Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

Substituting R = 1.987 × 10
-3
kcal mol
-1
deg
-1
and T = 298 K (corresponding to 25°C) gives
where ∆ G°
is here expressed in kilocalories per mole because of the choice of the units for R in equation 7. Thus, the
standard free energy and the equilibrium constant of a reaction are related by a simple expression. For example, an
equilibrium constant of 10 gives a standard free-energy change of -1.36 kcal mol
-1
(-5.69 kJ mol
-1
) at 25°C (Table 8.4).
Note that, for each 10-fold change in the equilibrium constant, the ∆ G°
changes by 1.36 kcal mol
-1
(5.69 kJ mol
-1
).
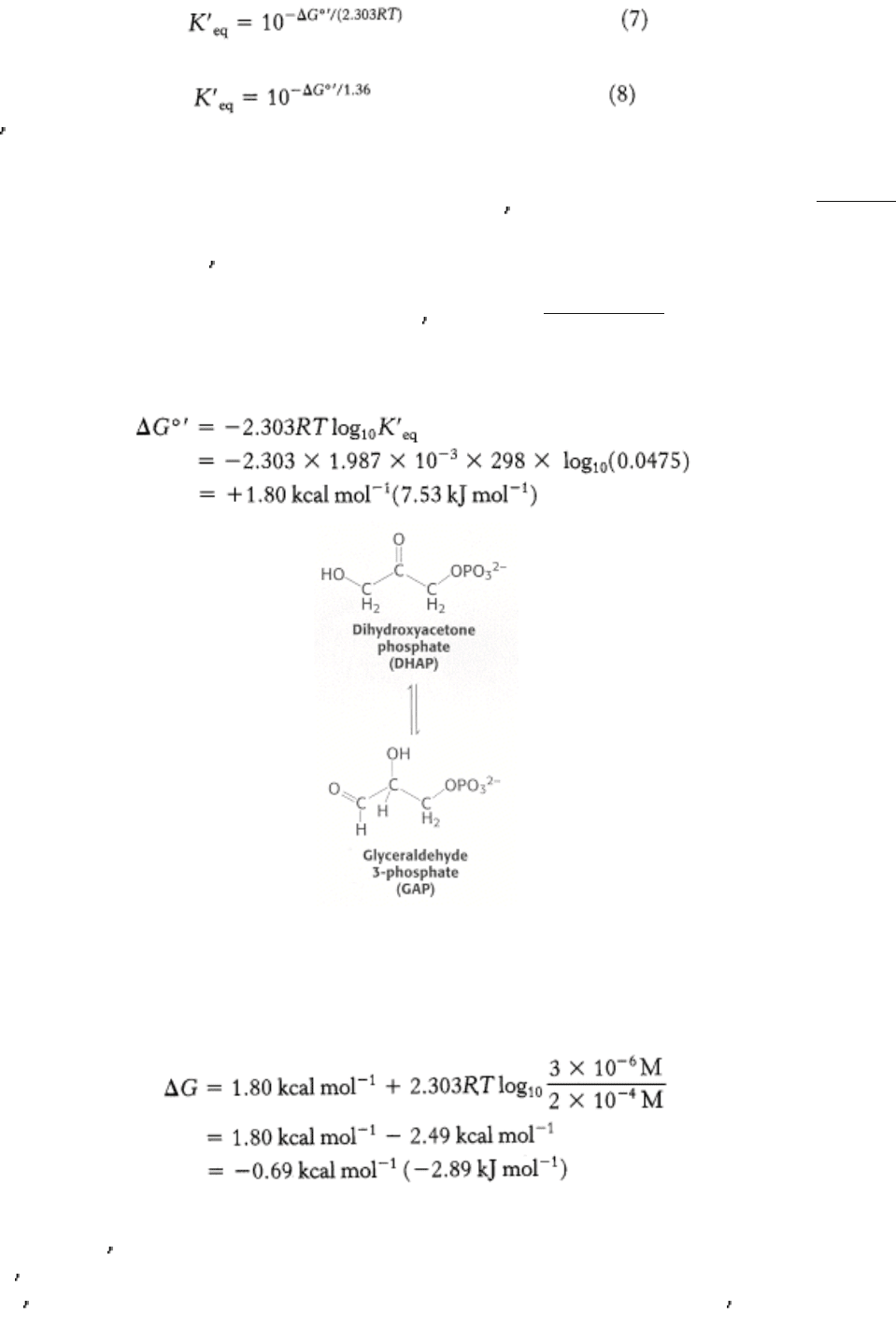
As an example, let us calculate ∆ G°
and ∆ G for the isomerization of dihydroxyacetone phosphate (DHAP) to
glyceraldehyde 3-phosphate (GAP). This reaction takes place in glycolysis (Section 16.1.4). At equilibrium, the ratio of
GAP to DHAP is 0.0475 at 25°C (298 K) and pH 7. Hence, K
eq
= 0.0475. The standard free-energy change for this
reaction is then calculated from equation 6:
Under these conditions, the reaction is endergonic. DHAP will not spontaneously convert to GAP.
