Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

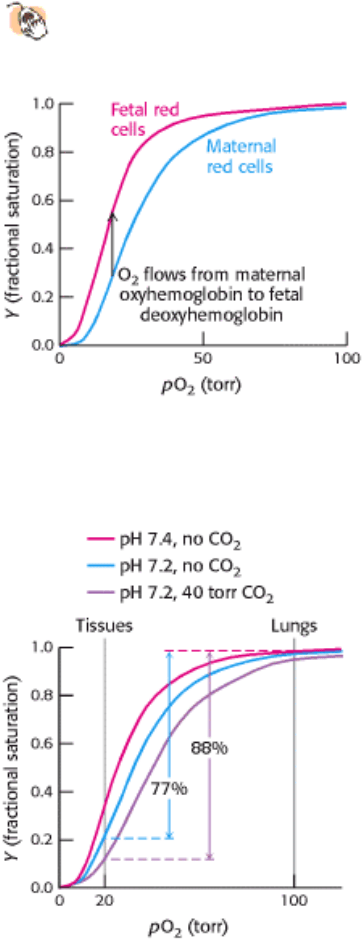
Figure 10.23. Mode of Binding of 2,3-BPG to Human Deoxyhemoglobin. 2,3-BPG binds in the central cavity of
deoxyhemoglobin. There, it interacts with three positively charged groups on each β chain.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Figure 10.24. Oxygen Affinity of Fetal Red Blood Cells. Fetal red blood cells have a higher oxygen affinity than that
of maternal red blood cells because fetal hemoglobin does not bind 2,3-BPG as well as maternal hemoglobin does.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Figure 10.25. Effect of pH and CO
2
concentration on the oxygen affinity of hemoglobin. Lowering the pH from 7.4
(red curve) to 7.2 (blue curve) results in the release of O
2
from oxyhemoglobin. Raising the CO
2
partial pressure from 0
to 40 torr (purple curve) also favors the release of O
2
from oxyhemoglobin.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.2. Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
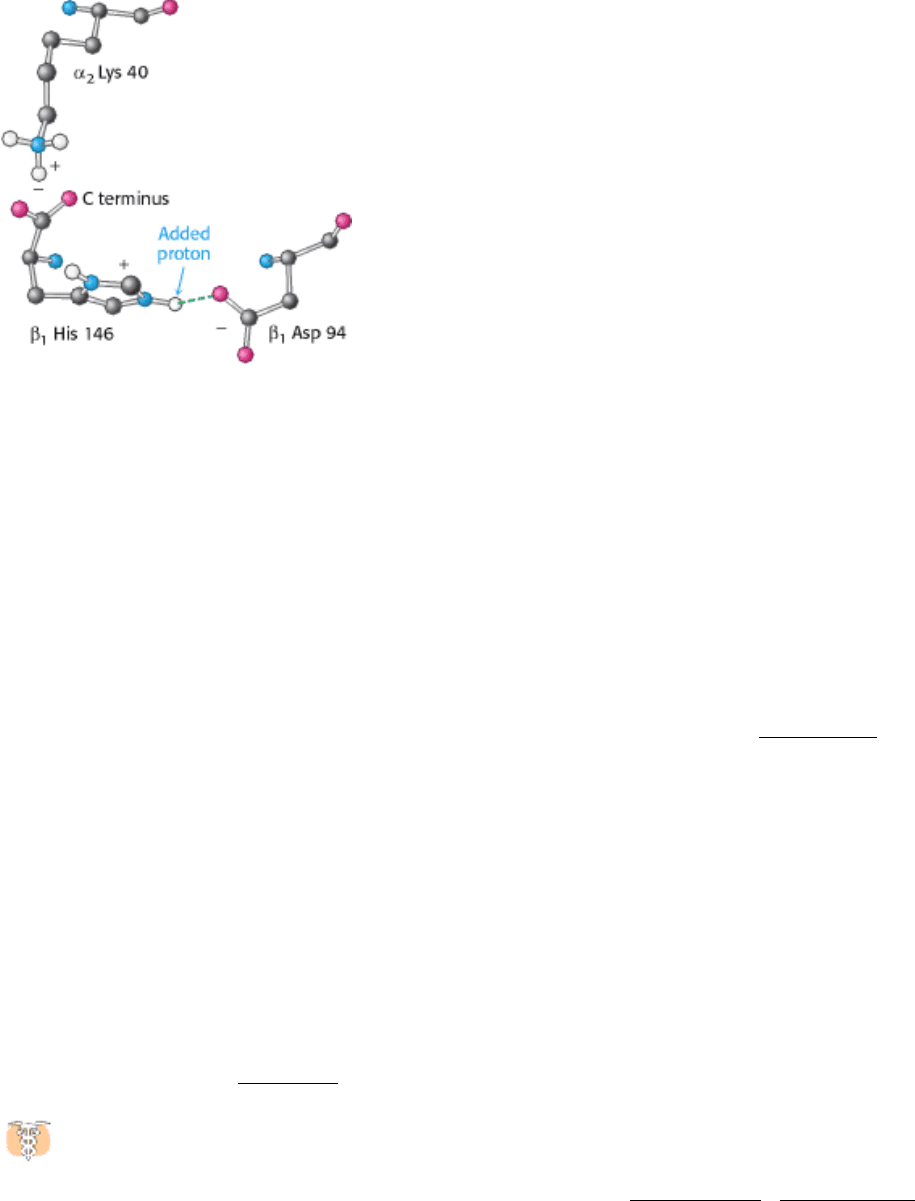
Figure 10.26. Chemical Basis of the Bohr Effect. In deoxyhemoglobin, shown here, three amino acid residues form
two salt bridges that stabilize the T quaternary structure. The formation of one of the salt bridges depends on the
presence of an added proton on histidine β146. The proximity of the negative charge on aspartate 94 favors protonation
of histidine β146 in deoxyhemoglobin.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin
10.3. Isozymes Provide a Means of Regulation Specific to Distinct Tissues and
Developmental Stages
Isozymes or isoenzymes, are enzymes that differ in amino acid sequence yet catalyze the same reaction. Usually, these
enzymes display different kinetic parameters, such as K
M
, or different regulatory properties. They are encoded by
different genetic loci, which usually arise through gene duplication and divergence (Section 2.2.5). Isozymes differ from
allozymes, which are enzymes that arise from allelic variation at one gene locus. Isozymes can often be distinguished
from one another by biochemical properties such as electrophoretic mobility.
