Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

22.
Cooperative, more or less. (a) Suppose you purify the same enzyme from two different species and find that
one is a tetramer (n = 4) and the other a hexamer (n = 6). All other things being equal, which would you
expect would show the greater cooperativity? (b) To your surprise, both enzymes show similar cooperativity.
Explain how differences in the MWC parameter c could explain this. (Hint: Make sure you do the exercise in
Section 4 of the Conceptual Insights module on cooperative binding and kinetics.)
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin Problems
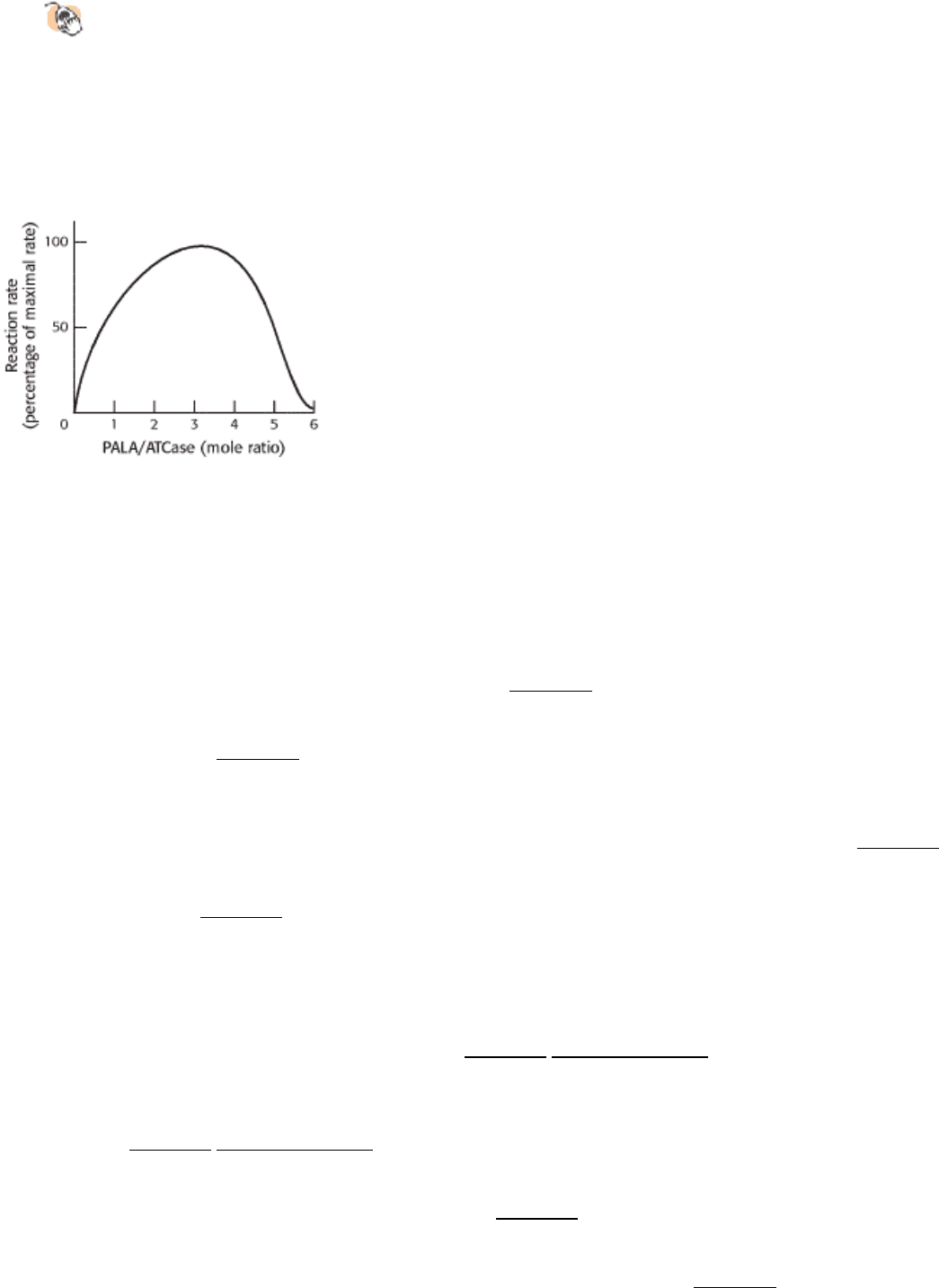
Effect of PALA on ATCase rate.