Now let us calculate ∆ G for this reaction when the initial concentration of DHAP is 2 × 10
-4
M and the initial
concentration of GAP is 3 × 10
-6
M. Substituting these values into equation 1 gives
This negative value for the ∆ G indicates that the isomerization of DHAP to GAP is exergonic and can occur
spontaneously when these species are present at the aforestated concentrations. Note that ∆ G for this reaction is
negative, although ∆ G
°
is positive. It is important to stress that whether the ∆ G for a reaction is larger, smaller, or the
same as ∆ G
°
depends on the concentrations of the reactants and products. The criterion of spontaneity for a reaction is
∆ G, not ∆ G
°
. This point is important because reactions that are not spontaneous based on ∆ G
°
can be made
spontaneous by adjusting the concentrations of reactants and products. This principle is the basis of the coupling of
reactions to form metabolic pathways (Chapter 14).
8.2.3. Enzymes Alter Only the Reaction Rate and Not the Reaction Equilibrium
Because enzymes are such superb catalysts, it is tempting to ascribe to them powers that they do not have. An enzyme
cannot alter the laws of thermodynamics and consequently cannot alter the equilibrium of a chemical reaction. This
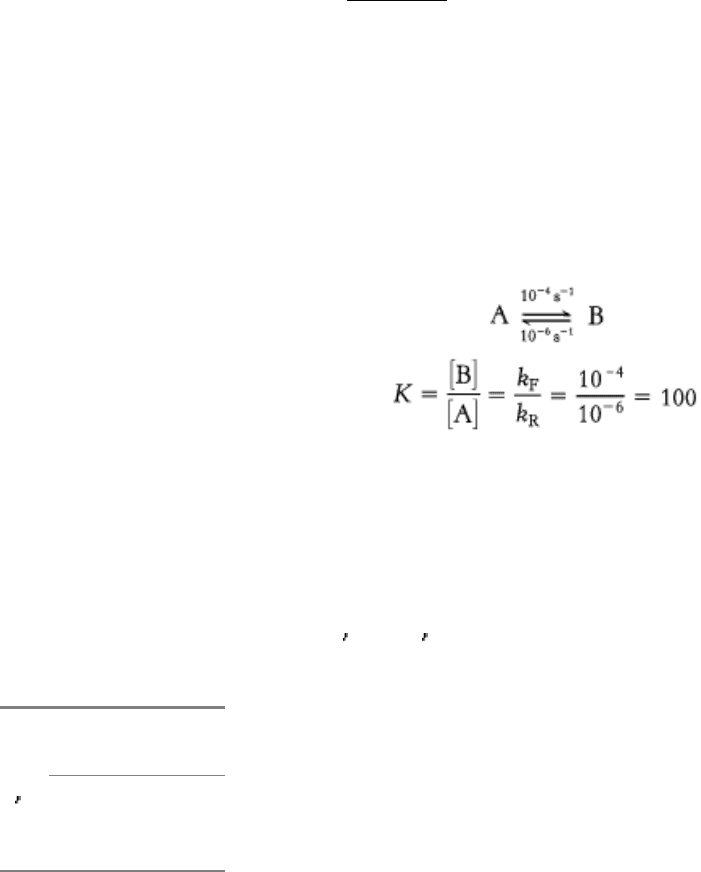
inability means that an enzyme accelerates the forward and reverse reactions by precisely the same factor. Consider the
interconversion of A and B. Suppose that, in the absence of enzyme, the forward rate constant (k
F
) is 10
-4
s
-1
and the
reverse rate constant (k
R
) is 10
-6
s
-1
. The equilibrium constant K is given by the ratio of these rate constants:
The equilibrium concentration of B is 100 times that of A, whether or not enzyme is present. However, it might take
considerable time to approach this equilibrium without enzyme, whereas equilibrium would be attained rapidly in the
presence of a suitable enzyme. Enzymes accelerate the attainment of equilibria but do not shift their positions. The
equilibrium position is a function only of the free-energy difference between reactants and products.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.2. Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes
Table 8.4. Relation between ∆ G° and K
eq
(at 25°C)
DG°
K
eq
kcal mol
-
1
kJ/mol
-
1
10
-
5
6.82 28.53
10
-
4
5.46 22.84
10
-
3
4.09 17.11
10
-
2
2.73 11.42
10
-
1
1.36 5.69
1 0 0
10 -1.36 -5.69
10
2
-2.73 -11.42
10
3
-4.09 -17.11
10
4
-5.46 -22.84
10
5
-6.82 -28.53
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition
State
The free-energy difference between reactants and products accounts for the equilibrium of the reaction, but enzymes
accelerate how quickly this equilibrium is attained. How can we explain the rate enhancement in terms of
thermodynamics? To do so, we have to consider not the end points of the reaction but the chemical pathway between the
end points.