The existence of isozymes permits the fine-tuning of metabolism to meet the particular needs of a given tissue or
developmental stage. Consider the example of lactate dehydrogenase (LDH), an enzyme that functions in anaerobic
glucose metabolism and glucose synthesis. Human beings have two isozymic polypeptide chains for this enzyme: the H
isozyme highly expressed in heart and the M isozyme found in skeletal muscle. The amino acid sequences are 75%
identical. The functional enzyme is tetrameric, and many different combinations of the two subunits are possible. The H
4
isozyme, found in the heart, has a higher affinity for substrates than does the M
4
isozyme. The two isozymes also differ
in that high levels of pyruvate allosterically inhibit the H
4
but not the M
4
isozyme. The other combinations, such as
H
3
M, have intermediate properties depending on the ratio of the two kinds of chains. We will consider these isozymes in
their biological context in Chapter 16.
The M
4
isozyme functions optimally in an anaerobic environment, whereas the H
4
isozyme does so in an aerobic
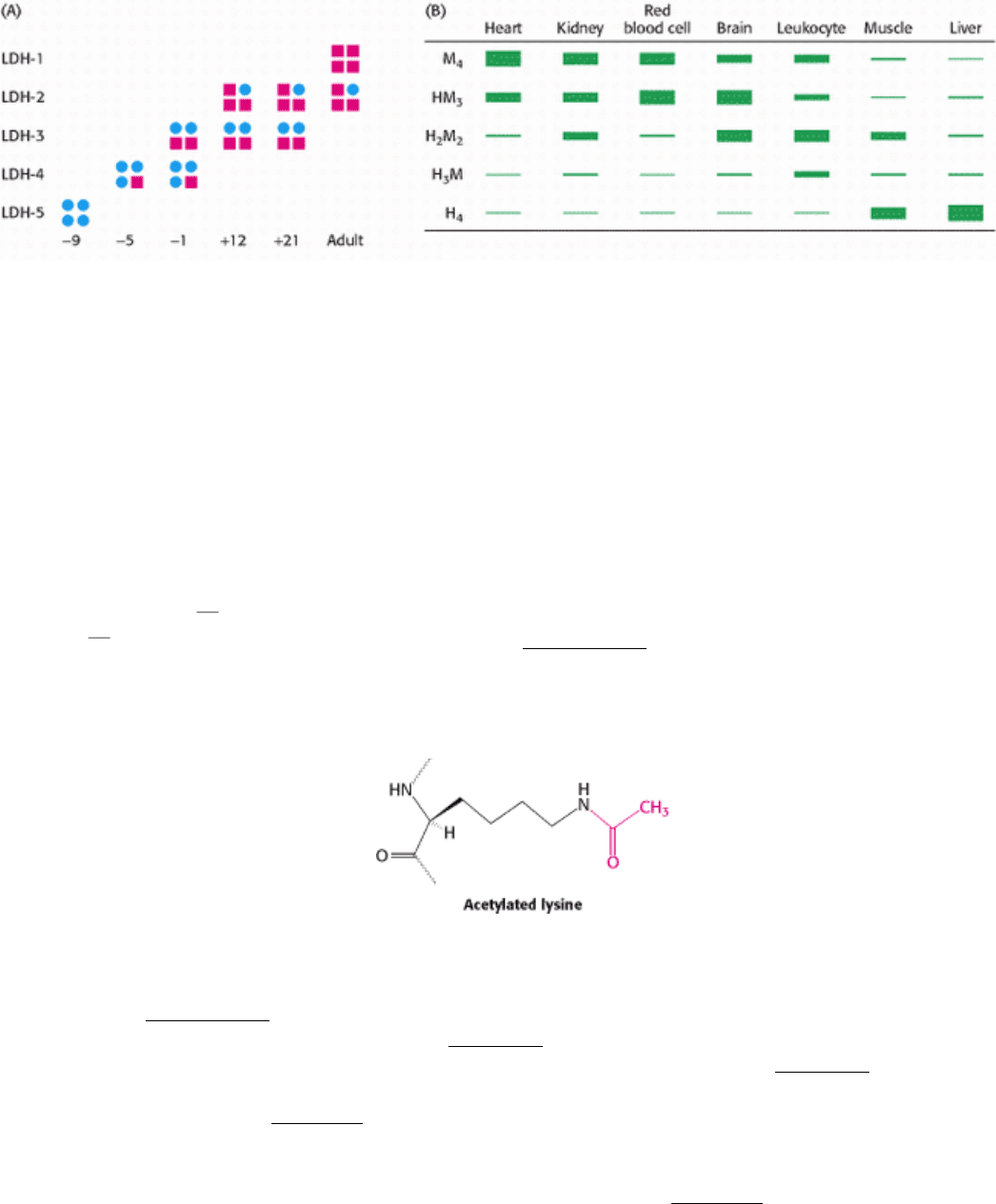
environment. Indeed, the proportions of these isozymes are altered in the course of development of the rat heart as
the tissue switches from an anaerobic environment to an aerobic one (Figure 10.27A). Figure 10.27 B shows the tissue-
specific forms of lactate dehydrogenase in adult rat tissues. The appearance of some isozymes in the blood is indicative
of tissue damage and can be used for clinical diagnosis. For instance, an increase in serum levels of H
4
relative to H
3
M is
an indication that a myocardial infarction, or heart attack, has occurred.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.3. Isozymes Provide a Means of Regulation Specific to Distinct Tissues and Developmental Stages
Figure 10.27. Isozymes of Lactate Dehydrogenase. (A) The rat heart LDH isozyme profile changes in the course of
development. The H isozyme is represented by squares and the M isozyme by circles. The negative and positive numbers
denote the days before and after birth, respectively. (B) LDH isozyme content varies by tissue. [(A) After W.-H. Li,
Molecular Evolution (Sinauer, 1997), p. 283; (B) After K. Urich, Comparative Animal Biochemistry (Springer Verlag,
1990), p. 542.]
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin
10.4. Covalent Modification Is a Means of Regulating Enzyme Activity
The covalent attachment of another molecule can modify the activity of enzymes and many other proteins. In these
instances, a donor molecule provides a functional moiety that modifies the properties of the enzyme. Most modifications
are reversible. Phosphorylation and dephosphorylation are the most common but not the only means of covalent
modification. Histones
proteins that assist in the packaging of DNA into chromosomes as well as in gene
regulation are rapidly acetylated and deacetylated in vivo (Section 31.3.4). More heavily acetylated histones are
associated with genes that are being actively transcribed. The acetyltransferase and deacetylase enzymes are themselves
regulated by phosphorylation, showing that the covalent modification of histones may be controlled by the covalent
modification of the modifying enzymes.
