Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
Problems
1.
Hydrolytic driving force. The hydrolysis of pyrophosphate to orthophosphate is important in driving forward
biosynthetic reactions such as the synthesis of DNA. This hydrolytic reaction is catalyzed in Escherichia coli by a
pyrophosphatase that has a mass of 120 kd and consists of six identical subunits. For this enzyme, a unit of activity is
defined as the amount of enzyme that hydrolyzes 10 µmol of pyrophosphate in 15 minutes at 37°C under standard
assay conditions. The purified enzyme has a V
max
of 2800 units per milligram of enzyme.
(a) How many moles of substrate are hydrolyzed per second per milligram of enzyme when the substrate
concentration is much greater than K
M
?
(b) How many moles of active site are there in 1 mg of enzyme? Assume that each subunit has one active site.
(c) What is the turnover number of the enzyme? Compare this value with others mentioned in this chapter.
See answer
2.
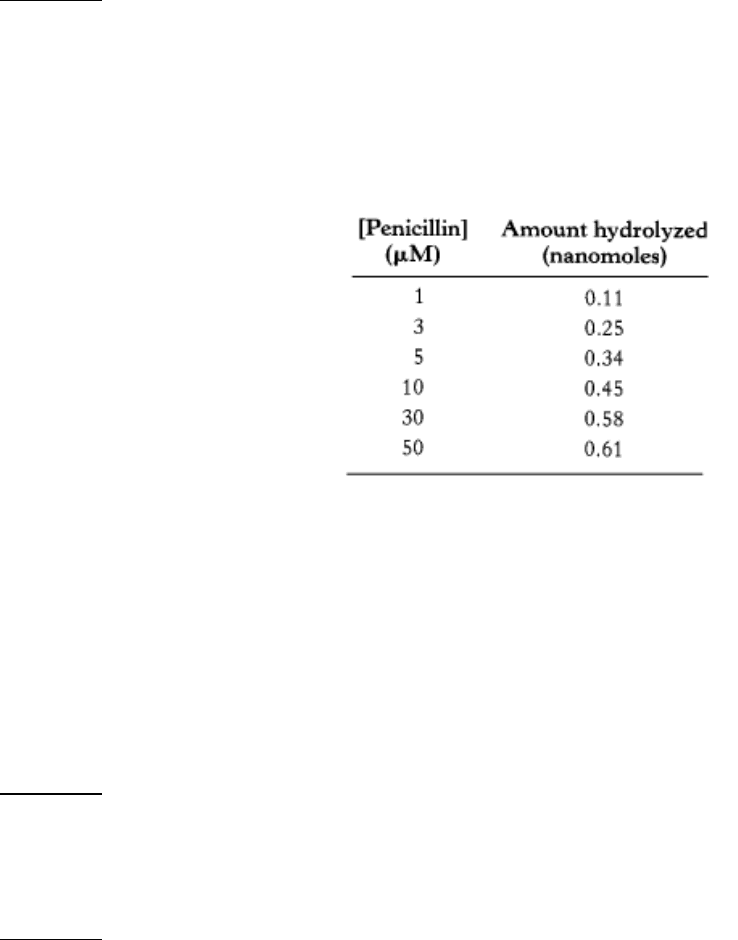
Destroying the Trojan horse. Penicillin is hydrolyzed and thereby rendered inactive by penicillinase (also known as
β-lactamase), an enzyme present in some resistant bacteria. The mass of this enzyme in Staphylococcus aureus is
29.6 kd. The amount of penicillin hydrolyzed in 1 minute in a 10-ml solution containing 10
-9
g of purified
penicillinase was measured as a function of the concentration of penicillin. Assume that the concentration of
penicillin does not change appreciably during the assay.
(a) Plot V
0
versus [S] and 1/V
0
versus 1/[S] for these data. Does penicillinase appear to obey Michaelis-Menten
kinetics? If so, what is the value of K
M
?
(b) What is the value of V
max
?
(c) What is the turnover number of penicillinase under these experimental conditions? Assume one active site per
enzyme molecule.
See answer
3.
Counterpoint. Penicillinase (β-lactamase) hydrolyzes penicillin. Compare penicillinase with glycopeptide
transpeptidase.
See answer

4.
Mode of inhibition. The kinetics of an enzyme are measured as a function of substrate concentration in the presence
and in the absence of 2 mM inhibitor (I).
(a) What are the values of V
max
and K
M
in the absence of inhibitor? In its presence?
(b) What type of inhibition is it?
(c) What is the binding constant of this inhibitor?
(d) If [S] = 10 µM and [I] = 2 mM, what fraction of the enzyme molecules have a bound substrate? A bound
inhibitor?
(e) If [S] = 30 µM, what fraction of the enzyme molecules have a bound substrate in the presence and in the absence
of 2 mM inhibitor? Compare this ratio with the ratio of the reaction velocities under the same conditions.
See answer
5.
A different mode. The kinetics of the enzyme considered in problem 4 are measured in the presence of a different
inhibitor. The concentration of this inhibitor is 100 µM.
(a) What are the values of V
max
and K
M
in the presence of this inhibitor? Compare them with those obtained in
problem 4.
