Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

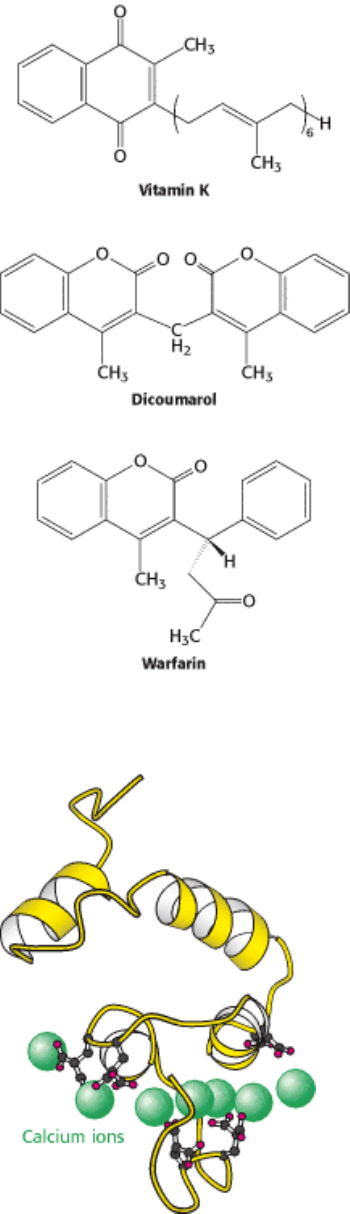
Figure 10.42. Structures of Vitamin K and Two Antagonists, Dicoumarol and Warfarin.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.5. Many Enzymes Are Activated by Specific Proteolytic Cleavage
Figure 10.43. The Calcium-Binding Region of Prothrombin. Prothrombin binds calcium ions with the modified
amino acid γ-carboxyglutamate (red).
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.5. Many Enzymes Are Activated by Specific Proteolytic Cleavage
Figure 10.44. Action of Antihemophilic Factor. Antihemophilic factor (VIII) stimulates the activation of factor X by
factor IX
a
. It is interesting to note that the activity of factor VIII is markedly increased by limited proteolysis by
thrombin and factor X
a
. This positive feedback amplifies the clotting signal and accelerates clot formation after a
threshold has been reached.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.5. Many Enzymes Are Activated by Specific Proteolytic Cleavage
Figure 10.45. Electron Micrograph of a Mast Cell. Heparin and other molecules in the dense granules are released
into the extracellular space when the cell is triggered to secrete. [Courtesy of Lynne Mercer.]
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.5. Many Enzymes Are Activated by Specific Proteolytic Cleavage
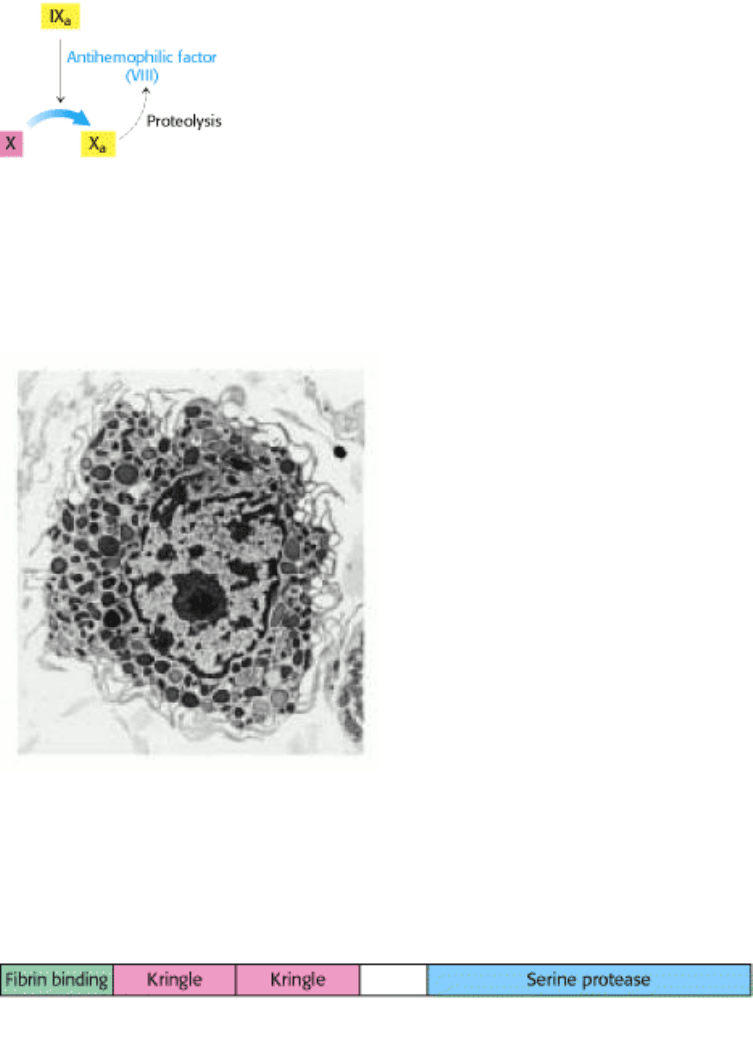
Figure 10.46. Modular Structure of Tissue-Type Plasminogen Activator (TPA).