Modification is not readily reversible in some cases. Some proteins in signal-transduction pathways, such as Ras and Src
(a protein tyrosine kinase), are localized to the cytoplasmic face of the plasma membrane by the irreversible attachment
of a lipid group (Section 12.5.3). Fixed in this location, the proteins are better able to receive and transmit information
that is being passed along their signaling pathways (Chapter 15). The attachment of ubiquitin, a protein comprising 72
amino acids, is a signal that a protein is to be destroyed, the ultimate means of regulation (Chapter 23). Cyclin, an
important protein in cell-cycle regulation, must be ubiquitinated and destroyed before a cell can enter anaphase and
proceed through the cell cycle (Table 10.1).
Virtually all the metabolic processes that we will examine are regulated in part by covalent modification. Indeed, the
allosteric properties of many enzymes are modified by covalent modification. Table 10.1 lists some of the common
covalent modifications.
10.4.1. Phosphorylation Is a Highly Effective Means of Regulating the Activities of
Target Proteins
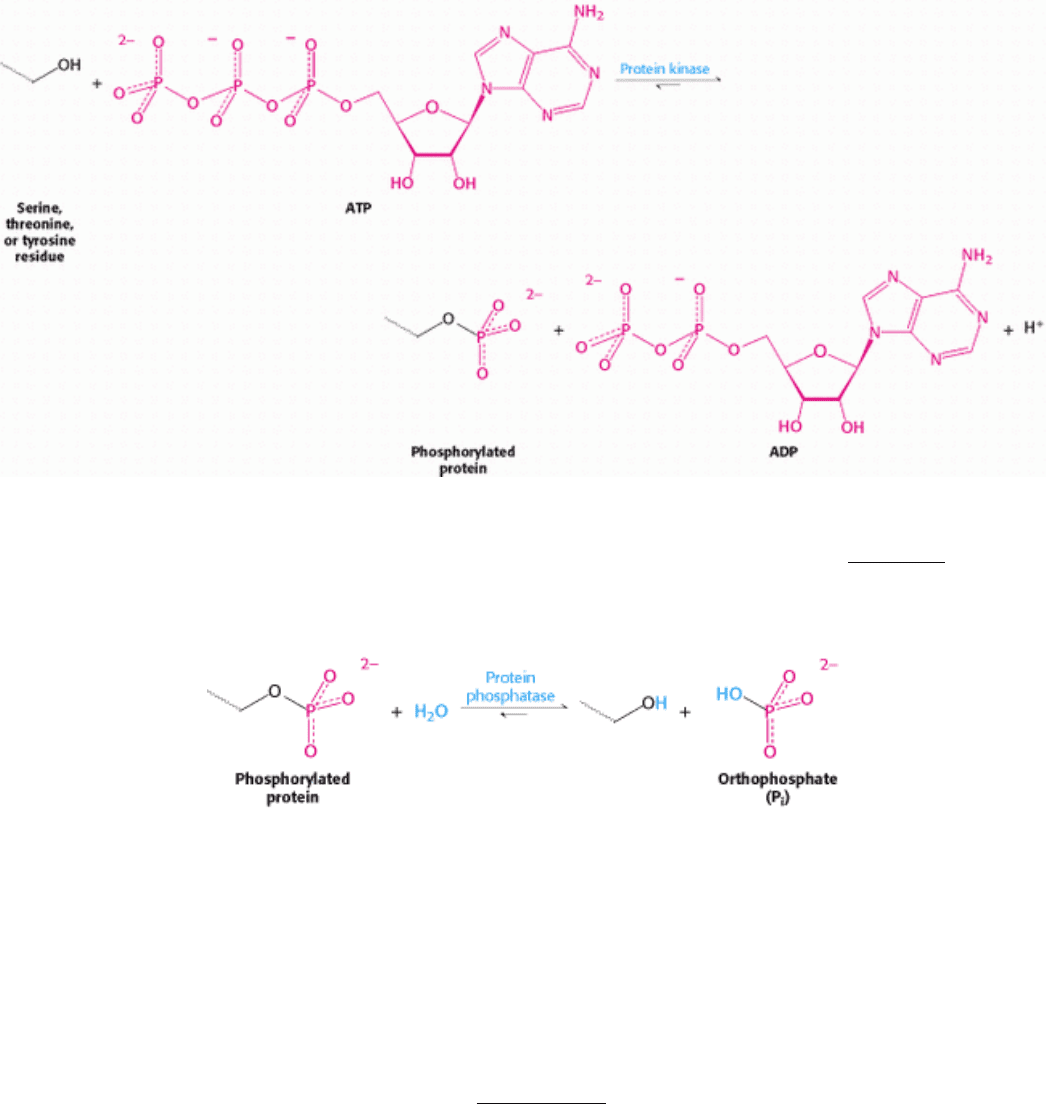
The activities of many enzymes, membrane channels, and other target proteins are regulated by phosphorylation, the
most prevalent reversible covalent modification. Indeed, we will see this regulatory mechanism in virtually every
metabolic process in eukaryotic cells. The enzymes catalyzing phosphorylation reactions are called protein kinases,
which constitute one of the largest protein families known, with more than 100 homologous enzymes in yeast and more
than 550 in human beings. This multiplicity of enzymes allows regulation to be fine-tuned according to a specific tissue,
time, or substrate.
The terminal (γ) phosphoryl group of ATP is transferred to specific serine and threonine residues by one class of protein
kinases and to specific tyrosine residues by another.
The acceptors in protein phosphorylation reactions are located inside cells, where the phosphoryl-group donor ATP is
abundant. Proteins that are entirely extracellular are not regulated by reversible phosphorylation. Table 10.2 lists a few of
the known protein kinases. Protein phosphatases reverse the effects of kinases by catalyzing the hydrolytic removal of
phosphoryl groups attached to proteins.
The unmodified hydroxyl-containing side chain is regenerated and orthophosphate (P
i
) is produced.