(b) What type of inhibition is it?
(c) What is the dissociation constant of this inhibitor?
(d) If [S] = 30 µM, what fraction of the enzyme molecules have a bound substrate in the presence and in the absence
of 100 µM inhibitor?
See answer
6.
A fresh view. The plot of 1/V
0
versus 1/[S] is sometimes called a Lineweaver-Burk plot. Another way of expressing
the kinetic data is to plot V
0
versus V
0
/[S], which is known as an Eadie-Hofstee plot.
(a) Rearrange the Michaelis-Menten equation to give V
0
as a function of V
0
/[S].
(b) What is the significance of the slope, the vertical intercept, and the horizontal intercept in a plot of V
0
versus V
0
/
[S]?
(c) Sketch a plot of V
0
versus V
0
/[S] in the absence of an inhibitor, in the presence of a competitive inhibitor, and in
the presence of a noncompetitive inhibitor.
See answer
7.
Potential donors and acceptors. The hormone progesterone contains two ketone groups. At pH 7, which side chains
of the receptor might form hydrogen bonds with progesterone?
See answer
8.
Competing substrates. Suppose that two substrates, A and B, compete for an enzyme. Derive an expression relating
the ratio of the rates of utilization of A and B, V
A
/V
B
, to the concentrations of these substrates and their values of k
2
and K
M
. (Hint: Express V
A
as a function of k
2
/K
M
for substrate A, and do the same for V
B
.) Is specificity
determined by K
M
alone?
See answer
9.
A tenacious mutant. Suppose that a mutant enzyme binds a substrate 100-fold as tightly as does the native enzyme.
What is the effect of this mutation on catalytic rate if the binding of the transition state is unaffected?
See answer
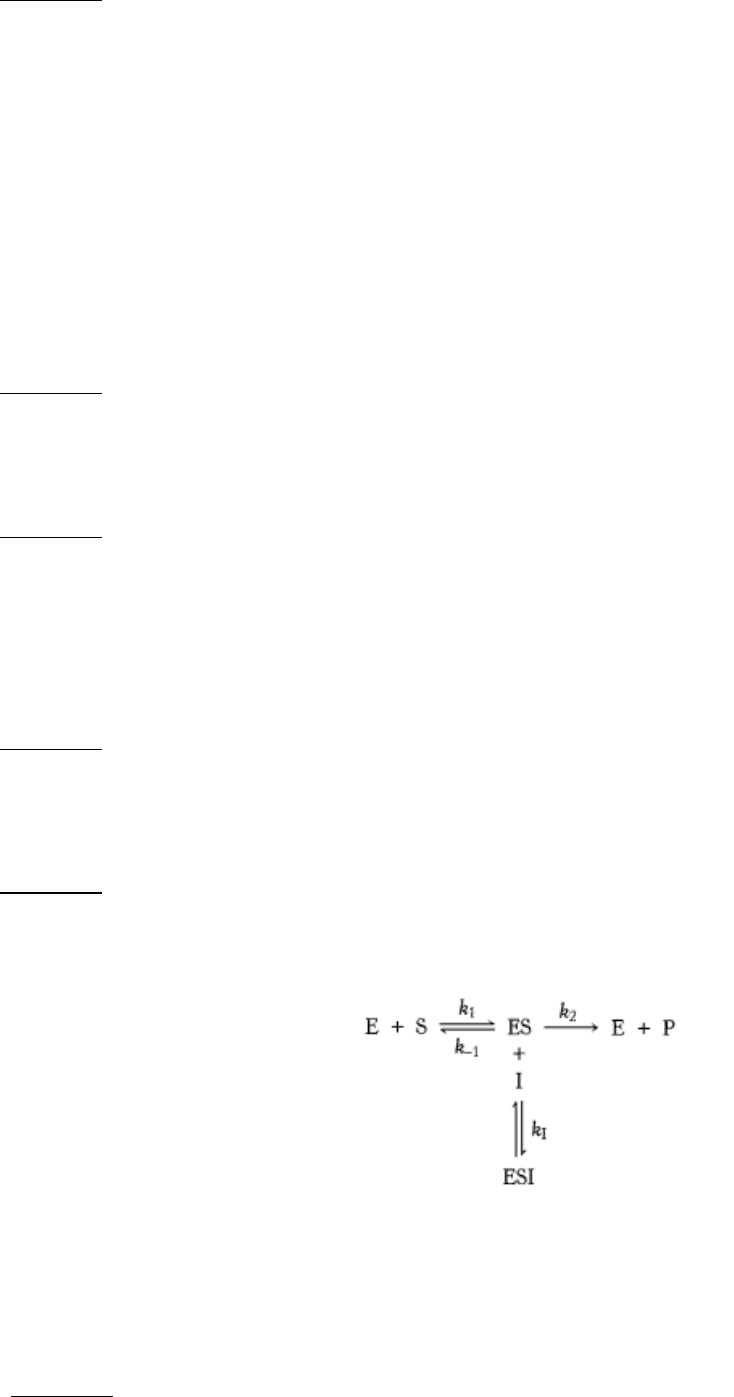
10.
Uncompetitive inhibition. The following reaction represents the mechanism of action of an uncompetitive inhibitor.
