Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

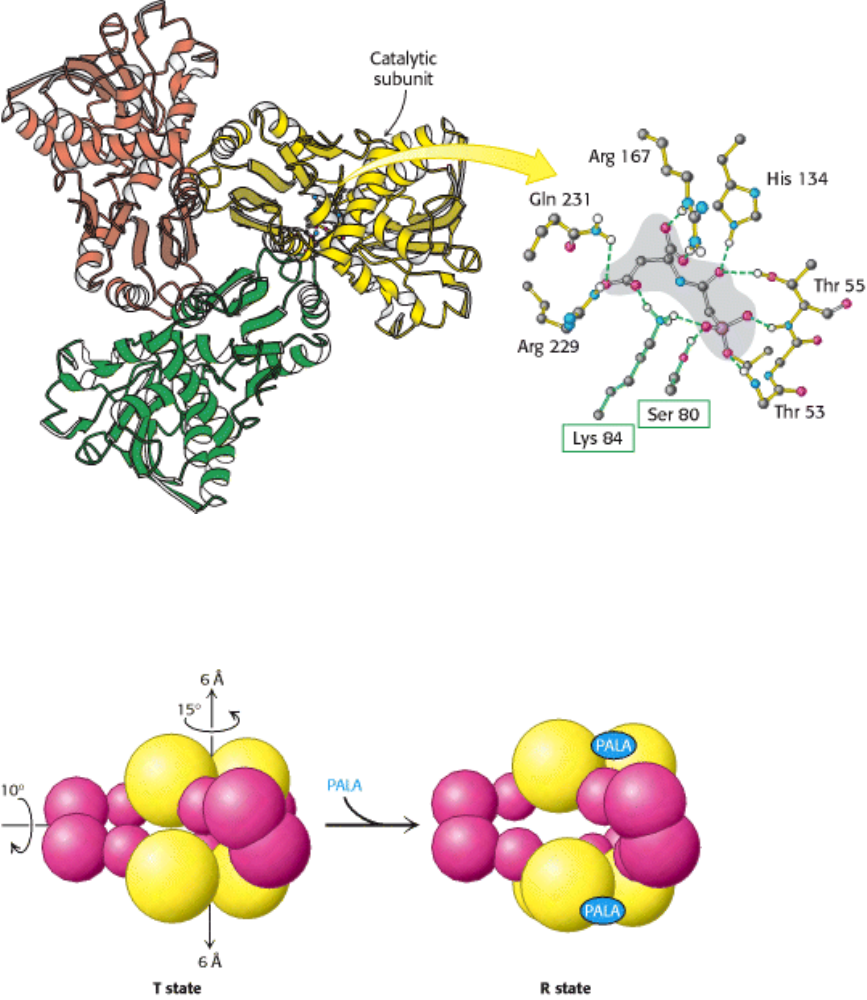
Figure 10.7. The Active Site of ATCase. Some of the crucial active-site residues are shown binding to the inhibitor
PALA. The active site is composed mainly of residues from one subunit, but an adjacent subunit also contributes
important residues (boxed in green).
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
Figure 10.8. The T-to-R State Transition in ATCase. Aspartate transcarbamoylase exists in two conformations: a
compact, relatively inactive form called the tense (T) state and an expanded form called the relaxed (R) state. PALA
binding stabilizes the R state.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
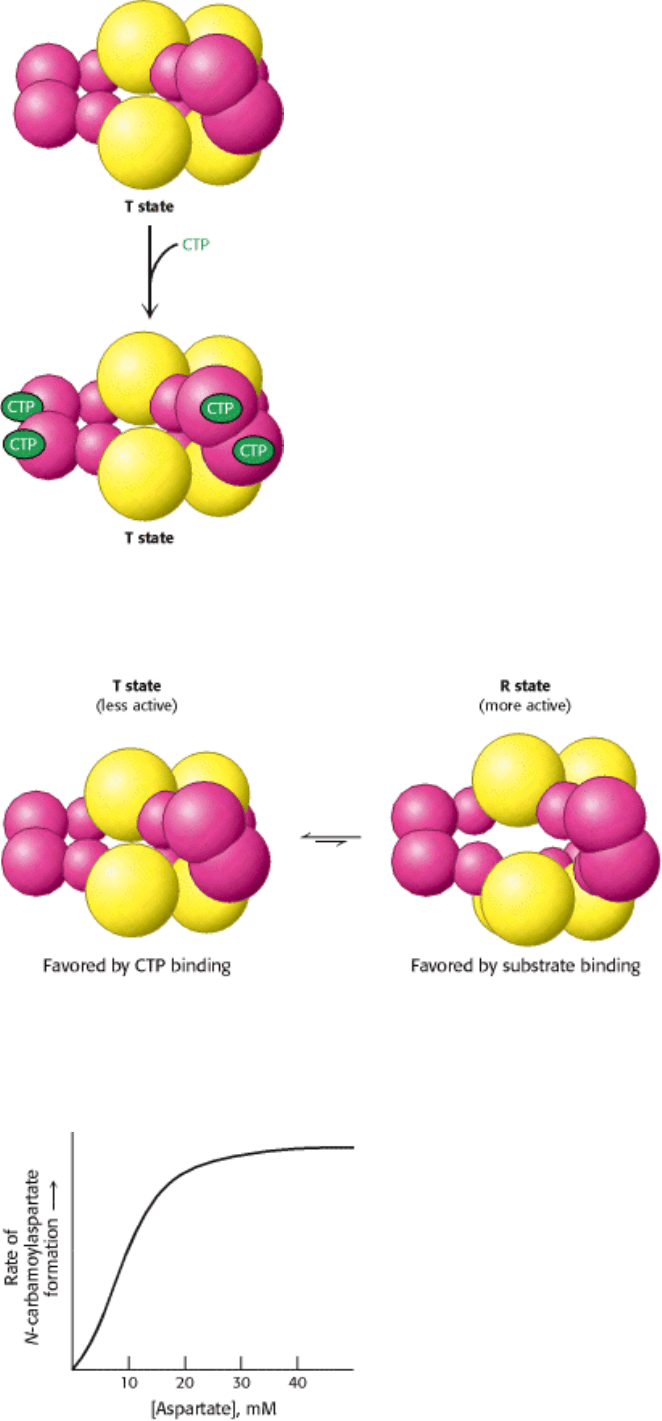
Figure 10.9. CTP Stabilizes the T State. The binding of CTP to the regulatory subunit of aspartate transcarbamoylase
stabilizes the T state.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
Figure 10.10. The R State and the T State Are in Equilibrium. Even in the absence of any substrate or regulators,
aspartate transcarbamoylase exists in an equilibrium between the R and the T states. Under these conditions, the T state
is favored by a factor of approximately 200.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
Figure 10.11. ATCase Displays Sigmoidal Kinetics. A plot of product formation as a function of substrate
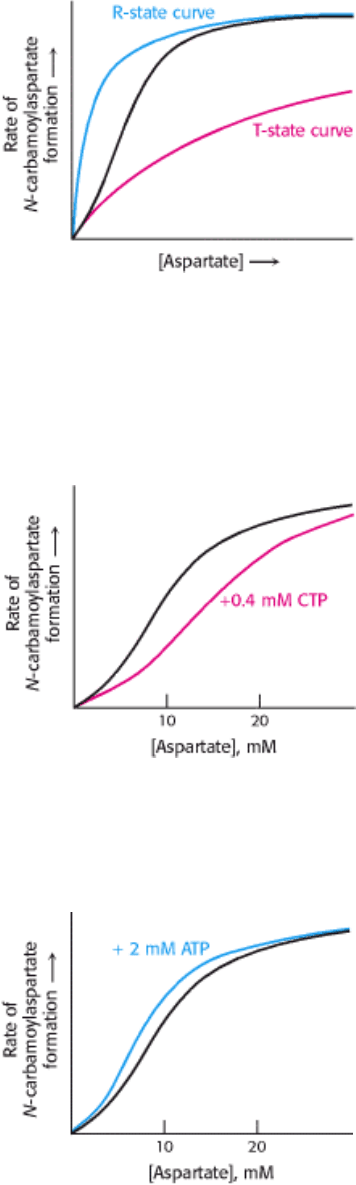
concentration produces a sigmoidal curve because the binding of substrate to one active site favors the conversion of the