It is important to note that phosphorylation and dephosphorylation are not the reverse of one another; each is essentially
irreversible under physiological conditions. Furthermore, both reactions take place at negligible rates in the absence of
enzymes. Thus, phosphorylation of a protein substrate will take place only through the action of a specific protein kinase
and at the expense of ATP cleavage, and dephosphorylation will result only through the action of a phosphatase. The rate
of cycling between the phosphorylated and the dephosphorylated states depends on the relative activities of kinases and
phosphatases. Note that the net outcome of the two reactions is the hydrolysis of ATP to ADP and P
i
, which has a ∆ G of
-12 kcal mol
-1
(-50 kJ mol
-1
) under cellular conditions (Section 14.1.2). This highly favorable free-energy change
ensures that target proteins cycle unidirectionally between unphosphorylated and phosphorylated forms.
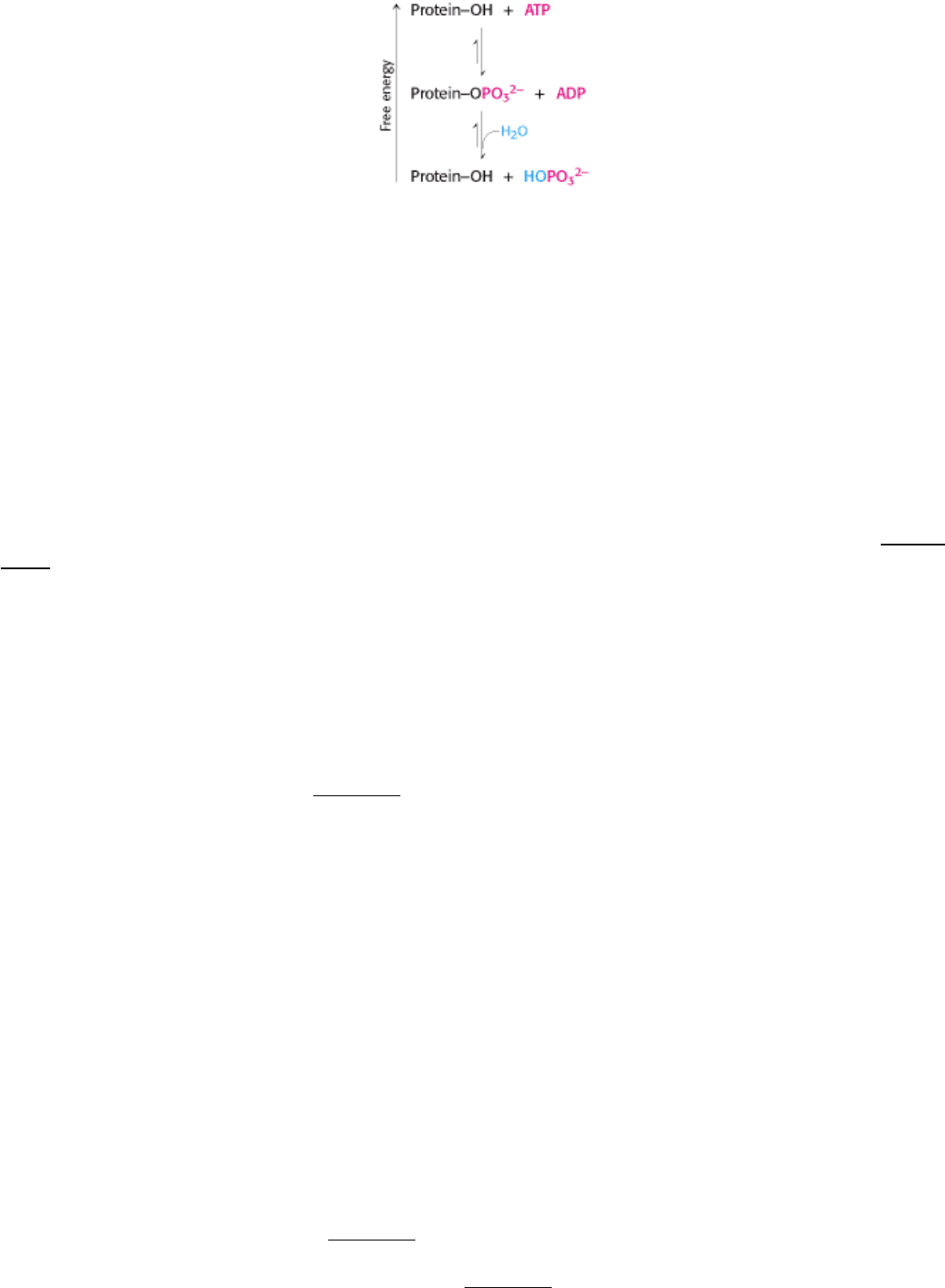
Phosphorylation is a highly effective means of controlling the activity of proteins for structural, thermodynamic, kinetic,
and regulatory reasons:
1. A phosphoryl group adds two negative charges to a modified protein. Electrostatic interactions in the unmodified
protein can be disrupted and new electrostatic interactions can be formed. Such structural changes can markedly alter
substrate binding and catalytic activity.
2. A phosphate group can form three or more hydrogen bonds. The tetrahedral geometry of the phosphoryl group makes
these hydrogen bonds highly directional, allowing for specific interactions with hydrogen-bond donors.
3. The free energy of phosphorylation is large. Of the -12 kcal mol
-1
(-50 kJ mol
-1
) provided by ATP, about half is
consumed in making phosphorylation irreversible; the other half is conserved in the phosphorylated protein. Recall that a
free-energy change of 1.36 kcal mol
-1
(5.69 kJ mol
-1
) corresponds to a factor of 10 in an equilibrium constant (Section
14.1.3). Hence, phosphorylation can change the conformational equilibrium between different functional states by a
large factor, of the order of 10
4
.
4. Phosphorylation and dephosphorylation can take place in less than a second or over a span of hours. The kinetics can
be adjusted to meet the timing needs of a physiological process.
5. Phosphorylation often evokes highly amplified effects. A single activated kinase can phosphorylate hundreds of target
proteins in a short interval. Further amplification can take place because the target proteins may be enzymes, each of
which can then transform a large number of substrate molecules.
6. ATP is the cellular energy currency (Chapter 14). The use of this compound as a phosphoryl group donor links the
energy status of the cell to the regulation of metabolism.