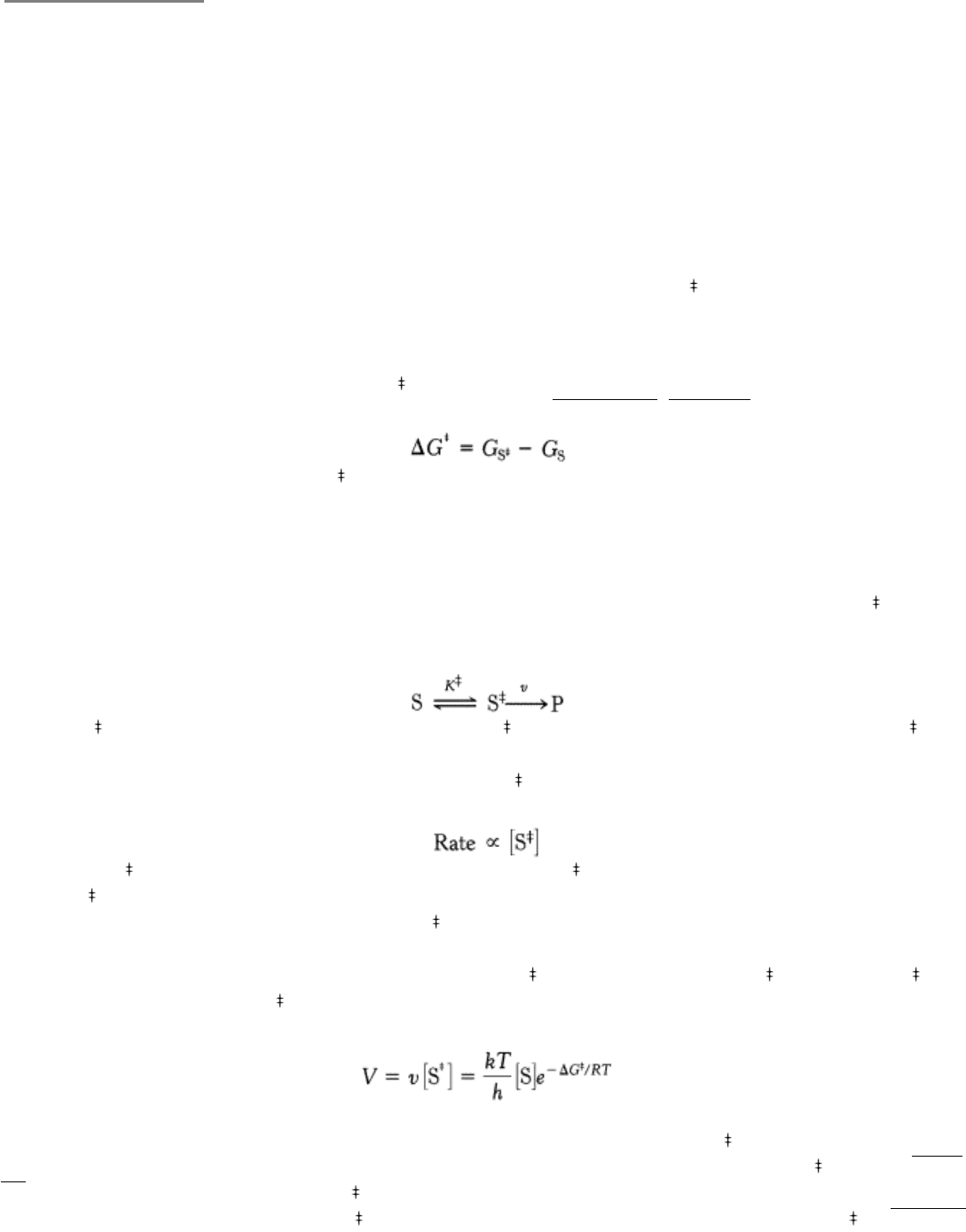
A chemical reaction of substrate S to form product P goes through a transition state S
that has a higher free energy than
does either S or P. The double dagger denotes a thermodynamic property of the transition state. The transition state is the
most seldom occupied species along the reaction pathway because it is the one with the highest free energy. The
difference in free energy between the transition state and the substrate is called the Gibbs free energy of activation or
simply the activation energy, symbolized by ∆ G
, as mentioned in Section 8.2.1 (Figure 8.3).
Note that the energy of activation, or ∆ G
, does not enter into the final ∆ G calculation for the reaction, because the
energy input required to reach the transition state is returned when the transition state forms the product. The activation-
energy barrier immediately suggests how enzymes enhance reaction rate without altering ∆ G of the reaction: enzymes
function to lower the activation energy, or, in other words, enzymes facilitate the formation of the transition state.
One approach to understanding how enzymes achieve this facilitation is to assume that the transition state (S
) and the
substrate (S) are in equilibrium.
in which K
is the equilibrium constant for the formation of S
, and v is the rate of formation of product from S
.
The rate of the reaction is proportional to the concentration of S
:
because only S
can be converted into product. The concentration of S
is in turn related to the free energy difference
between S
and S, because these two chemical species are assumed to be in equilibrium; the greater the difference
between these two states, the smaller the amount of S
.
Because the reaction rate is proportional to the concentration of S
, and the concentration of S
depends on ∆ G
, the
rate of reaction V depends on ∆ G
. Specifically,
In this equation, k is Boltzmann's constant, and h is Planck's constant. The value of kT/h at 25°C is 6.2 × 10
12
s
-1
.