entire enzyme into the R state, increasing the activity at the other active sites. Thus, the active sites show cooperativity.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
Figure 10.12. Basis for the Sigmoidal Curve. The generation of the sigmoidal curve by the property of cooperativity
can be understood by imagining an allosteric enzyme as a mixture of two Michaelis-Menten enzymes, one with a high
value of K
m
that corresponds to the T state and another with a low value of K
m
that corresponds to the R state. As the
concentration of substrate is increased, the equilibrium shifts from the T state to the R state, which results in a steep rise
in activity with respect to substrate concentration.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
Figure 10.13. Effect of CTP on ATCase Kinetics. Cytidine triphosphate (CTP) stabilizes the T state of aspartate
transcarbamoylase, making it more difficult for substrate binding to convert the enzyme into the R state. As a result, the
curve is shifted to the right, as shown in red.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
Figure 10.14. Effect of ATP on ATCase Kinetics. ATP is an allosteric activator of aspartate transcarbamoylase
because it stabilizes the R state, making it easier for substrate to bind. As a result, the curve is shifted to the left, as
shown in blue.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
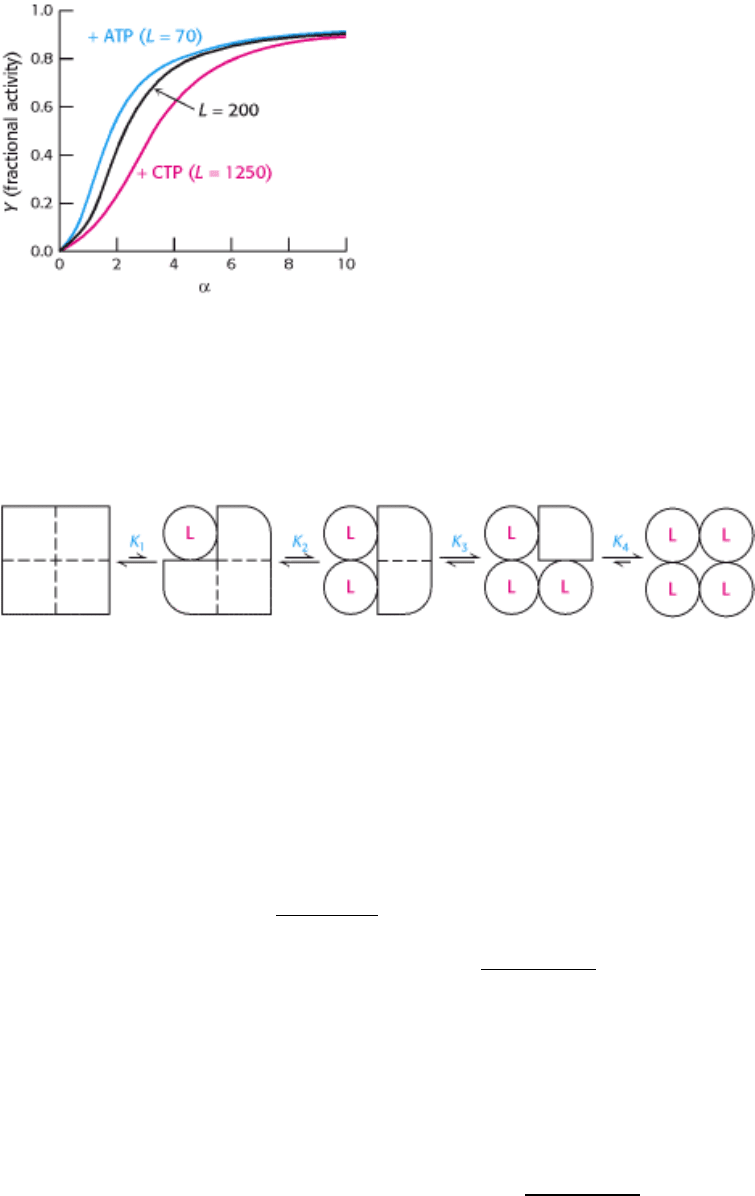