Protein kinases vary in their degree of specificity. Dedicated protein kinases phosphorylate a single protein or several
closely related ones. Multifunctional protein kinases modify many different targets; they have a wide reach and can
coordinate diverse processes. Comparisons of amino acid sequences of many phosphorylation sites show that a
multifunctional kinase recognizes related sequences. For example, the consensus sequence recognized by protein kinase
A is Arg-Arg-X-Ser-Z or Arg-Arg-X-Thr-Z, in which X is a small residue, Z is a large hydrophobic one, and Ser or Thr
is the site of phosphorylation. It should be noted that this sequence is not absolutely required. Lysine, for example, can
substitute for one of the arginine residues but with some loss of affinity. Short synthetic peptides containing a consensus
motif are nearly always phosphorylated by serine- threonine protein kinases. Thus, the primary determinant of specificity
is the amino acid sequence surrounding the serine or threonine phosphorylation site. However, distant residues can
contribute to specificity. For instance, changes in protein conformation may alter the accessibility of a possible
phosphorylation site.
10.4.2. Cyclic AMP Activates Protein Kinase A by Altering the Quaternary Structure
Protein kinases modulate the activity of many proteins, but what leads to the activation of a kinase? Activation is often a
multistep process initiated by hormones (Chapter 15). In some cases, the hormones trigger the formation of cyclic AMP,
a molecule formed by cyclization of ATP. Cyclic AMP serves as an intracellular messenger in mediating the
physiological actions of hormones, as will be discussed in Chapter 15. The striking finding is that most effects of cAMP
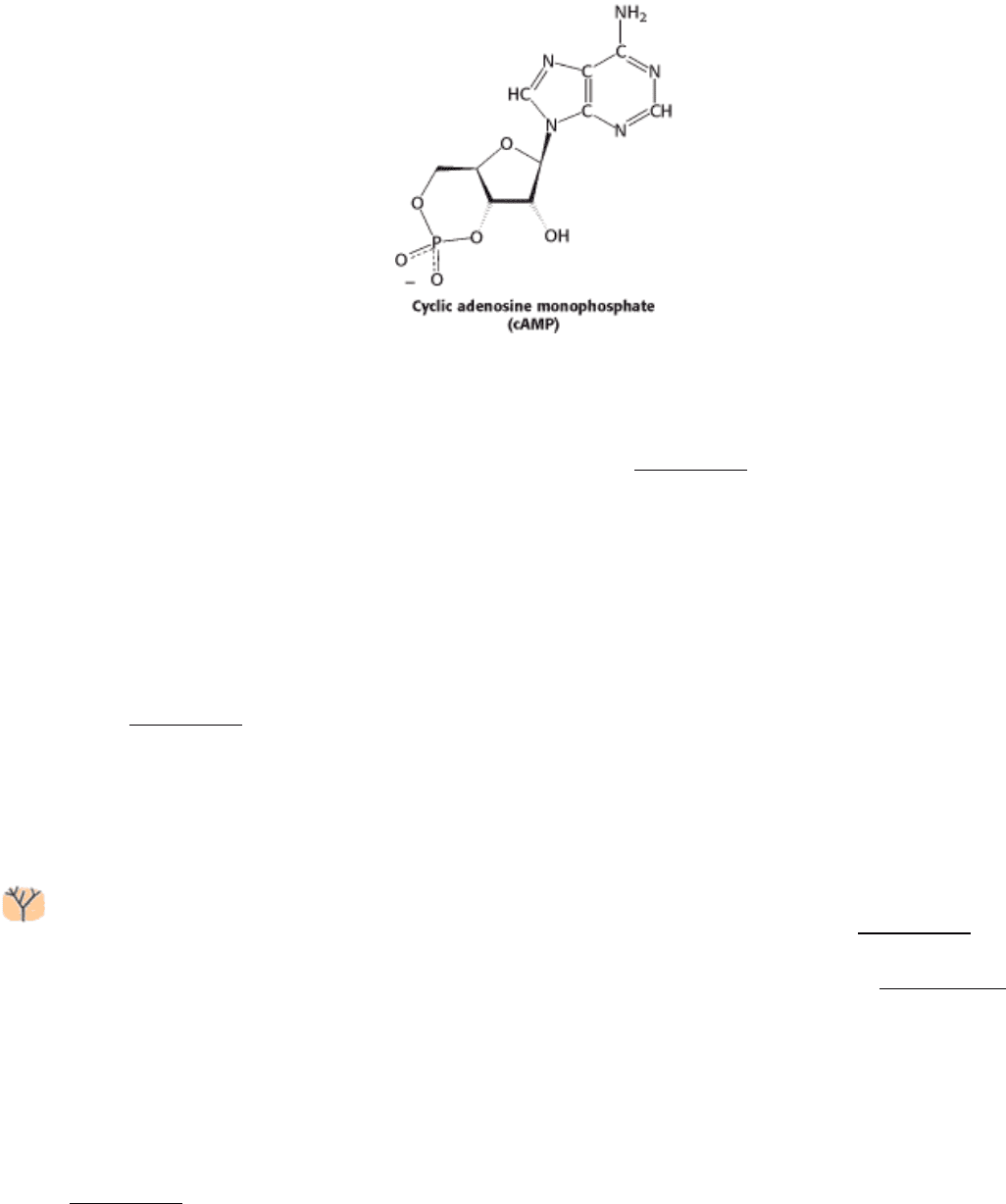
in eukaryotic cells are achieved through the activation by cAMP of a single protein kinase. This key enzyme is called
protein kinase A or PKA. The kinase alters the activities of target proteins by phosphorylating specific serine or
threonine residues. As we shall see, PKA provides a clear example of the integration of allosteric regulation and
phosphorylation.