Suppose that the free energy of activation is 6.82 kcal mol
-1
(28.53 kJ mol
-1
). The ratio [S
]/[S] is then 10
-5
(see Table
8.4). If we assume for simplicity's sake that [S] = 1 M, then the reaction rate V is 6.2 × 10
7
s
-1
. If ∆ G
were lowered by
1.36 kcal mol
-1
(5.69 kJ mol
-1
), the ratio [S
]/[S] is then 10
-4
, and the reaction rate would be 6.2 × 10
8
s
-1
. As Table 8.4
shows, a decrease of 1.36 kcal mol
-1
in ∆ G
yields a tenfold larger V. A relatively small decrease in ∆ G
(20% in this
particular reaction) results in a much greater increase in V.

"I think that enzymes are molecules that are complementary in
structure to the activated complexes of the reactions that they
catalyze, that is, to the molecular configuration that is intermediate
between the reacting substances and the products of reaction for
these catalyzed processes. The attraction of the enzyme molecule for
the activated complex would thus lead to a decrease in its energy and
hence to a decrease in the energy of activation of the reaction and to
an increase in the rate of reaction."
- Linus Pauling
Nature 161(1948):707
Thus, we see the key to how enzymes operate: Enzymes accelerate reactions by decreasing ∆ G
, the activation energy.
The combination of substrate and enzyme creates a new reaction pathway whose transition-state energy is lower than
that of the reaction in the absence of enzyme (see Figure 8.3). The lower activation energy means that more molecules
have the required energy to reach the transition state. Decreasing the activation barrier is analogous to lowering the
height of a high-jump bar; more athletes will be able to clear the bar. The essence of catalysis is specific binding of the
transition state.
8.3.1. The Formation of an Enzyme-Substrate Complex Is the First Step in Enzymatic
Catalysis
Much of the catalytic power of enzymes comes from their bringing substrates together in favorable orientations to
promote the formation of the transition states in enzyme-substrate (ES) complexes. The substrates are bound to a specific
region of the enzyme called the active site. Most enzymes are highly selective in the substrates that they bind. Indeed, the
catalytic specificity of enzymes depends in part on the specificity of binding.
What is the evidence for the existence of an enzyme-substrate complex?
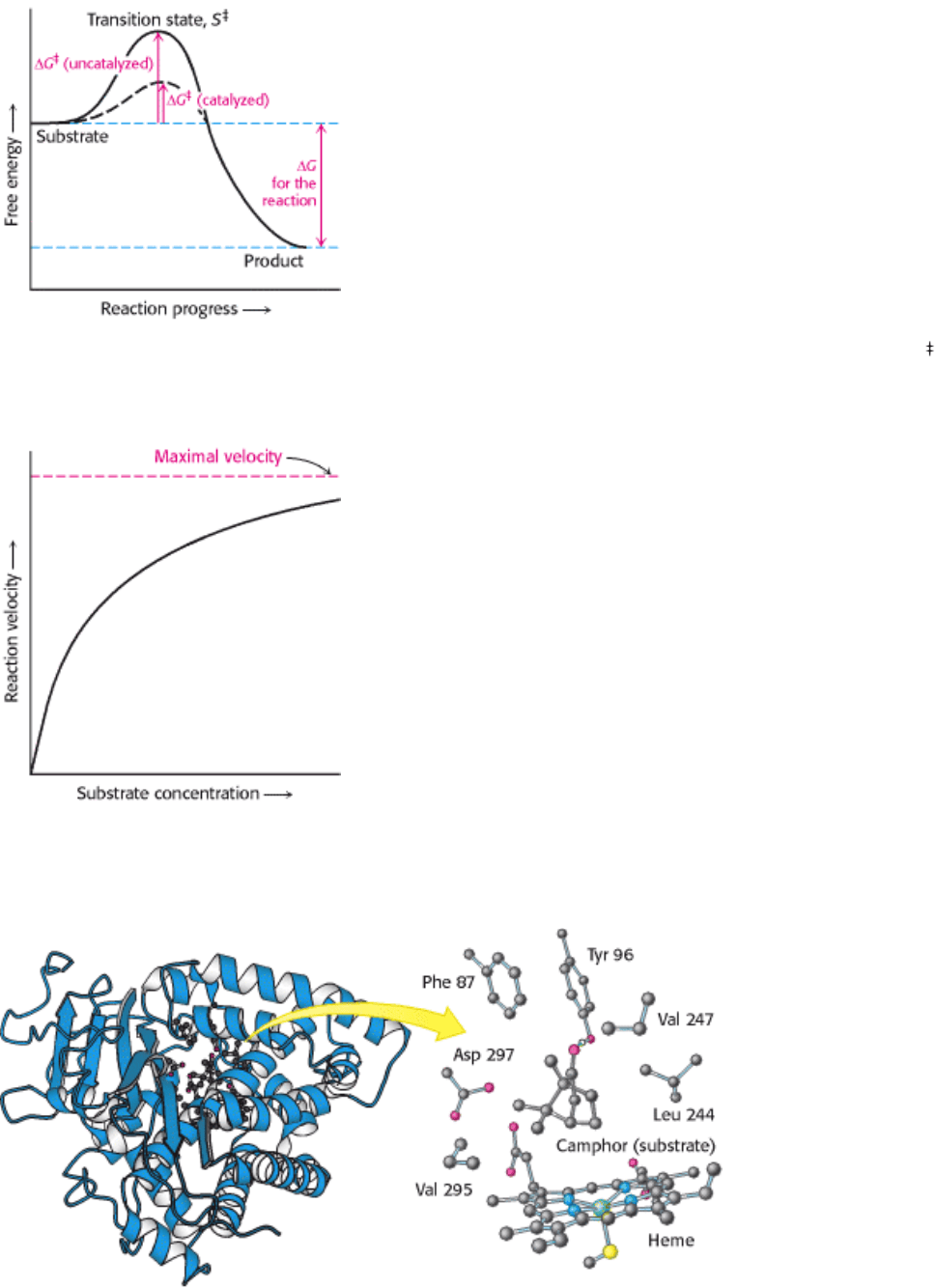
1. The first clue was the observation that, at a constant concentration of enzyme, the reaction rate increases with
increasing substrate concentration until a maximal velocity is reached (Figure 8.4). In contrast, uncatalyzed reactions do
not show this saturation effect. The fact that an enzyme-catalyzed reaction has a maximal velocity suggests the formation
of a discrete ES complex. At a sufficiently high substrate concentration, all the catalytic sites are filled and so the
reaction rate cannot increase. Although indirect, this is the most general evidence for the existence of ES complexes.