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin 10.5. Many Enzymes Are Activated by Specific Proteolytic Cleavage
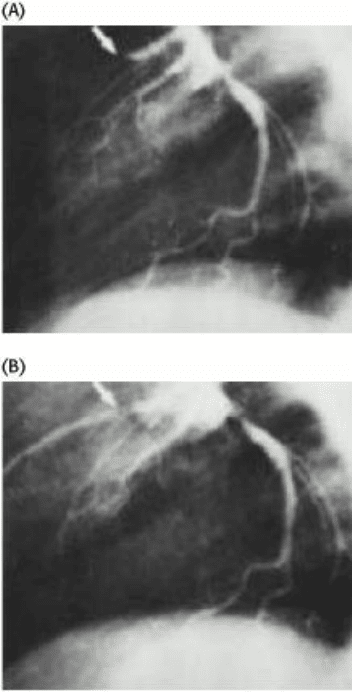
Figure 10.47. The Effect of Tissue-Type Plasminogen Factor. TPA leads to the dissolution of blood clots, as shown
by x-ray images of blood vessels in the heart (A) before and (B) 3 hours after the administration of TPA. The position of
the clot is marked by the arrow in part A. [After F. Van de Werf, P. A. Ludbrook, S. R. Bergmann, A. J. Tiefenbrunn, K.
A. A. Fox, H. de Geest, M. Verstraete, D. Collen, and B. E. Sobel. New Engl. J. Med. 310(1984):609.]
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin
Summary
Aspartate Transcarbamoylase Is Allosterically Inhibited by the End Product of Its
Pathway
Allosteric proteins constitute an important class of proteins whose biological activity can be regulated. Specific
regulatory molecules can modulate the activity of allosteric proteins by binding to distinct regulatory sites, separate from
the functional site. These proteins have multiple functional sites, which display cooperation as evidenced by a sigmoidal
dependence of function on substrate concentration. Aspartate transcarbamoylase (ATCase), one of the best-understood
allosteric enzymes, catalyzes the synthesis of N-carbamoylaspartate, the first intermediate in the synthesis of
pyrimidines. ATCase is feedback inhibited by cytidine triphosphate(CTP), the final product of the pathway. ATP
reverses this inhibition. ATCase consists of separable catalytic (c
3
) subunits (which bind the substrates) and regulatory
(r
2
) subunits (which bind CTP and ATP). The inhibitory effect of CTP, the stimulatory action of ATP, and the
cooperative binding of substrates are mediated by large changes in quaternary structure. On binding substrates, the c
3
subunits of the c
6
r
6
enzyme move apart and reorient themselves. This allosteric transition is highly concerted, as
postulated by the Monod-Wyman-Changeux (MWC) model. All subunits of an ATCase molecule simultaneously
interconvert from the T (low-affinity) to the R (high-affinity) state.

Hemoglobin Transports Oxygen Efficiently by Binding Oxygen Cooperatively
Hemoglobin, the oxygen carrier in the blood, is an allosteric protein. Hemoglobin consists of four polypeptide chains,
each with a heme group
a substituted porphyrin with a central iron. Hemoglobin A, the predominant hemoglobin in
adults, has the subunit structure α
2
β
2
. Hemoglobin transports H
+
and CO
2
in addition to O
2
. Hemoglobin exhibits
three kinds of allosteric effects. First, the oxygen-binding curve of hemoglobin is sigmoidal, which indicates that the
binding of oxygen is cooperative. The binding of oxygen to one heme group facilitates the binding of oxygen to the other
heme groups in the same molecule. Second, the binding of H
+
and CO
2
promotes the release of O
2
from hemoglobin, an
effect that is physiologically important in enhancing the release of O
2
in metabolically active tissues such as muscle.