PKA is activated by cAMP concentrations of the order of 10 nM. The activation mechanism is reminiscent of that of
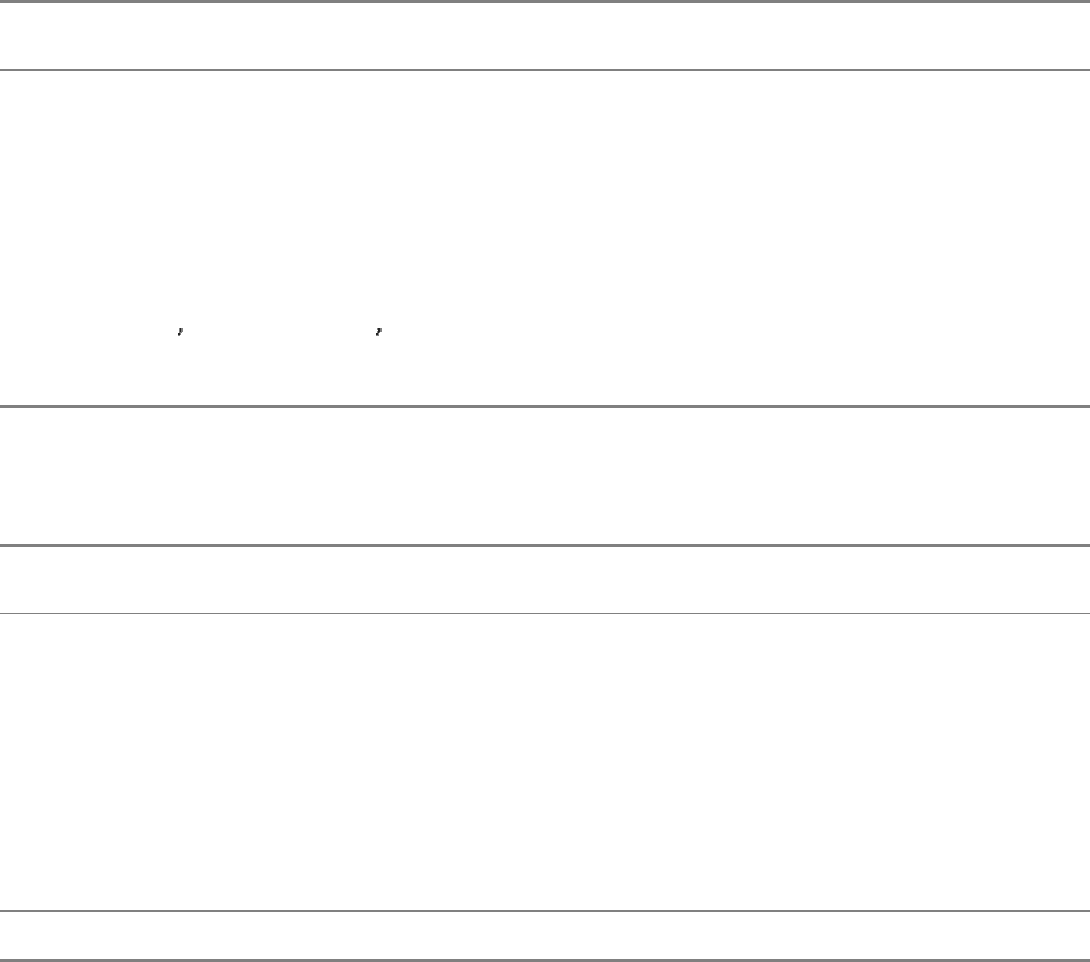
aspartate transcarbamoylase. Like that enzyme, PKA in muscle consists of two kinds of subunits: a 49-kd regulatory (R)
subunit, which has high affinity for cAMP, and a 38-kd catalytic (C) subunit. In the absence of cAMP, the regulatory and
catalytic subunits form an R
2
C
2
complex that is enzymatically inactive (Figure 10.28). The binding of two molecules of
cAMP to each of the regulatory subunits leads to the dissociation of R
2
C
2
into an R
2
subunit and two C subunits. These
free catalytic subunits are then enzymatically active. Thus, the binding of cAMP to the regulatory subunit relieves its
inhibition of the catalytic subunit. PKA and most other kinases exist in isozymic forms for finetuning regulation to meet
the needs of a specific cell or developmental stage.
How does the binding of cAMP activate the kinase? Each R chain contains the sequence Arg-Arg-Gly-Ala-Ile, which
matches the consensus sequence for phosphorylation except for the presence of alanine in place of serine. In the R
2
C
2
complex, this pseudosubstrate sequence of R occupies the catalytic site of C, thereby preventing the entry of protein
substrates (see Figure 10.28). The binding of cAMP to the R chains allosterically moves the pseudosubstrate sequences
out of the catalytic sites. The released C chains are then free to bind and phosphorylate substrate proteins.
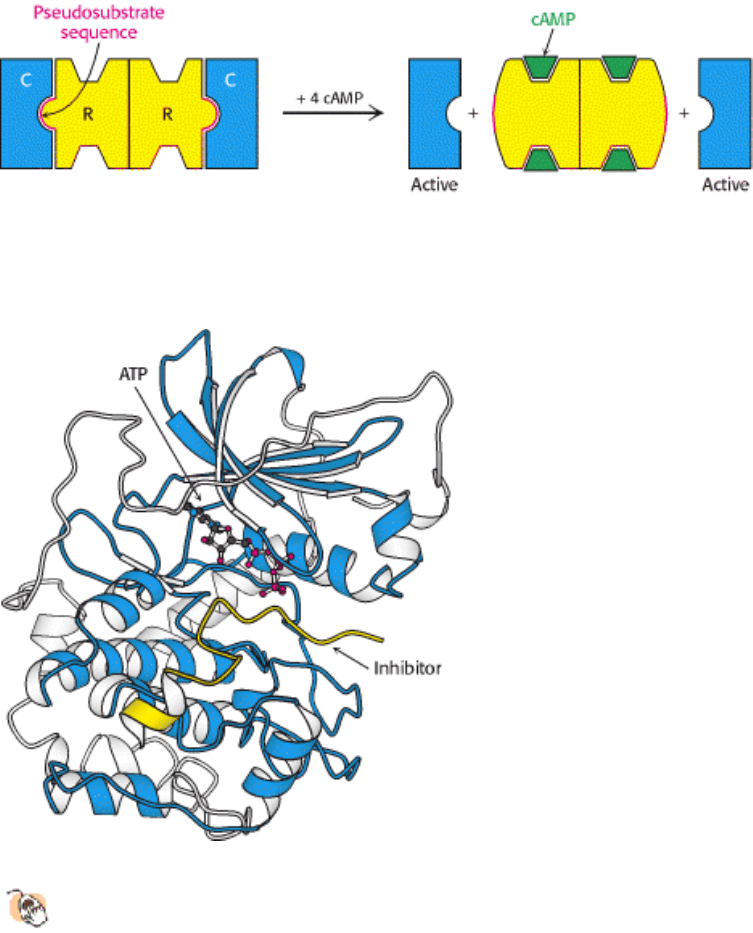
10.4.3. ATP and the Target Protein Bind to a Deep Cleft in the Catalytic Subunit of
Protein Kinase A
The three-dimensional structure of the catalytic subunit of PKA containing a bound 20-residue peptide inhibitor
was determined by x-ray crystallography. The 350-residue catalytic subunit has two lobes (Figure 10.29). ATP
and part of the inhibitor fill a deep cleft between the lobes. The smaller lobe makes many contacts with ATP-Mg
2+
,
whereas the larger lobe binds peptide and contributes the key catalytic residues. Like other kinases (Section 16.1.1), the
two lobes move closer to one another on substrate binding; mechanisms for restricting this domain closure provides a
means for regulating protein kinase activity. The PKA structure has broad significance because residues 40 to 280
constitute a conserved catalytic core that is common to essentially all known protein kinases. We see here an example of
a successful biochemical solution to the problem of protein phosphorylation being employed many times in the course of
evolution.