Figure 10.15. Quantitative Description of the MWC Model. Fractional activity, Y, is the fraction of active sites bound
to substrate and is directly proportional to reaction velocity; α is the ratio of [S] to the dissociation constant of S with the
enzyme in the R state; L is the ratio of the concentration of enzyme in the T state to that in the R state. The binding of the
regulators ATP and CTP to ATCase changes the value of L and thus the response to substrate concentration.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.1. Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its Pathway
Figure 10.16. Simple Sequential Model for a Tetrameric Allosteric Enzyme. The binding of a ligand (L) to a subunit
changes the conformation of that particular subunit from the T (square) to the R (circle) form. This transition affects the
affinity of the other subunits for the ligand.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin
10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Allostery is a property not limited to enzymes. The basic principles of allostery are also well illustrated by the oxygen-
transport protein hemoglobin (Section 7.2). The binding of oxygen to hemoglobin isolated from red blood cells displays
marked sigmoidal behavior (similar to that observed for the activity of ATCase, as a function of substrate concentration),
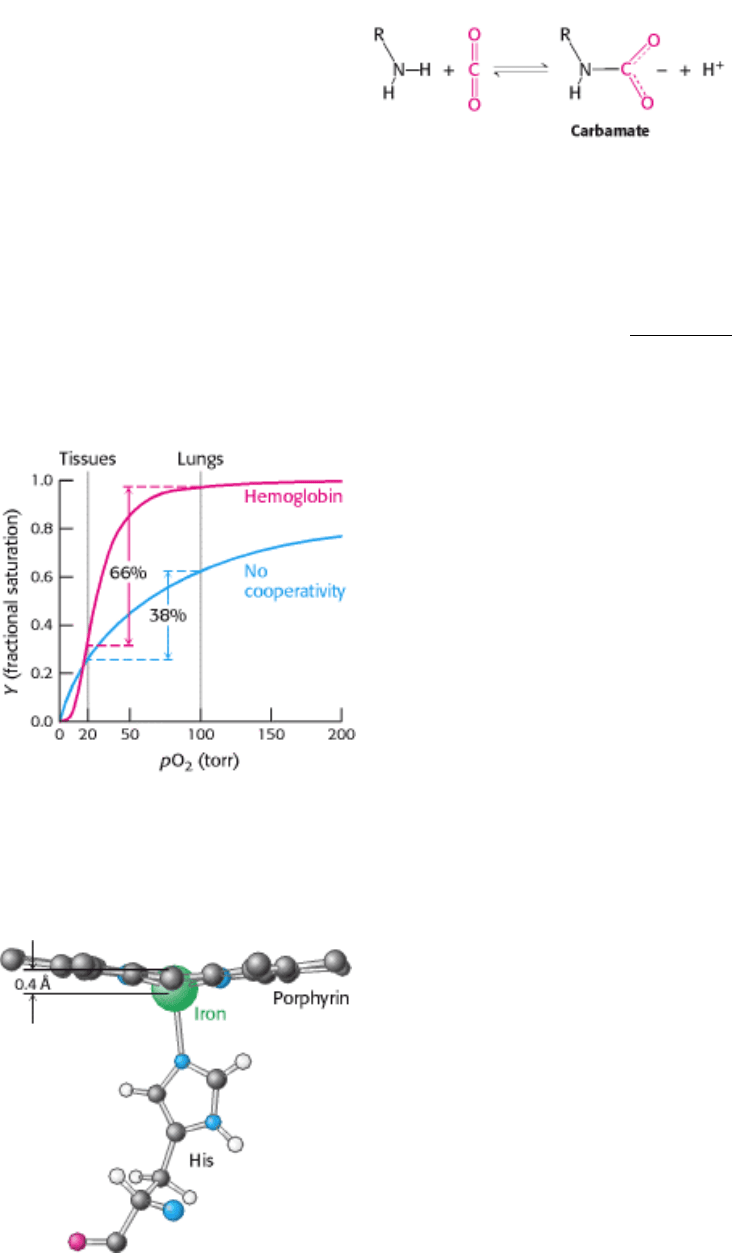
which is indicative of cooperation between subunits (Figure 10.17). What is the physiological significance of the