(a) Draw a standard Michaelis-Menton curve in the absence and in the presence of increasing amounts of inhibitor.
Repeat for a double-reciprocal plot.
(b) Explain the results obtained in part a.
See answer
11.
More Michaelis-Menten. For an enzyme that follows simple Michaelis-Menten kinetics, what is the value of V
max
if V
0
is equal to 1 µ mol/minute at 1/10 K
M
?
See answer
Data Interpretation Problems
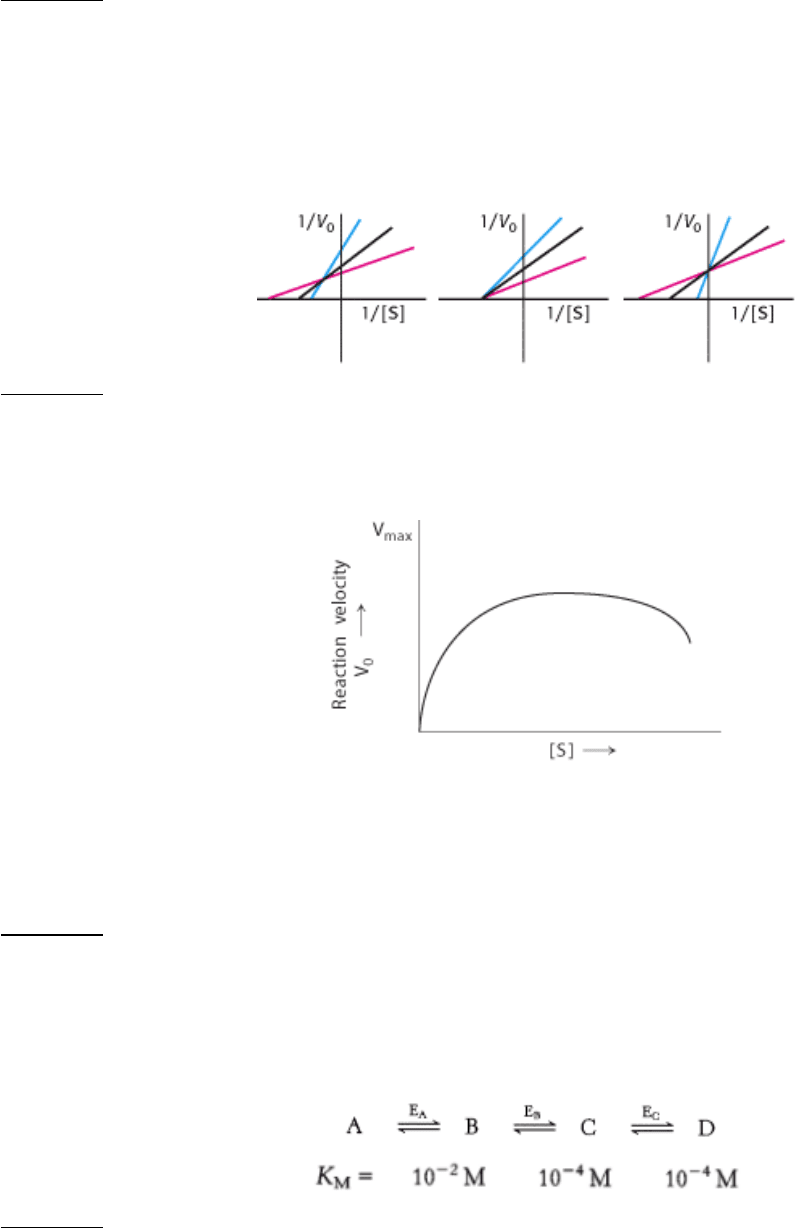
12.
Varying the enzyme. For a one-substrate, enzyme-catalyzed reaction, double-reciprocal plots were determined for
three different enzyme concentrations. Which of the following three families of curve would you expect to be
obtained? Explain.
See answer
13.
Too much of a good thing. A simple Michaelis-Menten enzyme, in the absence of any inhibitor, displayed the
following kinetic behavior. The expected value of V
max
is shown on the y-axis.
(a) Draw a double-reciprocal plot that corresponds to the velocity-versus-substrate curve.
(b) Provide an explanation for the kinetic results.
See answer
14.
Rate-limiting step. In the conversion of A into D in the following biochemical pathway, enzymes E
A
, E
B
, and E
C
have the K
M
values indicated under each enzyme. If all of the substrates and products are present at a
concentration of 10
-4
M, which step will be rate limiting and why?
See answer
Chapter Integration Problems
15.
Titration experiment. The effect of pH on the activity of an enzyme was examined. At its active site, the enzyme
has an ionizable group that must be negatively charged for substrate binding and catalysis to take place. The
ionizable group has a pK
a
of 6.0. The substrate is positively charged throughout the pH range of the experiment.
(a) Draw the V
0
-versus-pH curve when the substrate concentration is much greater than the enzyme K
M
.
(b) Draw the V
0
-versus-pH curve when the substrate concentration is much less than the enzyme K
M
.
(c) At which pH will the velocity equal one-half of the maximal velocity attainable under these condition?