The bound peptide in this crystal occupies the active site because it contains the pseudosubstrate sequence Arg-Arg-Asn-
Ala-Ile (Figure 10.30). The structure of the complex reveals the basis for the consensus sequence. The guanidinium
group of the first arginine residue forms an ion pair with the carboxylate side chain of a glutamate residue (Glu 127) of
the enzyme. The second arginine likewise interacts with two other carboxylates. The nonpolar side chain of isoleucine,
which matches Z in the consensus sequence, fits snugly in a hydrophobic groove formed by two leucine residues of the
enzyme.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.4. Covalent Modification Is a Means of Regulating Enzyme Activity
Table 10.1. Common covalent modifications of protein activity
Modification Donor molecule Example of modified protein Protein function
Phosphorylation ATP Glycogen phosphorylase Glucose homeostasis; energy
transduction
Acetylation Acetyl CoA Histones DNA packing; transcription
Myristoylation Myristoyl CoA Src Signal transduction
ADP-ribosylation NAD RNA polymerase Transcription
Farnesylation Farnesyl pyrophosphate Ras Signal transduction
γ-Carboxylation
HCO
3
-
Thrombin Blood clotting
Sulfation 3
-Phosphoadenosine-5 -phosphosulfate Fibrinogen Blood-clot formation
Ubiquitination Ubiquitin Cyclin Control of cell cycle
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.4. Covalent Modification Is a Means of Regulating Enzyme Activity
Table 10.2. Examples of serine and threonine kinases and their activating signals
Signal Enzyme
Cyclic nucleotides Cyclic AMP-dependent protein kinase
Cyclic GMP-dependent protein kinase
Ca
2+
and calmodulin Ca
2+
-calmodulin protein kinase
Phosphorylase kinase or glycogen synthase kinase 2
AMP AMP-activated kinase
Diacylglycerol Protein kinase C
Metabolic intermediates and other "local" effectors Many target specific enzymes, such as pyruvate dehydrogenase
kinase and branched-chain ketoacid dehydrogenase kinase
Source: After D. Fell, Understanding the Control of Metabolism (Portland Press, 1997), Table 7.2.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.4. Covalent Modification Is a Means of Regulating Enzyme Activity
Figure 10.28. Regulation of Protein Kinase A. The binding of four molecules of cAMP activates protein kinase A by
dissociating the inhibited holoenzyme (R
2
C
2
) into a regulatory subunit (R
2
) and two catalytically active subunits (C).
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.4. Covalent Modification Is a Means of Regulating Enzyme Activity
Figure 10.29. Protein Kinase A Bound to an Inhibitor.
Three-dimensional structure of a complex of the catalytic
subunit of protein kinase A and an inhibitor bearing a pseudosubstrate sequence. The inhibitor (yellow) binds in a
cleft between the domains of the enzyme. The bound ATP is adjacent to the active site.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.4. Covalent Modification Is a Means of Regulating Enzyme Activity
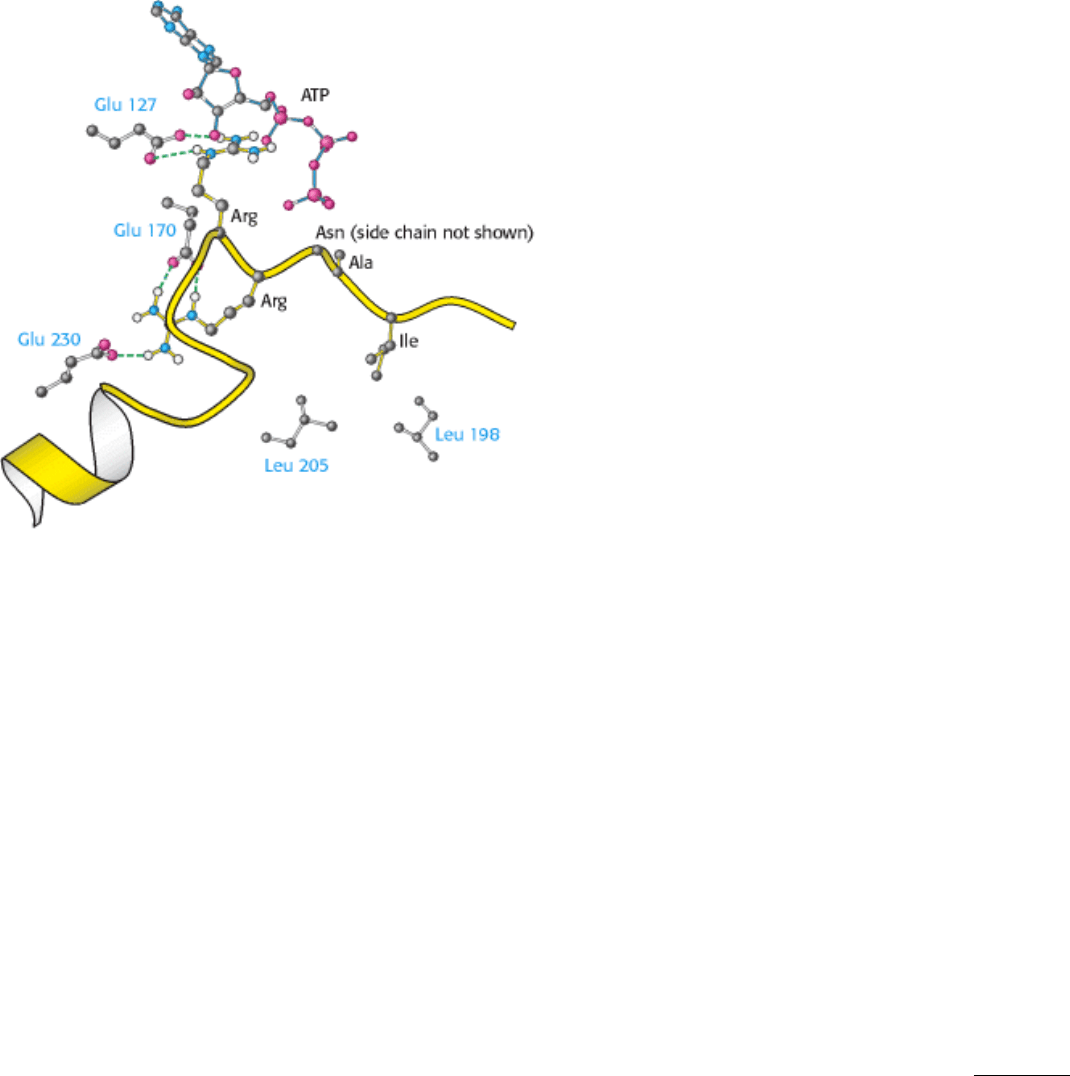
Figure 10.30. Binding of Pseudosubstrate to Protein Kinase A. The two arginine side chains of the pseudosubstrate
form salt bridges with three glutamate carboxylates. Hydrophobic interactions are also important in the recognition of
substrate. The isoleucine residue of the pseudosubstrate is in contact with a pair of leucine residues of the enzyme.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin
10.5. Many Enzymes Are Activated by Specific Proteolytic Cleavage
We turn now to a different mechanism of enzyme regulation. Many enzymes acquire full enzymatic activity as they
spontaneously fold into their characteristic three-dimensional forms. In contrast, other enzymes are synthesized as
inactive precursors that are subsequently activated by cleavage of one or a few specific peptide bonds. The inactive
precursor is called a zymogen (or a proenzyme). A energy source (ATP) is not needed for cleavage. Therefore, in contrast
with reversible regulation by phosphorylation, even proteins located outside cells can be activated by this means.