2. X-ray crystallography has provided high-resolution images of substrates and substrate analogs bound to the active
sites of many enzymes (Figure 8.5). In Chapter 9, we will take a close look at several of these complexes. X-ray studies
carried out at low temperatures (to slow reactions down) are providing revealing views of enzyme-substrate complexes
and their subsequent reactions. A new technique, time-resolved crystallography, depends on cocrystallizing a photolabile
substrate analog with the enzyme. The substrate analog can be converted to substrate light, and images of the enzyme-
substrate complex are obtained in a fraction of a second by scanning the crystal with intense, polychromatic x-rays from
a synchrotron.
3. The spectroscopic characteristics of many enzymes and substrates change on formation of an ES complex. These
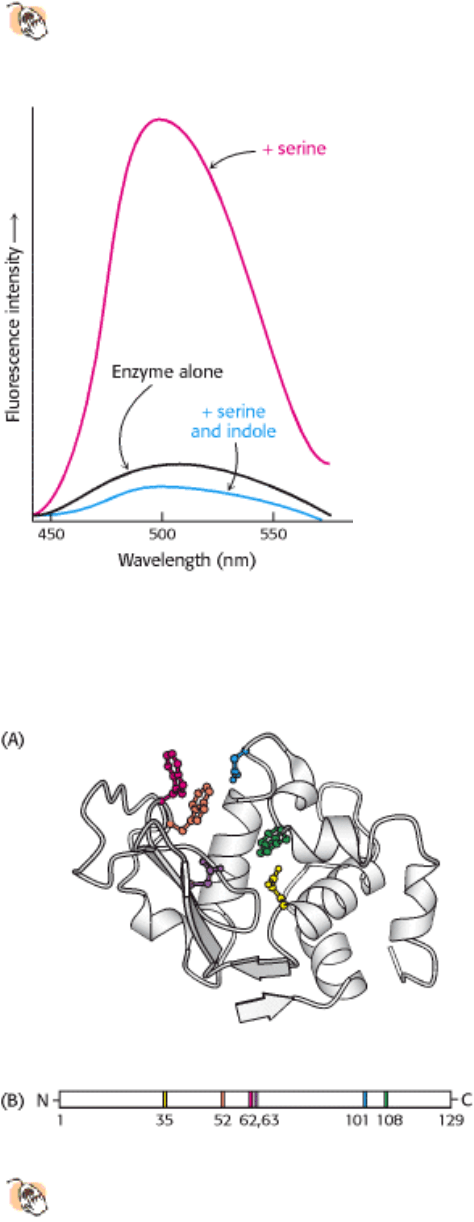
changes are particularly striking if the enzyme contains a colored prosthetic group. Tryptophan synthetase, a bacterial
enzyme that contains a pyridoxal phosphate (PLP) prosthetic group, provides a nice illustration. This enzyme catalyzes
the synthesis of l-tryptophan from l-serine and indole-derivative. The addition of l-serine to the enzyme produces a
marked increase in the fluorescence of the PLP group (Figure 8.6). The subsequent addition of indole, the second
substrate, reduces this fluorescence to a level even lower than that of the enzyme alone. Thus, fluorescence spectroscopy
reveals the existence of an enzyme-serine complex and of an enzyme-serine-indole complex. Other spectroscopic

techniques, such as nuclear magnetic resonance and electron spin resonance, also are highly informative about ES
interactions.