I. The Molecular Design of Life 10. Regulatory Strategies: Enzymes and Hemoglobin
Selected Readings
Where to start
E.R. Kantrowitz and W.N. Lipscomb. 1990. Escherichia coli aspartate transcarbamoylase: The molecular basis for a
concerted allosteric transition Trends Biochem. Sci. 15: 53-59. (PubMed)
H.K. Schachman. 1988. Can a simple model account for the allosteric transition of aspartate transcarbamoylase? J. Biol.
Chem. 263: 18583-18586. (PubMed)
Dickerson, R. E., and Geis, I.,1983. Hemoglobin: Structure, Function, Evolution and Pathology. Benjamin Cummings.
H. Neurath. 1989. Proteolytic processing and physiological regulation Trends Biochem. Sci. 14: 268-271. (PubMed)
W. Bode and R. Huber. 1992. Natural protein proteinase inhibitors and their interaction with proteinases Eur. J.
Biochem. 204: 433-451. (PubMed)
Aspartate transcarbamoylase and allosteric interactions
J.A. Endrizzi, P.T. Beernink, T. Alber, and H.K. Schachman. 2000. Binding of bisubstrate analog promotes large
structural changes in the unregulated catalytic trimer of aspartate transcarbamoylase: Implications for allosteric
regulation Proc. Natl. Acad. Sci. U. S. A. 97: 5077-5082. (PubMed) (Full Text in PMC)
P.T. Beernink, J.A. Endrizzi, T. Alber, and H.K. Schachman. 1999. Assessment of the allosteric mechanism of aspartate
transcarbamoylase based on the crystalline structure of the unregulated catalytic subunit Proc. Natl. Acad. Sci. U. S. A.
96: 5388-5393. (PubMed) (Full Text in PMC)
M.E. Wales, L.L. Madison, S.S. Glaser, and J.R. Wild. 1999. Divergent allosteric patterns verify the regulatory paradigm
for aspartate transcarbamylase J. Mol. Biol. 294: 1387-1400. (PubMed)
V.J. LiCata, D.S. Burz, N.J. Moerke, and N.M. Allewell. 1998. The magnitude of the allosteric conformational transition
of aspartate transcarbamylase is altered by mutations Biochemistry 37: 17381-17385. (PubMed)
E. Eisenstein, D.W. Markby, and H.K. Schachman. 1990. Heterotropic effectors promote a global conformational change
in aspartate transcarbamoylase Biochemistry 29: 3724-3731. (PubMed)
W.E. Werner and H.K. Schachman. 1989. Analysis of the ligand-promoted global conformational change in aspartate
transcarbamoylase: Evidence for a two-state transition from boundary spreading in sedimentation velocity experiments J.
Mol. Biol. 206: 221-230. (PubMed)
J.O. Newell, D.W. Markby, and H.K. Schachman. 1989. Cooperative binding of the bisubstrate analog N-
(phosphonacetyl)-l-aspartate to aspartate transcarbamoylase and the heterotropic effects of ATP and CTP J. Biol. Chem.
264: 2476-2481. (PubMed)
R.C. Stevens, K.M. Reinisch, and W.N. Lipscomb. 1991. Molecular structure of Bacillus subtilis aspartate
transcarbamoylase at 3.0 Å resolution Proc. Natl. Acad. Sci. U. S. A. 88: 6087-6091. (PubMed) (Full Text in PMC)
R.C. Stevens, J.E. Gouaux, and W.N. Lipscomb. 1990. Structural consequences of effector binding to the T state of
aspartate carbamoyltransferase: Crystal structures of the unligated and ATP- and CTP-complexed enzymes at 2.6-Å
resolution Biochemistry 29: 7691-7701. (PubMed)
J.E. Gouaux and W.N. Lipscomb. 1990. Crystal structures of phosphonoacetamide ligated T and phosphonoacetamide
and malonate ligated R states of aspartate carbamoyltransferase at 2.8-Å resolution and neutral pH Biochemistry 29: 389-
402. (PubMed)
B. Labedan, A. Boyen, M. Baetens, D. Charlier, P. Chen, R. Cunin, V. Durbeco, N. Glansdorff, G. Herve, C. Legrain, Z.
Liang, C. Purcarea, M. Roovers, R. Sanchez, T.L. Toong, M. Van de Casteele, F. van Vliet, Y. Xu, and Y.F. Zhang.