See answer
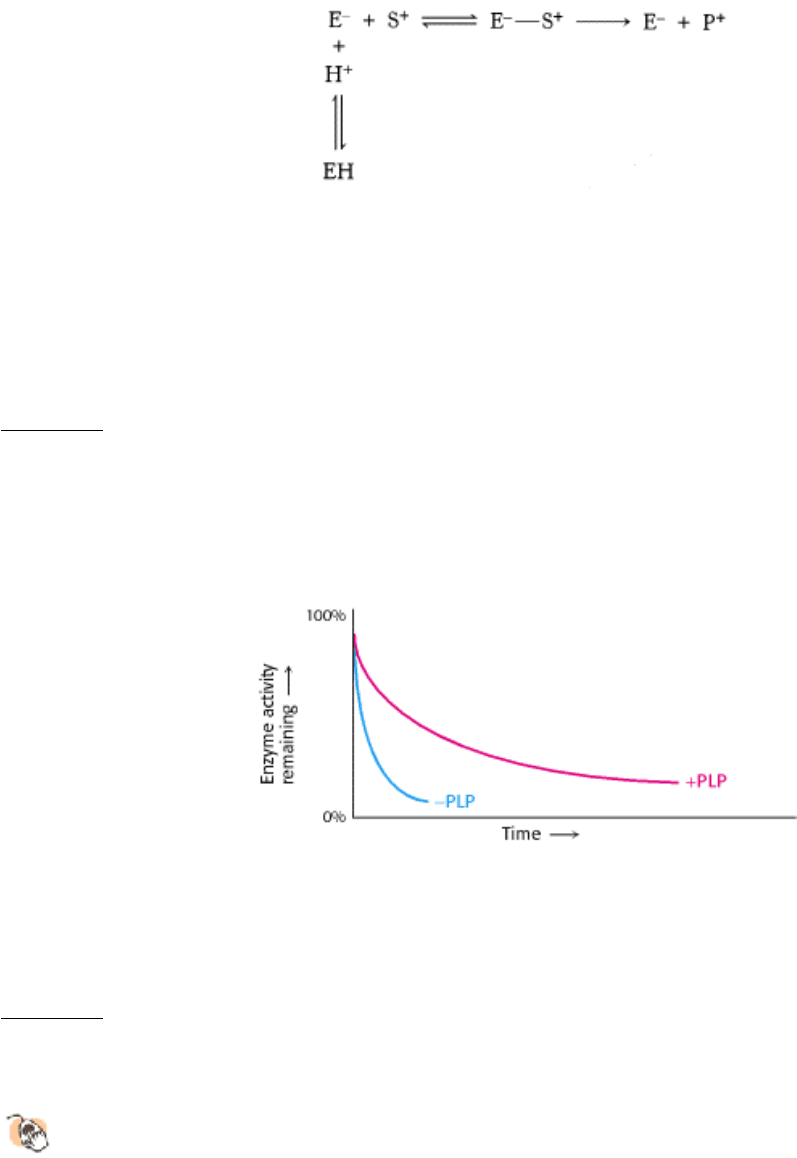
16.
A question of stability. Pyridoxal phosphate (PLP) is a coenzyme for the enzyme ornithine aminotransferase. The
enzyme was purified from cells grown in PLP-deficient media as well as from cells grown in media that contained
pyridoxal phosphate. The stability of the enzyme was then measured by incubating the enzyme at 37°C and
assaying for the amount of enzyme activity remaining. The following results were obtained.
(a) Why does the amount of active enzyme decrease with the time of incubation?
(b) Why does the amount of enzyme from the PLP-deficient cells decline more rapidly?
See answer
Media Problem
17.
Not all data points are created equal. Your lab partner, who is both systematic and frugal, decides to perform
a series of enzyme assays at substrate concentrations of 1, 2, 4, and 8 µM. You argue for doing the
experiments at [S] = 1, 4, 16, and 100 µM. Try both sets of experiments using the simulated enzyme kinetics lab in
the Steady-State Enzyme Kinetics Conceptual Insights module. Who had the better idea, and why?
I. The Molecular Design of Life 8. Enzymes: Basic Concepts and Kinetics
Selected Readings
Where to start
D.E. Koshland Jr. 1987. Evolution of catalytic function Cold Spring Harbor Symp. Quant. Biol. 52: 1-7. (PubMed)
W.P. Jencks. 1987. Economics of enzyme catalysis Cold Spring Harbor Symp. Quant. Biol. 52: 65-73. (PubMed)
R.A. Lerner and A. Tramontano. 1988. Catalytic antibodies Sci. Am. 258: (3) 58-70. (PubMed)
Books
Fersht, A., 1999. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding. W. H.
Freeman and Company.
Walsh, C., 1979. Enzymatic Reaction Mechanisms. W. H. Freeman and Company.
Page, M. I., and Williams, A. (Eds.), 1987. Enzyme Mechanisms. Royal Society of Chemistry.
Bender, M. L., Bergeron,R. J., and Komiyama, M., 1984. The Bioorganic Chemistry of Enzymatic Catalysis. Wiley-
Interscience.
Abelson, J. N., and Simon, M. I. (Eds.), 1992. Methods in Enzymology. Academic Press.