8.3.2. The Active Sites of Enzymes Have Some Common Features
The active site of an enzyme is the region that binds the substrates (and the cofactor, if any). It also contains the residues
that directly participate in the making and breaking of bonds. These residues are called the catalytic groups. In essence,
the interaction of the enzyme and substrate at the active site promotes the formation of the transition state. The active
site is the region of the enzyme that most directly lowers the ∆ G
of the reaction, which results in the rate enhancement
characteristic of enzyme action. Although enzymes differ widely in structure, specificity, and mode of catalysis, a
number of generalizations concerning their active sites can be stated:
1. The active site is a three-dimensional cleft formed by groups that come from different parts of the amino acid
sequence indeed, residues far apart in the sequence may interact more strongly than adjacent residues in the amino
acid sequence. In lysozyme, an enzyme that degrades the cell walls of some bacteria, the important groups in the active
site are contributed by residues numbered 35, 52, 62, 63, 101, and 108 in the sequence of the 129 amino acids (Figure
8.7).
2. The active site takes up a relatively small part of the total volume of an enzyme. Most of the amino acid residues in an
enzyme are not in contact with the substrate, which raises the intriguing question of why enzymes are so big. Nearly all
enzymes are made up of more than 100 amino acid residues, which gives them a mass greater than 10 kd and a diameter
of more than 25 Å. The "extra" amino acids serve as a scaffold to create the three-dimensional active site from amino
acids that are far apart in the primary structure. Amino acids near to one another in the primary structure are often
sterically constrained from adopting the structural relations necessary to form the active site. In many proteins, the
remaining amino acids also constitute regulatory sites, sites of interaction with other proteins, or channels to bring the
substrates to the active sites.
3. Active sites are clefts or crevices. In all enzymes of known structure, substrate molecules are bound to a cleft or
crevice. Water is usually excluded unless it is a reactant. The nonpolar character of much of the cleft enhances the
binding of substrate as well as catalysis. Nevertheless, the cleft may also contain polar residues. In the nonpolar
microenvironment of the active site, certain of these polar residues acquire special properties essential for substrate
binding or catalysis. The internal positions of these polar residues are biologically crucial exceptions to the general rule
that polar residues are exposed to water.
4. Substrates are bound to enzymes by multiple weak attractions. ES complexes usually have equilibrium constants that
range from 10
-2
to 10
-8
M, corresponding to free energies of interaction ranging from about -3 to -12 kcal mol
-1
(from -
13 to -50 kJ mol
-1
). The noncovalent interactions in ES complexes are much weaker than covalent bonds, which have
energies between -50 and -110 kcal mol
-1
(between -210 and -460 kJ mol
-1
). As discussed in Chapter 1 (Section 1.3.1),