These allosteric linkages between the binding of H
+
, CO
2
, and O
2
are known as the Bohr effect. Third, the affinity of
hemoglobin for O
2
is further regulated by 2,3-bisphosphoglycerate (2,3-BPG), a small molecule with a very high density
of negative charge. 2,3-Bisphosphoglycerate binds tightly to deoxyhemoglobin but not to oxyhemoglobin. Hence, 2,3-
BPG lowers the oxygen affinity of hemoglobin. Fetal hemoglobin (α
2
γ
2
) has a higher oxygen affinity than human adult
hemoglobin because fetal hemoglobin binds 2,3-BPG less tightly. Neither the sequential nor the concerted model
completely describes the allosteric behavior of hemoglobin. Rather, the behavior of hemoglobin is best described by a
combined model that employs features of both models.
Isozymes Provide a Means of Regulation Specific to Distinct Tissues and
Developmental Stages
Isozymes differ in structural characteristics but catalyze the same reaction. They provide a means of fine-tuning
metabolism to meet the needs of a given tissue or developmental stage. The results of gene-duplication events provide
the means for subtle regulation of enzyme function.
Covalent Modification Is a Means of Regulating Enzyme Activity
Covalent modification of proteins is a potent means of controlling the activity of enzymes and other proteins.
Phosphorylation is the most common type of reversible covalent modification. Signals can be highly amplified by
phosphorylation because a single kinase can act on many target molecules. The regulatory actions of protein kinases are
reversed by protein phosphatases, which catalyze the hydrolysis of attached phosphoryl groups.
Cyclic AMP serves as an intracellular messenger in the transduction of many hormonal and sensory stimuli. Cyclic AMP
switches on protein kinase A (PKA), a major multifunctional kinase, by binding to the regulatory subunit of the enzyme,
thereby releasing the active catalytic subunits of PKA. In the absence of cAMP, the catalytic sites of PKA are occupied
by pseudosubstrate sequences of the regulatory subunit.
Many Enzymes Are Activated by Specific Proteolytic Cleavage
The activation of an enzyme by proteolytic cleavage of one or a few peptide bonds is a recurring control mechanism seen
in processes as diverse as the activation of digestive enzymes and blood clotting. The inactive precursor is called a
zymogen (or a proenzyme). Trypsinogen is activated by enteropeptidase or trypsin, and trypsin then activates a host of
other zymogens, leading to the digestion of foodstuffs. For instance, trypsin converts chymotrypsinogen, a zymogen, into
active chymotrypsin by hydrolyzing a single peptide bond.
A striking feature of the clotting process is that it is accomplished by a cascade of zymogen conversions, in which the
activated form of one clotting factor catalyzes the activation of the next precursor. Many of the activated clotting factors
are serine proteases. In the final step of clot formation, fibrinogen, a highly soluble molecule in the plasma, is converted
by thrombin into fibrin by the hydrolysis of four arginine-glycine bonds. The resulting fibrin monomer spontaneously
forms long, insoluble fibers called fibrin. Zymogen activation is also essential in the lysis of clots. Plasminogen is
converted into plasmin, a serine protease that cleaves fibrin, by tissue-type plasminogen activator (TPA). Although
zymogen activation is irreversible, specific inhibitors of some proteases exert control. The irreversible protein inhibitor
antithrombin III holds blood clotting in check in the clotting cascade.
Key Terms
feedback (end-product) inhibition
regulatory site
concerted mechanism
cooperativity
homotropic effect
heterotropic effect
sequential model
heme
protoporphyrin
Bohr effect
isozyme (isoenzyme)
covalent modification
protein kinase
protein phosphatase
consensus sequence
protein kinase A (PKA)
pseudosubstrate sequence
zymogen (proenzyme)
enzymatic cascade
intrinsic clotting pathway
extrinsic clotting pathway

I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin
Problems
1.
Activity profile. A histidine residue in the active site of aspartate transcarbamoylase is thought to be important in
stabilizing the transition state of the bound substrates. Predict the pH dependence of the catalytic rate, assuming that
this interaction is essential and dominates the pH-activity profile of the enzyme.
See answer
2.
Allosteric switching. A substrate binds 100 times as tightly to the R state of an allosteric enzyme as to its T state.