Boyer, P. D. (Ed.), 1970. The Enzymes (3d ed.). Academic Press.
Friedmann, H. (ed.), 1981. Benchmark Papers in Biochemistry. Vol. 1, Enzymes. Hutchinson Ross.
Transition-state stabilization, analogs, and other enzyme inhibitors
V.L. Schramm. 1998. Enzymatic transition states and transition state analog design Annu. Rev. Biochem. 67: 693-720.
(PubMed)
L. Pauling. 1948. Nature of forces between large molecules of biological interest Nature 161: 707-709.
G.E. Leinhard. 1973. Enzymatic catalysis and transition-state theory Science 180: 149-154. (PubMed)
J. Kraut. 1988. How do enzymes work? Science 242: 533-540. (PubMed)
D.J. Waxman and J.L. Strominger. 1983. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics
Annu. Rev. Biochem. 52: 825-869. (PubMed)
E.P. Abraham. 1981. The β-lactam antibiotics Sci. Am. 244: 76-86. (PubMed)
C.T. Walsh. 1984. Suicide substrates, mechanism-based enzyme inactivators: Recent developments Annu. Rev. Biochem.
53: 493-535. (PubMed)
Catalytic antibodies
D. Hilvert. 2000. Critical analysis of antibody catalysis Annu. Rev. Biochem. 69: 751-794. (PubMed)
H. Wade and T.S. Scanlan. 1997. The structural and functional basis of antibody catalysis Annu. Rev. Biophys. Biomol.
Struct. 26: 461-493. (PubMed)
R.A. Lerner, S.J. Benkovic, and P.G. Schultz. 1991. At the crossroads of chemistry and immunology: Catalytic
antibodies Science 252: 659-667. (PubMed)
A.G. Cochran and P.G. Schultz. 1990. Antibody-catalyzed porphyrin metallation Science 249: 781-783. (PubMed)
Enzyme kinetics and mechanisms
X.S. Xie and H.P. Lu. 1999. Single-molecule enzymology J. Biol. Chem. 274: 15967-15970. (PubMed)
E.W. Miles, S. Rhee, and D.R. Davies. 1999. The molecular basis of substrate channeling J. Biol. Chem. 274: 12193-
12196. (PubMed)
A. Warshel. 1998. Electrostatic origin of the catalytic power of en-zymes and the role of preorganized active sites J.
Biol. Chem. 273: 27035-27038. (PubMed)
W.R. Cannon and S.J. Benkovic. 1999. Solvation, reorganization energy, and biological catalysis J. Biol. Chem. 273:
26257-26260. (PubMed)
W.W. Cleland, P.A. Frey, and J.A. Gerlt. 1998. The low barrier hydrogen bond in enzymatic catalysis J. Biol. Chem.
273: 25529- 25532. (PubMed)
F.E. Romesberg, B.D. Santarsiero, B. Spiller, J. Yin, D. Barnes, P.G. Schultz, and R.C. Stevens. 1998. Structural and
kinetic evidence for strain in biological catalysis Biochemistry 37: 14404- 14409. (PubMed)
H.P. Lu, L. Xun, and X.S. Xie. 1998. Single-molecule enzymatic dynamics Science 282: 1877-1882. (PubMed)
A.R. Fersht, R.J. Leatherbarrow, and T.N.C. Wells. 1986. Binding energy and catalysis: A lesson from protein
engineering of the tyrosyl-tRNA synthetase Trends Biochem. Sci. 11: 321-325.
W.P. Jencks. 1975. Binding energy, specificity, and enzymic catalysis: The Circe effect Adv. Enzymol. 43: 219-410.
(PubMed)
J.R. Knowles and W.J. Albery. 1976. Evolution of enzyme function and the development of catalytic efficiency
Biochemistry 15: 5631-5640. (PubMed)
I. The Molecular Design of Life
9. Catalytic Strategies
What are the sources of the catalytic power and specificity of enzymes? This chapter presents the catalytic strategies
used by four classes of enzymes: the serine proteases, carbonic anhydrases, restriction endonucleases, and nucleoside
monophosphate (NMP) kinases. The first three classes of enzymes catalyze reactions that require the addition of water to
a substrate. For the serine proteases, exemplified by chymotrypsin, the challenge is to promote a reaction that is almost
immeasurably slow at neutral pH in the absence of a catalyst. For carbonic anhydrases, the challenge is to achieve a high
absolute rate of reaction, suitable for integration with other rapid physiological processes. For restriction endonucleases
such as EcoRV, the challenge is to attain a very high level of specificity. Finally, for NMP kinases, the challenge is to
transfer a phosphoryl group from ATP to a nucleotide and not to water. The actions of these enzymes illustrate many
important principles of catalysis. The mechanisms of these enzymes have been revealed through the use of incisive
experimental probes, including the techniques of protein structure determination (Chapter 4) and site-directed
mutagenesis (Chapter 6). These mechanisms include the use of binding energy and induced fit as well as several specific
catalytic strategies. Properties common to an enzyme family reveal how their enzyme active sites have evolved and been
refined. Structural and mechanistic comparisons of enzyme action are thus sources of insight into the evolutionary
history of enzymes. These comparisons also reveal particularly effective solutions to biochemical problems that are used
repeatedly in biological systems. In addition, our knowledge of catalytic strategies has been used to develop practical
applications, including drugs that are potent and specific enzyme inhibitors. Finally, although we shall not consider
catalytic RNA molecules (Section 28.4) explicitly in this chapter, the principles apply to these catalysts in addition to
protein catalysts.