electrostatic interactions, hydrogen bonds, van der Waals forces, and hydrophobic interactions mediate reversible
interactions of biomolecules. Van der Waals forces become significant in binding only when numerous substrate atoms
simultaneously come close to many enzyme atoms. Hence, the enzyme and substrate should have complementary
shapes. The directional character of hydrogen bonds between enzyme and substrate often enforces a high degree of
specificity, as seen in the RNA-degrading enzyme ribonuclease (Figure 8.8).
5. The specificity of binding depends on the precisely defined arrangement of atoms in an active site. Because the
enzyme and the substrate interact by means of short-range forces that require close contact, a substrate must have a
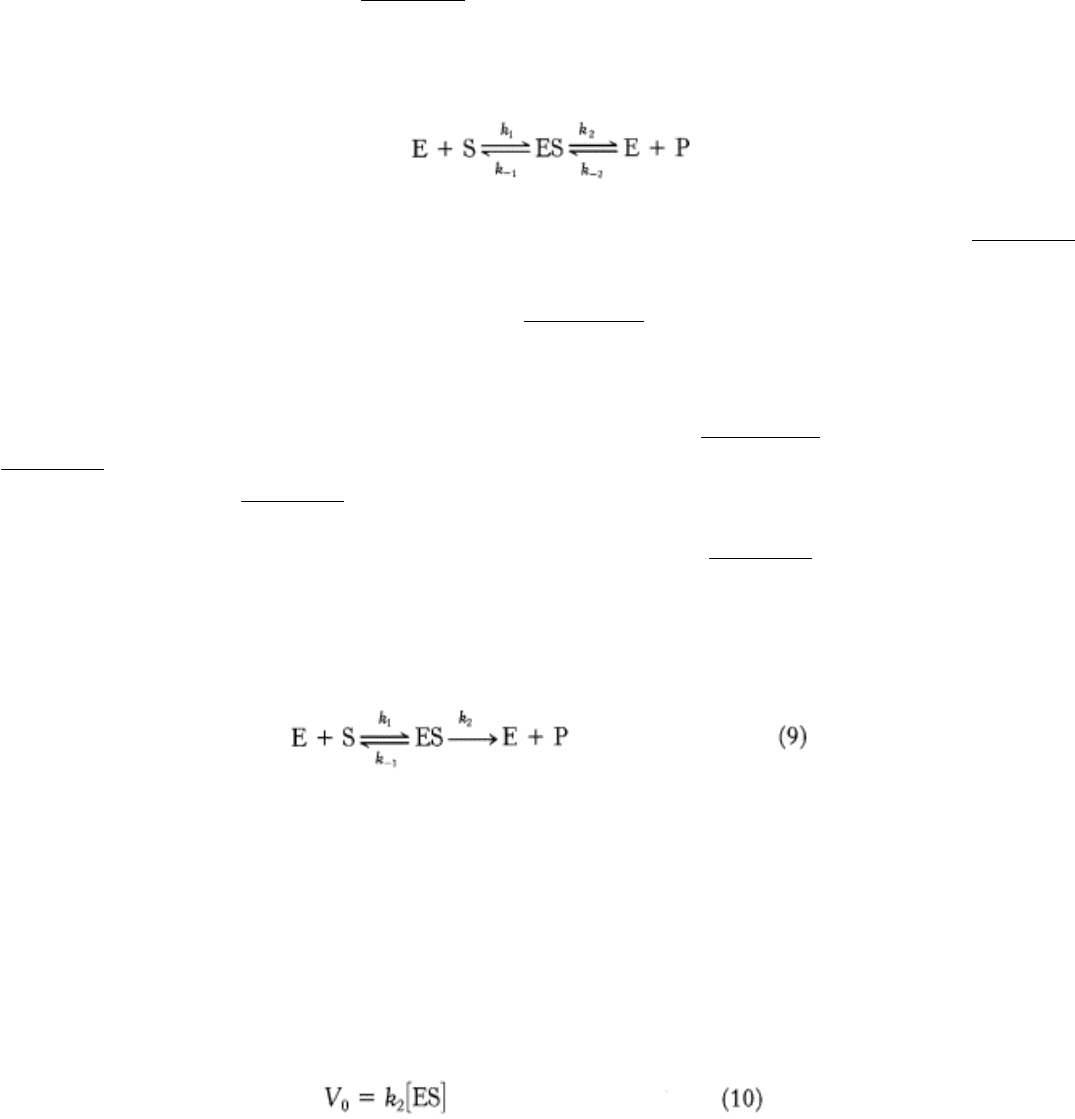
matching shape to fit into the site. Emil Fischer's analogy of the lock and key (Figure 8.9), expressed in 1890, has proved
to be highly stimulating and fruitful. However, we now know that enzymes are flexible and that the shapes of the active
sites can be markedly modified by the binding of substrate, as was postulated by Daniel E. Koshland, Jr., in 1958. The
active sites of some enzymes assume a shape that is complementary to that of the transition state only after the substrate
is bound. This process of dynamic recognition is called induced fit (Figure 8.10).
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Figure 8.3. Enzymes Decrease the Activation Energy. Enzymes accelerate reactions by decreasing ∆ G
, the free
energy of activation.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Figure 8.4. Reaction Velocity Versus Substrate Concentration in an Enzyme-Catalyzed Reaction. An enzyme-
catalyzed reaction reaches a maximal velocity.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Figure 8.5. Structure of an Enzyme-Substrate Complex. (Left) The enzyme cytochrome P-450 is illustrated bound to
its substrate camphor. (Right) In the active site, the substrate is surrounded by residues from the enzyme. Note
also the presence of a heme cofactor.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Figure 8.6. Change in Spectroscopic Characteristics with the Formation of an Enzyme-Substrate Complex.
Fluorescence intensity of the pyridoxal phosphate group at the active site of tryptophan synthetase changes on addition
of serine and indole, the substrates.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Figure 8.7. Active Sites May Include Distant Residues.
(A) Ribbon diagram of the enzyme lysozyme with several
components of the active site shown in color. (B) A schematic representation of the primary structure of lysozyme
shows that the active site is composed of residues that come from different parts of the polypeptide chain.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
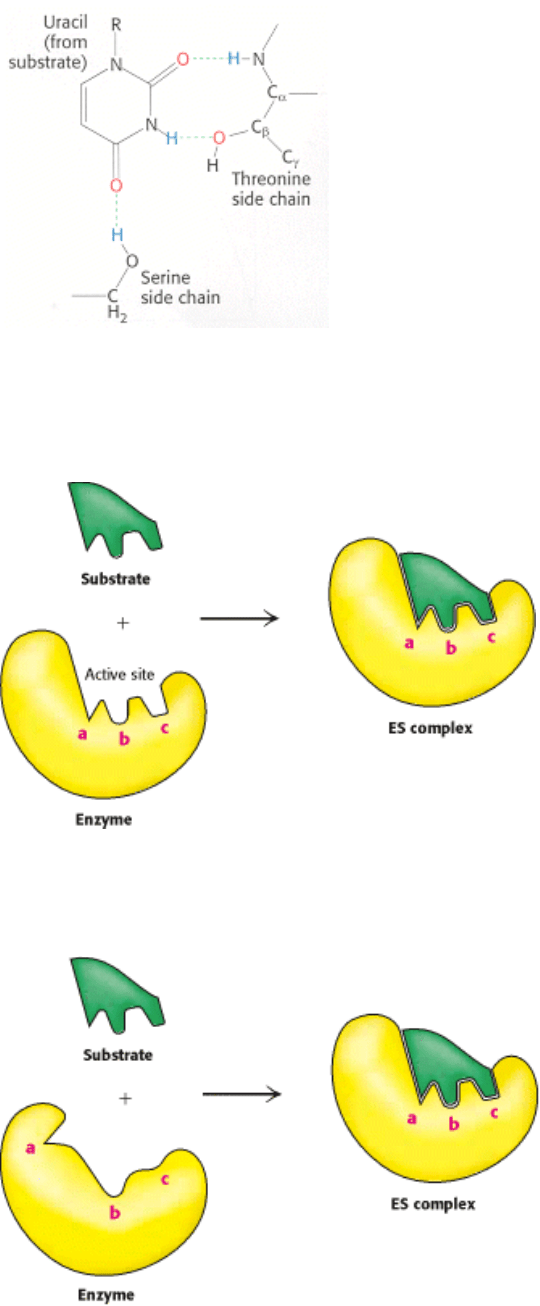
Figure 8.8. Hydrogen Bonds between an Enzyme and Substrate. The enzyme ribonuclease forms hydrogen bonds
with the uridine component of the substrate. [After F. M. Richards, H. W. Wyckoff, and N. Allewel. In The
Neurosciences: Second Study Program, F. O. Schmidt, Ed. (Rockefeller University Press, 1970), p. 970.]