cooperative binding of oxygen by hemoglobin? Oxygen must be transported in the blood from the lungs, where the
partial pressure of oxygen (pO
2
) is relatively high (approximately 100 torr), to the tissues, where the partial pressure of
oxygen is much lower (typically 20 torr). Let us consider how the cooperative behavior represented by the sigmoidal
curve leads to efficient oxygen transport. In the lungs, hemoglobin becomes nearly saturated with oxygen such that 98%
of the oxygen-binding sites are occupied. When hemoglobin moves to the tissues, the saturation level drops to 32%.
Thus, a total of 98 - 32 = 66% of the potential oxygen-binding sites contribute to oxygen transport. In comparison, for a
hypothetical noncooperative transport protein, the most oxygen that can be transported from a region in which pO
2
is
100 torr to one in which it is 20 torr is 63 - 25 = 38% (see Figure 10.17). Thus, the cooperative binding of oxygen by
hemoglobin enables it to deliver 1.7 times as much oxygen as it would if the sites were independent. The homotropic
regulation of hemoglobin by its ligand oxygen dramatically increases its physiological oxygen-carrying capacity.
Torr-
A unit of pressure equal to that exerted by a column of mercury 1
mm high at 0°C and standard gravity (1 mm Hg). Named after
Evangelista Torricelli (1608
1647), inventor of the mercury
barometer.
10.2.1. Oxygen Binding Induces Substantial Structural Changes at the Iron Sites in
Hemoglobin