1999. The evolutionary history of carbamoyltransferases: A complex set of paralogous genes was already present in the
last universal common ancestor J. Mol. Evol. 49: 461-473. (PubMed)
Hemoglobin
M.F. Perutz, A.J. Wilkinson, M. Paoli, and G.G. Dodson. 1998. The stereochemical mechanism of the cooperative
effects in hemoglobin revisited Annu. Rev. Biophys. Biomol. Struct. 27: 1-34. (PubMed)
G.K. Ackers. 1998. Deciphering the molecular code of hemoglobin allostery Adv. Protein Chem. 51: 185-253. (PubMed)
G.K. Ackers, M.L. Doyle, D. Myers, and M.A. Daugherty. 1992. Molecular code for cooperativity in hemoglobin
Science 255: 54-63. (PubMed)
G. Fermi, M.F. Perutz, B. Shaanan, and R. Fourme. 1984. The crystal structure of human deoxyhaemoglobin at 1.74 Å
resolution J. Mol. Biol. 175: 159-174. (PubMed)
J.V. Kilmartin and L. Rossi-Bernardi. 1973. Interaction of hemoglobin with hydrogen ions, carbon dioxide, and organic
phosphates Physiol. Rev. 53: 836-890. (PubMed)
Covalent modification
L.N. Johnson and D. Barford. 1993. The effects of phosphorylation on the structure and function of proteins Annu. Rev.
Biophys. Biomol. Struct. 22: 199-232. (PubMed)
M. Ziegler. 2000. New functions of a long-known molecule: Emerging roles of NAD in cellular signaling Eur. J.
Biochem. 267: 1550-1564. (PubMed)
H.H. Ng and A. Bird. 2000. Histone deacetylases: Silencers for hire Trends Biochem. Sci. 25: 121-126. (PubMed)
R.V. Raju, R. Kakkar, J.M. Radhi, and R.K. Sharma. 1997. Biological significance of phosphorylation and
myristoylation in the regulation of cardiac muscle proteins Mol. Cell. Biochem. 176: 135-143. (PubMed)
M.K. Jacobson and E.L. Jacobson. 1999. Discovering new ADPribose polymer cycles: Protecting the genome and more
Trends Biochem. Sci. 24: 415-417. (PubMed)
D. Barford, A.K. Das, and M.P. Egloff. 1998. The structure and mechanism of protein phosphatases: Insights into
catalysis and regulation Annu. Rev. Biophys. Biomol. Struct. 27: 133-164. (PubMed)
Protein kinase A
S.S. Taylor, D.R. Knighton, J. Zheng, J.M. Sowadski, C.S. Gibbs, and M.J. Zoller. 1993. A template for the protein
kinase family Trends Biochem. Sci. 18: 84-89. (PubMed)
C.S. Gibbs, D.R. Knighton, J.M. Sowadski, S.S. Taylor, and M.J. Zoller. 1992. Systematic mutational analysis of cAMP-
dependent protein kinase identifies unregulated catalytic subunits and defines regions important for the recognition of the
regulatory subunit J. Biol. Chem. 267: 4806-4814. (PubMed)
D.R. Knighton, J.H. Zheng, L. TenEyck, V.A. Ashford, N.H. Xuong, S.S. Taylor, and J.M. Sowadski. 1991. Crystal
structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase Science 253: 407-414.
