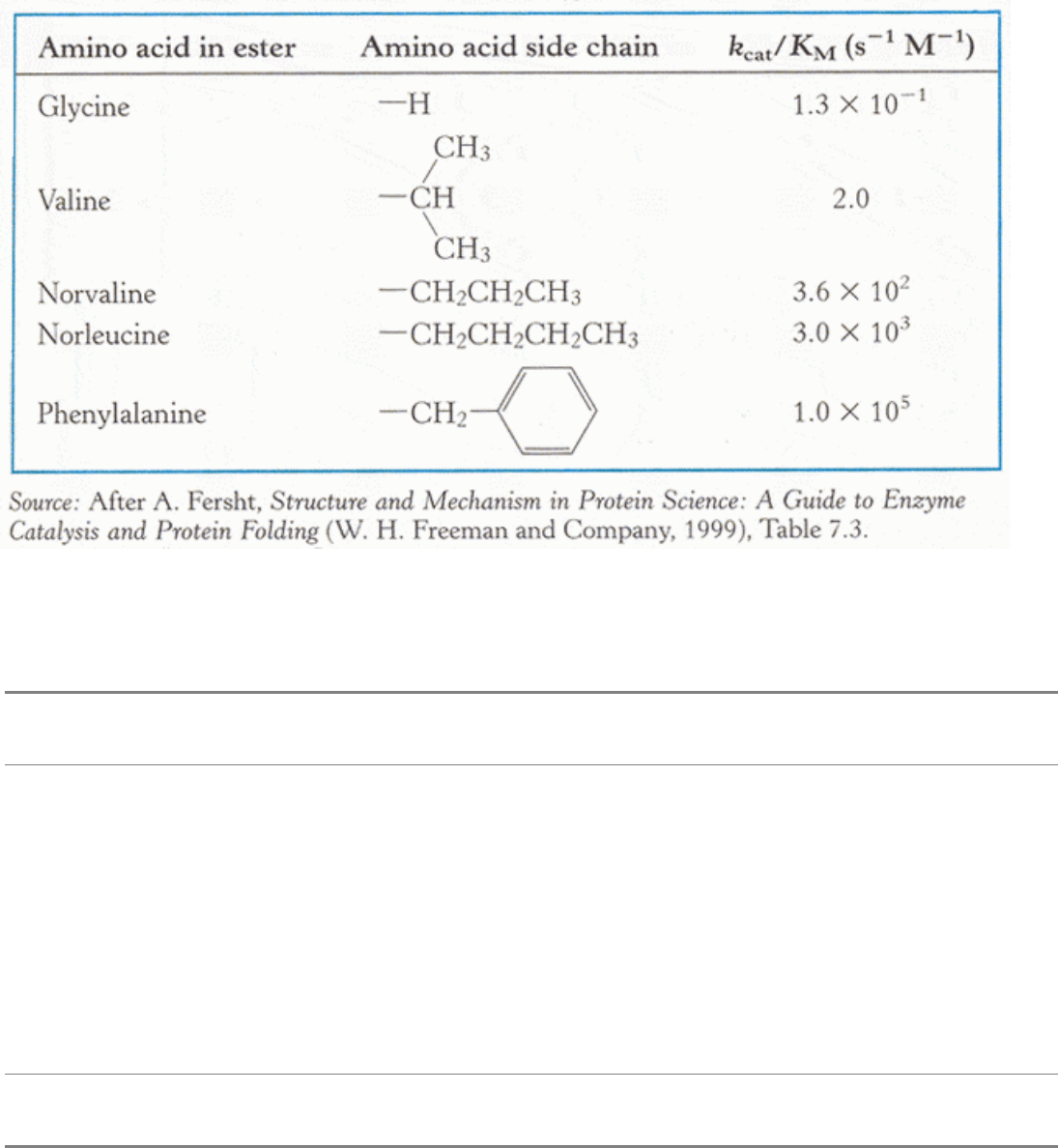
Table 8.7. Substrate preferences of chymotrypsin
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
Table 8.8. Enzymes for which k
cat
/K
M
is close to the diffusion-controlled rate of encounter
Enzyme
k
cat
/K
M
(s
-1
M
-1
)
Acetylcholinesterase
1.6 × 10
8
Carbonic anhydrase
8.3 × 10
7
Catalase
4 × 10
7
Crotonase
2.8 × 10
8
Fumarase
1.6 × 10
8
Triose phosphate isomerase
2.4 × 10
8
β-Lactamase
1 × 10
8
Superoxide dismutase
7 × 10
9
Source: After A. Fersht, Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding (W. H.
Freeman and Company, 1999), Table 4.5.