The results of structural studies pioneered by Max Perutz revealed the structure of hemoglobin in various forms. Human
hemoglobin A, present in adults, consists of four subunits: two α subunits and two β subunits. The α and β subunits are
homologous and have similar three-dimensional structures (Section 7.4). The capacity of hemoglobin to bind oxygen
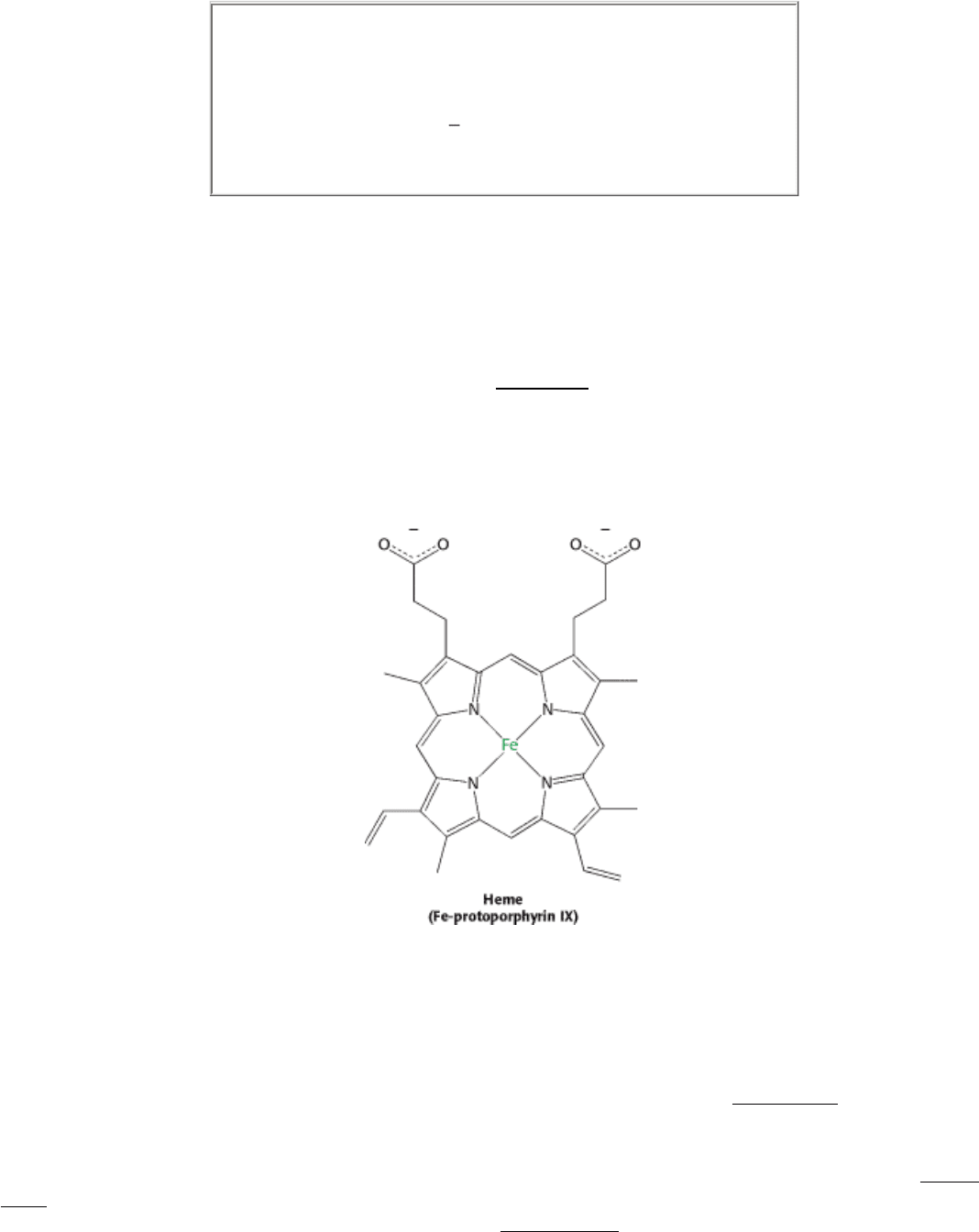
depends on the presence of a bound prosthetic group called heme. The heme group is responsible for the distinctive red
color of blood. The heme group consists of an organic component and a central iron atom. The organic component,
called protoporphyrin, is made up of four pyrrole rings linked by methene bridges to form a tetrapyrrole ring. Four
methyl groups, two vinyl groups, and two propionate side chains are attached.
The iron atom lies in the center of the protoporphyrin, bonded to the four pyrrole nitrogen atoms. Under normal
conditions, the iron is in the ferrous (Fe
2+
) oxidation state. The iron ion can form two additional bonds, one on each side
of the heme plane. These binding sites are called the fifth and sixth coordination sites. In hemoglobin, the fifth
coordination site is occupied by the imidazole ring of a histidine residue from the protein. In deoxyhemoglobin, the sixth
coordination site remains unoccupied. The iron ion lies approximately 0.4 Å outside the porphyrin plane because iron, in
this form, is slightly too large to fit into the well-defined hole within the porphyrin ring (Figure 10.18).