9.0.1. A Few Basic Catalytic Principles Are Used by Many Enzymes
In Chapter 8, we learned that enzymatic catalysis begins with substrate binding. The binding energy is the free energy
released in the formation of a large number of weak interactions between the enzyme and the substrate. We can envision
this binding energy as serving two purposes: it establishes substrate specificity and increases catalytic efficiency. Only
the correct substrate can participate in most or all of the interactions with the enzyme and thus maximize binding energy,
accounting for the exquisite substrate specificity exhibited by many enzymes. Furthermore, the full complement of such
interactions is formed only when the substrate is in the transition state. Thus, interactions between the enzyme and the
substrate not only favor substrate binding but stabilize the transition state, thereby lowering the activation energy. The
binding energy can also promote structural changes in both the enzyme and the substrate that facilitate catalysis, a
process referred to as induced fit.
Enzymes commonly employ one or more of the following strategies to catalyze specific reactions:
1. Covalent catalysis. In covalent catalysis, the active site contains a reactive group, usually a powerful nucleophile that
becomes temporarily covalently modified in the course of catalysis. The proteolytic enzyme chymotrypsin provides an
excellent example of this mechanism (Section 9.1.2).
2. General acid-base catalysis. In general acid-base catalysis, a molecule other than water plays the role of a proton
donor or acceptor. Chymotrypsin uses a histidine residue as a base catalyst to enhance the nucleophilic power of serine
(Section 9.1.3).
3. Metal ion catalysis. Metal ions can function catalytically in several ways. For instance, a metal ion may serve as an
electrophilic catalyst, stabilizing a negative charge on a reaction intermediate. Alternatively, the metal ion may generate
a nucleophile by increasing the acidity of a nearby molecule, such as water in the hydration of CO
2
by carbonic
anhydrase (Section 9.2.2). Finally, the metal ion may bind to substrate, increasing the number of interactions with the
enzyme and thus the binding energy. This strategy is used by NMP kinases (Section 9.4.2).
4. Catalysis by approximation. Many reactions include two distinct substrates. In such cases, the reaction rate may be
considerably enhanced by bringing the two substrates together along a single binding surface on an enzyme. NMP
kinases bring two nucleotides together to facilitate the transfer of a phosphoryl group from one nucleotide to the other
(Section 9.4.3).
I. The Molecular Design of Life 9. Catalytic Strategies
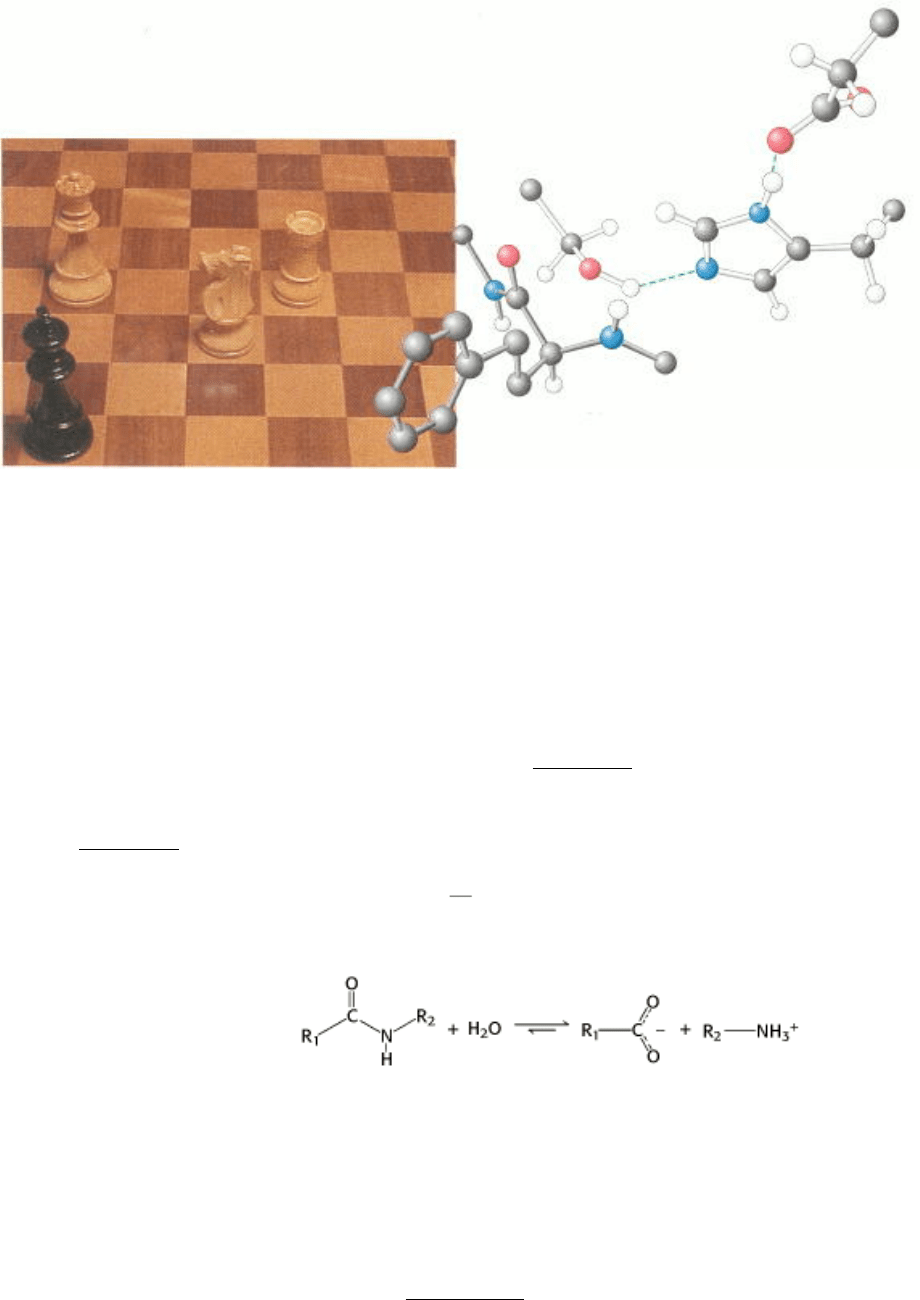