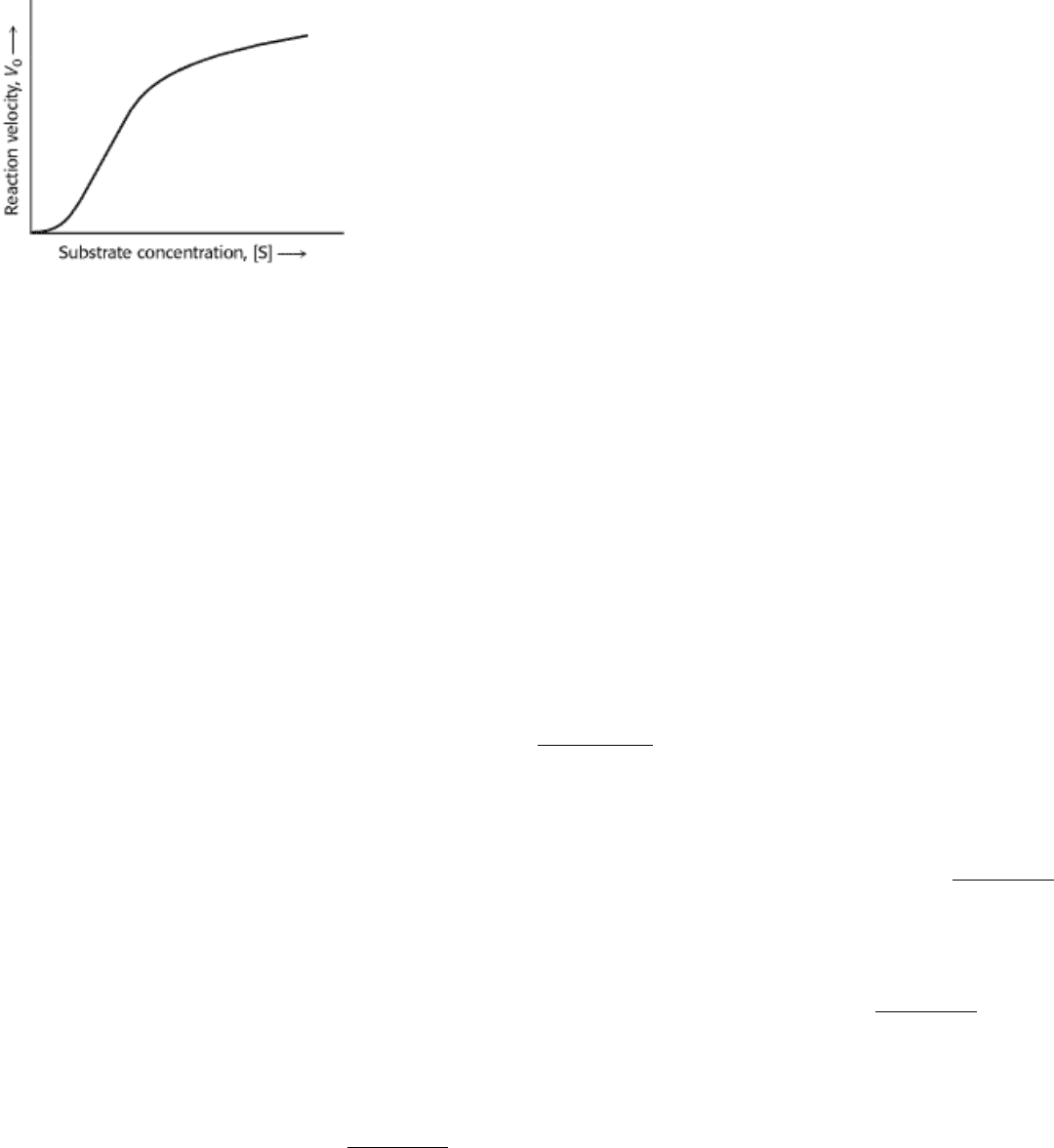
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes
Figure 8.14. Kinetics for an Allosteric Enzyme. Allosteric enzymes display a sigmoidal dependence of reaction
velocity on substrate concentration.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
8.5. Enzymes Can Be Inhibited by Specific Molecules
The activity of many enzymes can be inhibited by the binding of specific small molecules and ions. This means of
inhibiting enzyme activity serves as a major control mechanism in biological systems. The regulation of allosteric
enzymes typifies this type of control. In addition, many drugs and toxic agents act by inhibiting enzymes. Inhibition by
particular chemicals can be a source of insight into the mechanism of enzyme action: specific inhibitors can often be
used to identify residues critical for catalysis. The value of transition-state analogs as potent inhibitors will be discussed
shortly.
Enzyme inhibition can be either reversible or irreversible. An irreversible inhibitor dissociates very slowly from its
target enzyme because it has become tightly bound to the enzyme, either covalently or noncovalently. Some irreversible
inhibitors are important drugs. Penicillin acts by covalently modifying the enzyme transpeptidase, thereby preventing the
synthesis of bacterial cell walls and thus killing the bacteria (Section 8.5.5). Aspirin acts by covalently modifying the
enzyme cyclooxygenase, reducing the synthesis of inflammatory signals.
Reversible inhibition, in contrast with irreversible inhibition, is characterized by a rapid dissociation of the enzyme-
inhibitor complex. In competitive inhibition, an enzyme can bind substrate (forming an ES complex) or inhibitor (EI) but
not both (ESI). The competitive inhibitor resembles the substrate and binds to the active site of the enzyme (Figure 8.15).
The substrate is thereby prevented from binding to the same active site. A competitive inhibitor diminishes the rate of
catalysis by reducing the proportion of enzyme molecules bound to a substrate. At any given inhibitor concentration,
competitive inhibition can be relieved by increasing the substrate concentration. Under these conditions, the substrate
"outcompetes" the inhibitor for the active site. Methotrexate is a structural analog of tetrahydrofolate, a coenzyme for the
enzyme dihydrofolate reductase, which plays a role in the biosynthesis of purines and pyrimidines (Figure 8.16). It binds
to dihydrofolate reductase 1000-fold more tightly than the natural substrate and inhibits nucleotide base synthesis. It is
used to treat cancer.
In noncompetitive inhibition, which also is reversible, the inhibitor and substrate can bind simultaneously to an enzyme
molecule at different binding sites (see Figure 8.16). A noncompetitive inhibitor acts by decreasing the turnover number
rather than by diminishing the proportion of enzyme molecules that are bound to substrate. Noncompetitive inhibition, in
contrast with competitive inhibition, cannot be overcome by increasing the substrate concentration. A more complex
pattern, called mixed inhibition, is produced when a single inhibitor both hinders the binding of substrate and decreases
the turnover number of the enzyme.
8.5.1. Competitive and Noncompetitive Inhibition Are Kinetically Distinguishable
How can we determine whether a reversible inhibitor acts by competitive or noncompetitive inhibition? Let us consider