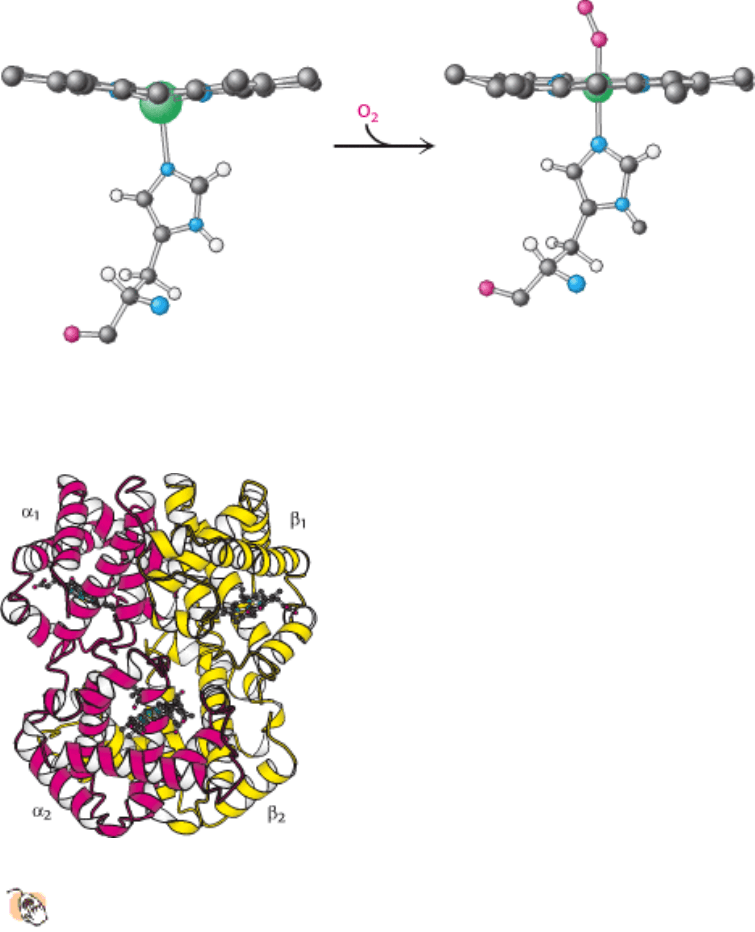
The binding of the oxygen molecule at the sixth coordination site of the iron ion substantially rearranges the electrons
within the iron so that the ion becomes effectively smaller, allowing it to move into the plane of the porphyrin (Figure
10.19). This change in electronic structure is paralleled by changes in the magnetic properties of hemoglobin, which are
the basis for functional magnetic resonance imaging (fMRI; Section 32.1.3). Indeed, the structural changes that take
place on oxygen binding were anticipated by Linus Pauling, based on magnetic measurements in 1936, nearly 25 years
before the three-dimensional structure of hemoglobin was elucidated.
10.2.2. Oxygen Binding Markedly Changes the Quaternary Structure of Hemoglobin

The three-dimensional structure of hemoglobin is best described as a pair of identical α β dimers (α
1
β
1
and α
2
β
2
)
that associate to form the hemoglobin tetramer (Figure 10.20). In deoxyhemoglobin, these α β dimers are linked by an
extensive interface, which includes, among other regions, the carboxyl terminus of each chain. The heme groups are well
separated in the tetramer with iron-iron distances ranging from 24 to 40 Å. The deoxy form corresponds to the T state in
the context of either the concerted or the sequential model for hemoglobin cooperativity. On oxygen binding, there are
substantial changes in quaternary structure that correspond to the T-to-R state transition (Figure 10.21).
The α
1
β
1
and α
2
β
2
dimers rotate approximately 15 degrees with respect to one another. The dimers themselves are
relatively unchanged, although localized conformational shifts do occur. Thus, the interface between the α
1
β
1
and α
2
β
2
dimers is most effected by this structural transition.
How does oxygen binding lead to the structural transition from the T state to the R state? When the iron ion moves into
the plane of the porphyrin, the histidine residue bound in the fifth coordination site moves with it. This histidine residue
is part of an α helix, which also moves (Figure 10.22). The carboxyl terminal end of this α helix lies in the interface
between the two α β dimers. Consequently, the structural transition at the iron ion is directly transmitted to the other
subunits. The rearrangement of the dimer interface provides a pathway for communication between subunits, enabling
the cooperative binding of oxygen.
Is the cooperative binding of oxygen by hemoglobin best described by the concerted or the sequential model? Neither
model in its pure form fully accounts for the behavior of hemoglobin. Instead, a combined model is required.
Hemoglobin behavior is concerted in that hemoglobin with three sites occupied by oxygen is in the quaternary structure
associated with the R state. The remaining open binding site has an affinity for oxygen more than 20-fold as great as that
of fully deoxygenated hemoglobin binding its first oxygen. However, the behavior is not fully concerted, because
hemoglobin with oxygen bound to only one of four sites remains in the T-state quaternary structure. Yet, this molecule
binds oxygen 3 times as strongly as does fully deoxygenated hemoglobin, an observation consistent only with a
sequential model. These results highlight the fact that the concerted and sequential models represent idealized limiting
cases, which real systems may approach but rarely attain.
10.2.3. Tuning the Oxygen Affinity of Hemoglobin: The Effect of 2,3-
Bisphosphoglycerate
Examination of the oxygen binding of hemoglobin fully purified from red blood cells revealed that the oxygen affinity of
purified hemoglobin is much greater than that for hemoglobin within red blood cells. This dramatic difference is due to
the presence within these cells of 2,3-bisphosphoglycerate (2,3-BPG) (also known as 2,3-diphosphoglycerate or 2,3-
DPG). This highly anionic compound is present in red blood cells at approximately the same concentration as that of
hemoglobin (~ 2 mM). Without 2,3-BPG, hemoglobin would be an extremely inefficient oxygen transporter, releasing
only 8% of its cargo in the tissues.