Strategy and tactics. Chess and enzymes have in common the use of strategy, consciously thought out in the game of
chess and selected by evolution for the action of an enzyme. The three amino acid residues at the right, denoted by the
white bonds, constitute a catalytic triad found in the active site of a class of enzymes that cleave peptide bonds. The
substrate, represented by the molecule with black bonds, is as hopelessly trapped as the king in the photograph of a chess
match at the left and is sure to be cleaved.[(Left) Courtesy of Wendie Berg.]
I. The Molecular Design of Life 9. Catalytic Strategies
9.1. Proteases: Facilitating a Difficult Reaction
Protein turnover is an important process in living systems (Chapter 23). Proteins that have served their purpose must be
degraded so that their constituent amino acids can be recycled for the synthesis of new proteins. Proteins ingested in the
diet must be broken down into small peptides and amino acids for absorption in the gut. Furthermore, as described in
detail in Chapter 10, proteolytic reactions are important in regulating the activity of certain enzymes and other proteins.
Proteases cleave proteins by a hydrolysis reaction
the addition of a molecule of water to a peptide bond:
Although the hydrolysis of peptide bonds is thermodynamically favored, such hydrolysis reactions are extremely slow.
In the absence of a catalyst, the half-life for the hydrolysis of a typical peptide at neutral pH is estimated to be between
10 and 1000 years. Yet, peptide bonds must be hydrolyzed within milliseconds in some biochemical processes.
The chemical bonding in peptide bonds is responsible for their kinetic stability. Specifically, the resonance structure that
accounts for the planarity of a peptide bond (Section 3.2.2) also makes such bonds resistant to hydrolysis. This resonance
structure endows the peptide bond with partial double-bond character:
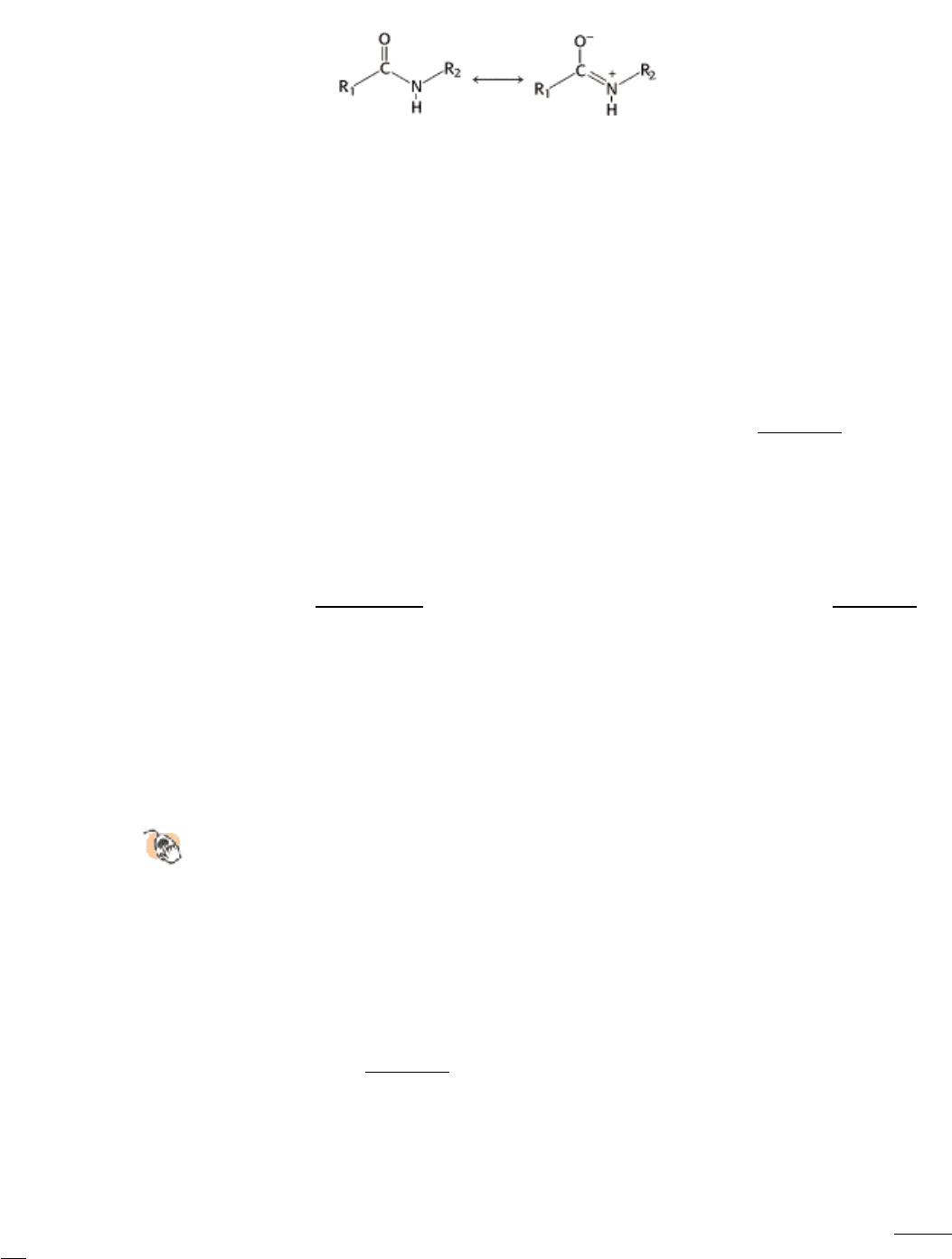
The carbon-nitrogen bond is strengthened by its double-bond character, and the carbonyl carbon atom is less
electrophilic and less susceptible to nucleophilic attack than are the carbonyl carbon atoms in compounds such as
carboxylate esters. Consequently, to promote peptide-bond cleavage, an enzyme must facilitate nucleophilic attack at a
normally unreactive carbonyl group.
9.1.1. Chymotrypsin Possesses a Highly Reactive Serine Residue
A number of proteolytic enzymes participate in the breakdown of proteins in the digestive systems of mammals and
other organisms. One such enzyme, chymotrypsin, cleaves peptide bonds selectively on the carboxylterminal side of the
large hydrophobic amino acids such as tryptophan, tyrosine, phenylalanine, and methionine (Figure 9.1). Chymotrypsin