Assume that the concerted (MWC) model applies to this enzyme.
See answer
(a) By what factor does the binding of one substrate molecule per enzyme molecule alter the ratio of the concentrations
of enzyme molecules in the R and T states?
(b) Suppose that L, the ratio of [T] to [R] in the absence of substrate, is 10
7
and that the enzyme contains four binding
sites for substrate. What is the ratio of enzyme molecules in the R state to that in the T state in the presence of
saturating amounts of substrate, assuming that the concerted model is obeyed?
3.
Allosteric transition. Consider an allosteric protein that obeys the concerted model. Suppose that the ratio of T to R
formed in the absence of ligand is 10
5
, K
T
= 2 mM, and K
R
= 5 µM. The protein contains four binding sites for
ligand. What is the fraction of molecules in the R form when 0, 1, 2, 3, and 4 ligands are bound?
See answer
4.
Negative cooperativity. You have isolated a dimeric enzyme that contains two identical active sites. The binding of
substrate to one active site decreases the substrate affinity of the other active site. Which allosteric model best
accounts for this negative cooperativity?
See answer
5.
Paradoxical at first glance. Recall that phosphonacetyl-l-aspartate (PALA) is a potent inhibitor of ATCase because
it mimics the two physiological substrates. However, low concentrations of this unreactive bisubstrate analog
increase the reaction velocity. On the addition of PALA, the reaction rate increases until an average of three
molecules of PALA are bound per molecule of enzyme. This maximal velocity is 17-fold as great as it is in the
absence of PALA. The reaction rate then decreases to nearly zero on the addition of three more molecules of PALA
per molecule of enzyme. Why do low concentrations of PALA activate ATCase?
See answer
6.
R versus T. An allosteric enzyme that follows the concerted mechanism has a T/R ratio of 300 in the absence of
substrate. Suppose that a mutation reversed the ratio. How would this mutation affect the relation between the rate of
the reaction and substrate concentration?
See answer

7.
Tuning oxygen affinity. What is the effect of each of the following treatments on the oxygen affinity of hemoglobin
A in vitro?
(a) Increase in pH from 7.2 to 7.4.
(b) Increase in pCO
2
from 10 to 40 torr.
(c) Increase in [2,3-BPG] from 2 × 10
-4
to 8 × 10
-4
M.
(d) Dissociation of α
2
β
2
into monomeric subunits.
See answer
8.
Avian and reptilian counterparts. The erythrocytes of birds and turtles contain a regulatory molecule different from
2,3-BPG. This substance is also effective in reducing the oxygen affinity of human hemoglobin stripped of 2,3-BPG.
Which of the following substances would you predict to be most effective in this regard?
(a) Glucose 6-phosphate
(b) Inositol hexaphosphate
(c) HPO
4
2-
(d) Malonate
(e) Arginine
(f) Lactate
See answer
9.
Tuning proton affinity. The pK of an acid depends partly on its environment. Predict the effect of the following
environmental changes on the pK of a glutamic acid side chain.
(a) A lysine side chain is brought into close proximity.
(b) The terminal carboxyl group of the protein is brought into close proximity.
(c) The glutamic acid side chain is shifted from the outside of the protein to a nonpolar site inside.
See answer
10.
Zymogen activation. When very low concentrations of pepsinogen are added to acidic media, how does the half-
time for activation depend on zymogen concentration?
See answer

11.
A revealing assay. Suppose that you have just examined a young boy with a bleeding disorder highly suggestive of
classic hemophilia (factor VIII deficiency). Because of the late hour, the laboratory that carries out specialized
coagulation assays is closed. However, you happen to have a sample of blood from a classic hemophiliac whom
you admitted to the hospital an hour earlier. What is the simplest and most rapid test that you can perform to
determine whether your present patient also is deficient in factor VIII activity?
See answer
12.
Counterpoint. The synthesis of factor X, like that of prothrombin, requires vitamin K. Factor X also contains γ-
carboxyglutamate residues in its amino-terminal region. However, activated factor X, in contrast with thrombin,
retains this region of the molecule. What is a likely functional consequence of this difference between the two
activated species?
See answer
13.
A discerning inhibitor. Antithrombin III forms an irreversible complex with thrombin but not with prothrombin.
What is the most likely reason for this difference in reactivity?
See answer
14.