How does 2,3-BPG affect oxygen affinity so significantly? Examination of the crystal structure of deoxyhemoglobin in
the presence of 2,3-BPG reveals that a single molecule of 2,3-BPG binds in a pocket, present only in the T form, in the
center of the hemoglobin tetramer (Figure 10.23). On T-to-R transition, this pocket collapses. Thus, 2,3-BPG binds
preferentially to deoxyhemoglobin and stabilizes it, effectively reducing the oxygen affinity. In order for the structural

transition from T to R to take place, the bonds between hemoglobin and 2,3-BPG must be broken and 2,3-BPG must be
expelled.
2,3-BPG binding to hemoglobin has other crucial physiological consequences. The globin gene expressed by
fetuses differs from that expressed by human adults; fetal hemoglobin tetramers include two α chains and two γ
chains. The γ chain, a result of another gene duplication, is 72% identical in amino acid sequence with the β chain. One
noteworthy change is the substitution of a serine residue for His 143 in the β chain of the 2,3-BPG-binding site. This
change removes two positive charges from the 2,3-BPG-binding site (one from each chain) and reduces the affinity of
2,3-BPG for fetal hemoglobin, thereby increasing the oxygen-binding affinity of fetal hemoglobin relative to that of
maternal (adult) hemoglobin (Figure 10.24). This difference in oxygen affinity allows oxygen to be effectively
transferred from maternal to fetal red cells. We see again an example of where gene duplication and specialization
produced a ready solution to a biological challenge in this case, the transport of oxygen from mother to fetus.
10.2.4. The Bohr Effect: Hydrogen Ions and Carbon Dioxide Promote the Release of
Oxygen
Rapidly metabolizing tissues, such as contracting muscle, have a high need for oxygen and generate large amounts of
hydrogen ions and carbon dioxide as well (Sections 16.1.9 and 17.1). Both of these species are heterotropic effectors of
hemoglobin that enhance oxygen release. The oxygen affinity of hemoglobin decreases as pH decreases from the value
of 7.4 found in the lungs (Figure 10.25). Thus, as hemoglobin moves into a region of low pH, its tendency to release
oxygen increases. For example, transport from the lungs, with pH 7.4 and an oxygen partial pressure of 100 torr, to
active muscle, with a pH of 7.2 and an oxygen partial pressure of 20 torr, results in a release of oxygen amounting to
77% of total carrying capacity. Recall that only 66% of the oxygen would be released in the absence of any change in
pH. In addition, hemoglobin responds to carbon dioxide with a decrease in oxygen affinity, thus facilitating the release of
oxygen in tissues with a high carbon dioxide concentration. In the presence of carbon dioxide at a partial pressure of 40
torr, the amount of oxygen released approaches 90% of the maximum carrying capacity. Thus, the heterotropic
regulation of hemoglobin by hydrogen ions and carbon dioxide further increases the oxygen-transporting efficiency of
this magnificent allosteric protein.
The regulation of oxygen binding by hydrogen ions and carbon dioxide is called the Bohr effect after Christian Bohr,
who described this phenomenon in 1904. The results of structural and chemical studies have revealed much about the
chemical basis of the Bohr effect. At least two sets of chemical groups are responsible for the effect of protons: the
amino termini and the side chains of histidines β146 and α122, which have pK
a
values near pH 7. Consider histidine
β146. In deoxyhemoglobin, the terminal carboxylate group of β146 forms a salt bridge with a lysine residue in the α
subunit of the other α β dimer. This interaction locks the side chain of histidine β146 in a position where it can
participate in a salt bridge with negatively charged aspartate 94 in the same chain, provided that the imidazole group of
the histidine residue is protonated (Figure 10.26). At high pH, the side chain of histidine β146 is not protonated and the
salt bridge does not form. As the pH drops, however, the side chain of histidine β146 becomes protonated, the salt bridge
with aspartate β94 forms, and the quaternary structure characteristic of deoxyhemoglobin is stabilized, leading to a
greater tendency for oxygen to be released at actively metabolizing tissues. No significant change takes place in
oxyhemoglobin over the same pH range.
Carbon dioxide also stabilizes deoxyhemoglobin by reacting with the terminal amino groups to form carbamate groups,
which are negatively charged, in contrast with the neutral or positive charges on the free amino groups. The amino
termini lie at the interface between the α β dimers, and these negatively charged carbamate groups participate in salt-
bridge interactions, characteristic of the T-state structure, which stabilize deoxyhemoglobin's structure and favor the
release of oxygen.
Hemoglobin with bound carbon dioxide and hydrogen ions is carried in the blood back to the lungs, where it releases the
hydrogen ions and carbon dioxide and rebinds oxygen. Thus, hemoglobin helps to transport hydrogen ions and carbon
dioxide in addition to transporting oxygen. However, transport by hemoglobin accounts for only about 14% of the total
transport of these species; both hydrogen ions and carbon dioxide are also transported in the blood as bicarbonate (HCO
3
-
) formed spontaneously or through the action of carbonic anhydrase (Section 9.2), an abundant enzyme in red blood
cells.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Figure 10.17. Cooperativity Enhances Oxygen Delivery by Hemoglobin. Because of cooperativity between O
2
-
binding sites, hemoglobin delivers more O
2
to tissues than would a noncooperative protein (pO
2
, partial pressure of
oxygen.)