Another noteworthy difference is that proteolytic activation, in contrast with allosteric control and reversible covalent
modification, occurs just once in the life of an enzyme molecule.
Specific proteolysis is a common means of activating enzymes and other proteins in biological systems. For example:
1. The digestive enzymes that hydrolyze proteins are synthesized as zymogens in the stomach and pancreas (Table 10.3).
2. Blood clotting is mediated by a cascade of proteolytic activations that ensures a rapid and amplified response to
trauma.
3. Some protein hormones are synthesized as inactive precursors. For example, insulin is derived from proinsulin by
proteolytic removal of a peptide.
4. The fibrous protein collagen, the major constituent of skin and bone, is derived from procollagen, a soluble precursor.
5. Many developmental processes are controlled by the activation of zymogens. For example, in the metamorphosis of a
tadpole into a frog, large amounts of collagen are resorbed from the tail in the course of a few days. Likewise, much
collagen is broken down in a mammalian uterus after delivery. The conversion of procollagenase into collagenase, the
active protease, is precisely timed in these remodeling processes.
6. Programmed cell death, or apoptosis, is mediated by proteolytic enzymes called caspases, which are synthesized in

precursor form as procaspases. When activated by various signals, caspases function to cause cell death in most
organisms, ranging from C. elegans (Section 2.4.3) to human beings. Apoptosis provides a means sculpting the shapes of
body parts in the course of development and a means of eliminating cells producing anti-self antibodies or infected with
pathogens as well as cells containing large amounts of damaged DNA.
We next examine the activation and control of zymogens, using as examples several digestive enzymes as well as blood-
clot formation.
10.5.1. Chymotrypsinogen Is Activated by Specific Cleavage of a Single Peptide Bond
Chymotrypsin is a digestive enzyme that hydrolyzes proteins in the small intestine. Its mechanism of action was
discussed in detail in Chapter 9. Its inactive precursor, chymotrypsinogen, is synthesized in the pancreas, as are several
other zymogens and digestive enzymes. Indeed, the pancreas is one of the most active organs in synthesizing and
secreting proteins. The enzymes and zymogens are synthesized in the acinar cells of the pancreas and stored inside
membrane-bounded granules (Figure 10.31). The zymogen granules accumulate at the apex of the acinar cell; when the
cell is stimulated by a hormonal signal or a nerve impulse, the contents of the granules are released into a duct leading
into the duodenum.
Chymotrypsinogen, a single polypeptide chain consisting of 245 amino acid residues, is virtually devoid of enzymatic
activity. It is converted into a fully active enzyme when the peptide bond joining arginine 15 and isoleucine 16 is cleaved
by trypsin (Figure 10.32). The resulting active enzyme, called π-chymotrypsin, then acts on other π-chymotrypsin
molecules. Two dipeptides are removed to yield α-chymotrypsin, the stable form of the enzyme. The three resulting
chains in α-chymotrypsin remain linked to one another by two interchain disulfide bonds. The striking feature of this
activation process is that cleavage of a single specific peptide bond transforms the protein from a catalytically inactive
form into one that is fully active.
10.5.2. Proteolytic Activation of Chymotrypsinogen Leads to the Formation of a
Substrate-Binding Site
How does cleavage of a single peptide bond activate the zymogen? Key conformational changes, which were revealed
by the elucidation of the three-dimensional structure of chymotrypsinogen, result from the cleavage of the peptide bond
between amino acids 15 and 16.
1. The newly formed amino-terminal group of isoleucine 16 turns inward and forms an ionic bond with aspartate 194 in
the interior of the chymotrypsin molecule (Figure 10.33). Protonation of this amino group stabilizes the active form of
chymotrypsin.
2. This electrostatic interaction triggers a number of conformational changes. Methionine 192 moves from a deeply
buried position in the zymogen to the surface of the active enzyme, and residues 187 and 193 become more extended.
These changes result in the formation of the substrate-specificity site for aromatic and bulky nonpolar groups. One side
of this site is made up of residues 189 through 192. This cavity for binding part of the substrate is not fully formed in the
zymogen.
3. The tetrahedral transition state in catalysis by chymotrypsin is stabilized by hydrogen bonds between the negatively
charged carbonyl oxygen atom of the substrate and two NH groups of the main chain of the enzyme (Section 9.1.3). One
of these NH groups is not appropriately located in chymotrypsinogen, and so the oxyanion hole is incomplete in the
zymogen.
4. The conformational changes elsewhere in the molecule are very small. Thus, the switching on of enzymatic activity in
a protein can be accomplished by discrete, highly localized conformational changes that are triggered by the hydrolysis
of a single peptide bond.