Repeating heptads. Each of the three types of fibrin chains contains repeating heptapeptide units (abcdefg) in which
residues a and d are hydrophobic. Propose a reason for this regularity.
See answer
15.
Drug design. A drug company has decided to use recombinant DNA methods to prepare a modified α
1
-antitrypsin
that will be more resistant to oxidation than is the naturally occurring inhibitor. Which single amino acid
substitution would you recommend?
See answer
Data Interpretation Problems
16.
Distinguishing between models. The graph below shows the fraction of an allosteric enzyme in the R state (f
R
) and
the fraction of active sites bound to substrate (Y) as a function of substrate concentration. Which model, the
concerted or sequential, best explains these results?
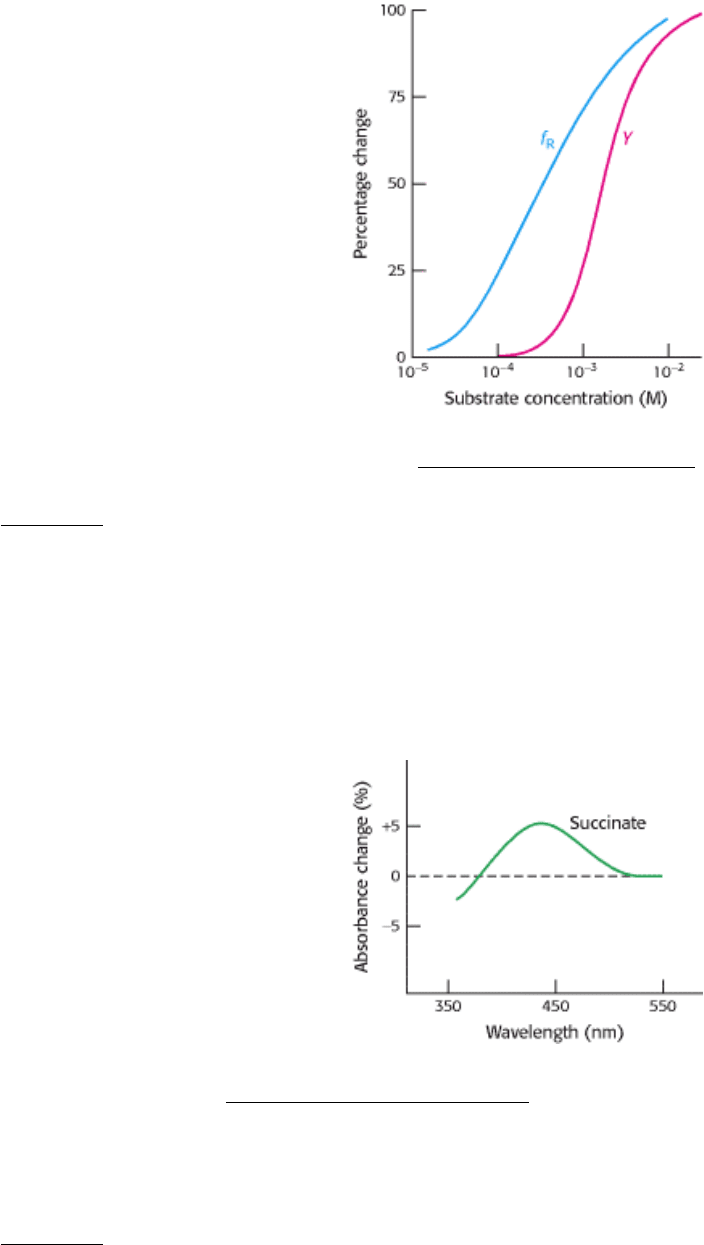
[From M.W. Kirschner and H.K. Schachman, Biochemistry 12 (1966):2997.]
See answer
17.
Reporting live from ATCase. ATCase was reacted with nitromethane to form a colored nitrotyrosine group (λ
max
=
430 nm) in each of its catalytic chains. The absorption by this reporter group depends on its immediate
environment. An essential lysine residue at each catalytic site also was modified to block the binding of substrate.
Catalytic trimers from this doubly modified enzyme were then combined with native trimers to form a hybrid
enzyme. The absorption by the nitrotyrosine group was measured on addition of the substrate analog succinate.
What is the significance of the alteration in the absorbance at 430 nm?
[After H.K. Schachman. J. Biol. Chem. 263 (1988):18583]
The binding of succinate, an unreactive analog of aspartate, to one trimer changed the visible absorption spectrum
of nitrotyrosine residues attached to the other trimer.