is a good example of the use of covalent modification as a catalytic strategy. The enzyme employs a powerful
nucleophile to attack the unreactive carbonyl group of the substrate. This nucleophile becomes covalently attached to the
substrate briefly in the course of catalysis.
What is the nucleophile that chymotrypsin employs to attack the substrate carbonyl group? A clue came from the fact
that chymotrypsin contains an extraordinarily reactive serine residue. Treatment with organofluorophosphates such as
diisopropylphosphofluoridate (DIPF) (Section 8.5.2) was found to inactivate the enzyme irreversibly (Figure 9.2).
Despite the fact that the enzyme possesses 28 serine residues, only one, serine 195, was modified, resulting in a total loss
of enzyme activity. This chemical modification reaction suggested that this unusually reactive serine residue plays a
central role in the catalytic mechanism of chymotrypsin.
9.1.2. Chymotrypsin Action Proceeds in Two Steps Linked by a Covalently Bound
Intermediate
Conceptual Insights, Enzyme Kinetics. See the section entitled "Pre-Steady-
State Kinetics" in Conceptual Insights module to better understand why a
"burst" phase at short reaction times implies the existence of an enzyme-
substrate intermediate.
How can we elucidate the role of serine 195 in chymotrypsin action? A study of the enzyme's kinetics provided a second
clue to chymotrypsin's catalytic mechanism and the role of serine 195. The kinetics of enzyme action are often easily
monitored by having the enzyme act on a substrate analog that forms a colored product. For chymotrypsin, such a
chromogenic substrate is N-acetyl-
l-phenylalanine p-nitrophenyl ester. This substrate is an ester rather than an amide,
but many proteases will also hydrolyze esters. One of the products formed by chymotrypsin's cleavage of this substrate is
p- nitrophenolate, which has a yellow color (Figure 9.3). Measurements of the absorbance of light revealed the amount
of p-nitrophenolate being produced.
Under steady-state conditions, the cleavage of this substrate obeys Michaelis-Menten kinetics with a K
M
of 20 µM and a
k
cat
of 77 s
-1
. The initial phase of the reaction was examined by using the stopped-flow method. This technique permits
the rapid mixing of enzyme and substrate and allows almost instantaneous monitoring of the reaction. At the beginning
of the reaction, this method revealed a "burst" phase during which the colored product was produced rapidly (Figure
9.4). Product was then produced more slowly as the reaction reached the steady state. These results suggest that
hydrolysis proceeds in two steps. The burst is observed because, for this substrate, the first step is more rapid than the
second step.