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Figure 8.9. Lock-and-Key Model of Enzyme-Substrate Binding. In this model, the active site of the unbound enzyme
is complementary in shape to the substrate.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics 8.3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Figure 8.10. Induced-Fit Model of Enzyme-Substrate Binding. In this model, the enzyme changes shape on substrate
binding. The active site forms a shape complementary to the substrate only after the substrate has been bound.
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
8.4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many
Enzymes
The primary function of enzymes is to enhance rates of reactions so that they are compatible with the needs of the
organism. To understand how enzymes function, we need a kinetic description of their activity. For many enzymes, the
rate of catalysis V
0
, which is defined as the number of moles of product formed per second, varies with the substrate
concentration [S] in a manner shown in Figure 8.11. The rate of catalysis rises linearly as substrate concentration
increases and then begins to level off and approach a maximum at higher substrate concentrations. Before we can
accurately interpret this graph, we need to understand how it is generated. Consider an enzyme that catalyzes the S to P
by the following pathway:
The extent of product formation is determined as a function of time for a series of substrate concentrations (Figure 8.12).
As expected, in each case, the amount of product formed increases with time, although eventually a time is reached when
there is no net change in the concentration of S or P. The enzyme is still actively converting substrate into product and
visa versa, but the reaction equilibrium has been attained. Figure 8.13A illustrates the changes in concentration observed
in all of the reaction participants with time until equilibrium has been reached.
Enzyme kinetics are more easily approached if we can ignore the back reaction. We define V
0
as the rate of increase in
product with time when [P] is low; that is, at times close to zero (hence, V
0
) (Figure 8.13B). Thus, for the graph in
Figure 8.11, V
0
is determined for each substrate concentration by measuring the rate of product formation at early times
before P accumulates (see Figure 8.12).
We begin our kinetic examination of enzyme activity with the graph shown in Figure 8.11. At a fixed concentration of
enzyme, V
0
is almost linearly proportional to [S] when [S] is small but is nearly independent of [S] when [S] is large. In
1913, Leonor Michaelis and Maud Menten proposed a simple model to account for these kinetic characteristics. The
critical feature in their treatment is that a specific ES complex is a necessary intermediate in catalysis. The model
proposed, which is the simplest one that accounts for the kinetic properties of many enzymes, is
An enzyme E combines with substrate S to form an ES complex, with a rate constant k
1
. The ES complex has two
possible fates. It can dissociate to E and S, with a rate constant k
-1
, or it can proceed to form product P, with a rate
constant k
2
. Again, we assume that almost none of the product reverts to the initial substrate, a condition that holds in
the initial stage of a reaction before the concentration of product is appreciable.
We want an expression that relates the rate of catalysis to the concentrations of substrate and enzyme and the rates of the
individual steps. Our starting point is that the catalytic rate is equal to the product of the concentration of the ES complex
and k
2
.
Now we need to express [ES] in terms of known quantities. The rates of formation and breakdown of ES are given by:
To simplify matters, we will work under the steady-state assumption. In a steady state, the concentrations of
intermediates, in this case [ES], stay the same even if the concentrations of starting materials and products are changing.
This occurs when the rates of formation and breakdown of the ES complex are equal. Setting the right-hand sides of
equations 11 and 12 equal gives
By rearranging equation 13, we obtain
Equation 14 can be simplified by defining a new constant, K
M
, called the Michaelis constant:
Note that K
M
has the units of concentration. K
M
is an important characteristic of enzyme-substrate interactions and is
independent of enzyme and substrate concentrations.
Inserting equation 15 into equation 14 and solving for [ES] yields
Now let us examine the numerator of equation 16. The concentration of uncombined substrate [S] is very nearly equal to
the total substrate concentration, provided that the concentration of enzyme is much lower than that of substrate. The
concentration of uncombined enzyme [E] is equal to the total enzyme concentration [E]
T
minus the concentration of the
ES complex.
Substituting this expression for [E] in equation 16 gives
Solving equation 18 for [ES] gives