See answer
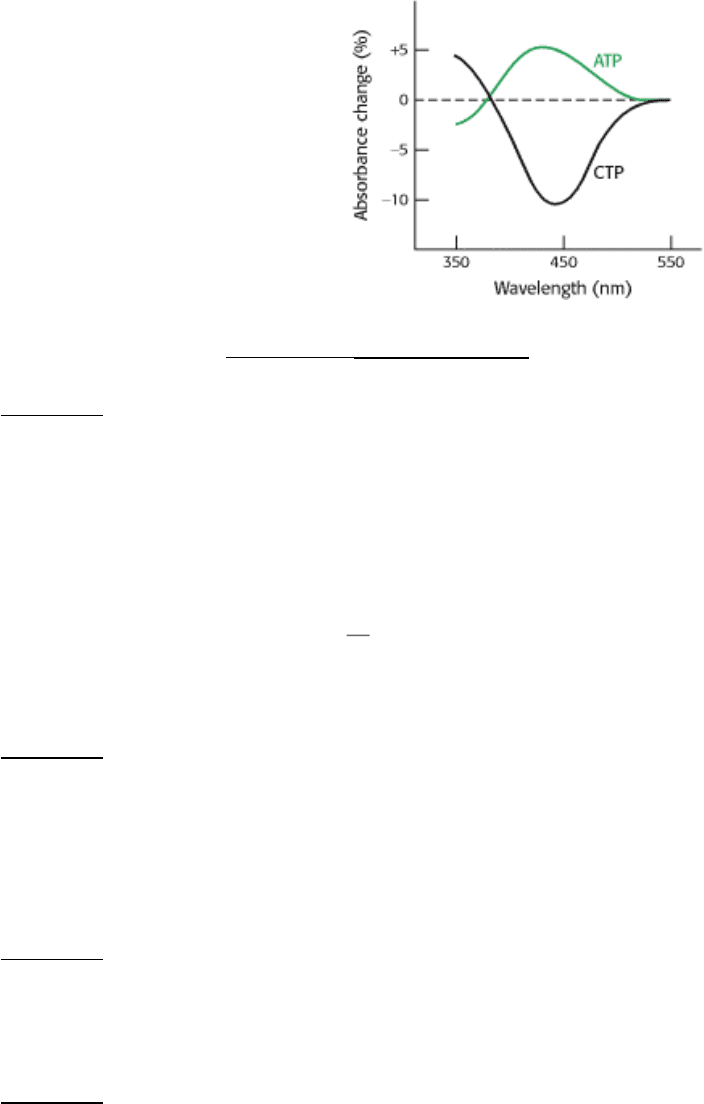
18.
Reporting live from ATCase 2. A different ATCase hybrid was constructed to test the effects of allosteric activators
and inhibitors. Normal regulatory subunits were combined with nitrotyrosine-containing catalytic subunits. The
addition of ATP in the absence of substrate increased the absorbance at 430 nm, the same change elicited by the
addition of succinate (see the graph in Problem 17). Conversely, CTP in the absence of substrate decreased the
absorbance at 430 nm. What is the significance of the changes in absorption of the reporter groups?.
[After H.K. Schachman. J. Biol. Chem. 263(1988):18583.]
See answer
Chapter Integration Problem
19.
Sticky patches. The substitution of valine for glutamate at position 6 of the β chains of hemoglobin places a
nonpolar residue on the outside of hemoglobin S, the version of hemoglobin responsible for sickle-cell anemia. The
oxygen affinity and allosteric properties of hemoglobin are virtually unaffected by this change. However, the valine
side chain of hemoglobin S interacts with a complementary sticky patch (formed by phenyl-alanine β85 and leucine
β88) on another hemoglobin molecule
a patch that is exposed in deoxygenated but not in oxygenated
hemoglobin. What is the chemical basis for the interaction between the hemoglobin molecules? What would be the
effect of this interaction?
See answer
Mechanism Problems
20.
Aspartate transcarbamoylase. Write the mechanism (in detail) for the conversion of aspartate and carbamoyl
phosphate into N-carbamoylaspartate. Include a role for the residue histidine present in the active site.
See answer
21.
Protein kinases. Write a mechanism (in detail) for the phosphorylation of a serine residue by ATP catalyzed by a
protein kinase. What groups might you expect to find in the enzyme active site?
See answer
Media Problem