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Figure 10.18. Position of Iron in Deoxyhemoglobin. The iron ion lies slightly outside the plane of the porphyrin in
heme.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Figure 10.19. Oxygen Binding Initiates Structural Changes. The iron ion moves into the plane of the heme on
oxygenation. The proximal histidine is pulled along with the iron ion.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Figure 10.20. Quaternary Structure of Hemoglobin.
Hemoglobin, which is composed of two α chains and two β
chains, functions as a pair of α β dimers.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
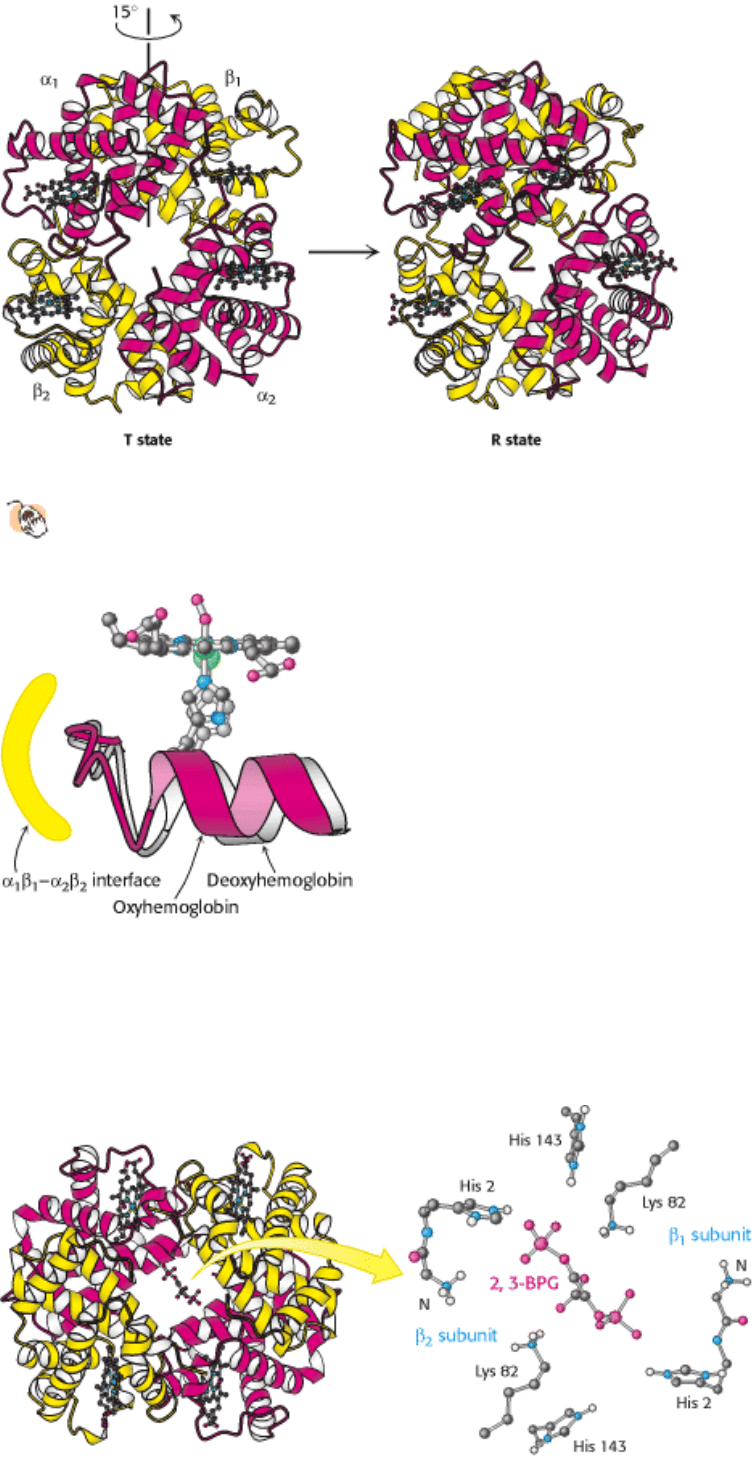
Figure 10.21. Transition from T to R State in Hemoglobin.
On oxygenation, one pair of α β subunits shifts with
respect to the other by a rotation of 15 degrees.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Figure 10.22. Conformational Changes in Hemoglobin. The movement of the iron ion on oxygenation brings the iron-
associated histidine residue toward the porphyrin ring. The corresponding movement of the histidine-containing α helix
alters the interface between the α β pairs, instigating other structural changes. For comparison, the deoxyhemoglobin
structure is shown in gray behind the oxyhemoglobin structure in color.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