(PubMed)
D.R. Knighton, J.H. Zheng, L. TenEyck, N.H. Xuong, S.S. Taylor, and J.M. Sowadski. 1991. Structure of a peptide
inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase Science 253: 414-
420. (PubMed)
S.R. Adams, A.T. Harootunian, Y.J. Buechler, S.S. Taylor, and R.Y. Tsien. 1991. Fluorescence ratio imaging of cyclic
AMP in single cells Nature 349: 694-697. (PubMed)
Zymogen activation
H. Neurath. 1986. The versatility of proteolytic enzymes J. Cell. Biochem. 32: 35-49. (PubMed)
Bode, W., and Huber, R., 1986. Crystal structure of pancreatic serine endopeptidases. In Molecular and Cellular Basis of
Digestion (pp. 213 234), edited by P. Desnuelle, H. Sjostrom, and O. Noren. Elsevier.
R. Huber and W. Bode. 1978. Structural basis of the activation and action of trypsin Acc. Chem. Res. 11: 114-122.
R.M. Stroud, A.A. Kossiakoff, and J.L. Chambers. 1977. Mechanism of zymogen activation Annu. Rev. Biophys. Bioeng.
6: 177-193. (PubMed)
A.R. Sielecki, M. Fujinaga, R.J. Read, and M.N. James. 1991. Refined structure of porcine pepsinogen at 1.8 Å
resolution J. Mol. Biol. 219: 671-692. (PubMed)
Protease inhibitors
R. Carrell and J. Travis. 1985. α
1
-Antitrypsin and the serpins: Variation and countervariation Trends Biochem. Sci. 10:
20-24.
H. Carp, F. Miller, J.R. Hoidal, and A. Janoff. 1982. Potential mechanism of emphysema: α
1
-Proteinase inhibitor
recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity
Proc. Natl. Acad. Sci. U. S. A. 79: 2041-2045. (PubMed)
M.C. Owen, S.O. Brennan, J.H. Lewis, and R.W. Carrell. 1983. Mutation of antitrypsin to antithrombin New Engl. J.
Med. 309: 694-698. (PubMed)
J. Travis and G.S. Salvesen. 1983. Human plasma proteinase inhibitors Annu. Rev. Biochem. 52: 655-709. (PubMed)
Clotting cascade
P. Fuentes-Prior, Y. Iwanaga, R. Huber, R. Pagila, G. Rumennik, M. Seto, J. Morser, D.R. Light, and W. Bode. 2000.
Structural basis for the anticoagulant activity of the thrombin-thrombomodulin complex Nature. 404: 518-525.
(PubMed)
R.W. Herzog and K.A. High. 1998. Problems and prospects in gene therapy for hemophilia Curr. Opin. Hematol. 5: 321-
326. (PubMed)
R.F. Doolittle. 1981. Fibrinogen and fibrin Sci. Am. 245: (12) 126-135. (PubMed)
R.M. Lawn and G.A. Vehar. 1986. The molecular genetics of hemophilia Sci. Am. 254: (3) 48-65. (PubMed)
J.H. Brown, N. Volkmann, G. Jun, A.H. Henschen-Edman, and C. Cohen. 2000. The crystal structure of modified bovine
fibrinogen Proc. Natl. Acad. Sci. U. S. A. 97: 85-90. (PubMed) (Full Text in PMC)
M.T. Stubbs, H. Oschkinat, I. Mayr, R. Huber, H. Angliker, S.R. Stone, and W. Bode. 1992. The interaction of thrombin
with fibrinogen: A structural basis for its specificity Eur. J. Biochem. 206: 187-195. (PubMed)
T.J. Rydel, A. Tulinsky, W. Bode, and R. Huber. 1991. Refined structure of the hirudin-thrombin complex J. Mol. Biol.
221: 583-601. (PubMed)
I. The Molecular Design of Life
11. Carbohydrates
Let us take an overview of carbohydrates, one of the four major classes of biomolecules along with proteins, nucleic
acids, and lipids. Carbohydrates are aldehyde or ketone compounds with multiple hydroxyl groups. They make up most
of the organic matter on Earth because of their extensive roles in all forms of life. First, carbohydrates serve as energy
stores, fuels, and metabolic intermediates. Second, ribose and deoxyribose sugars form part of the structural framework
of RNA and DNA. Third, polysaccharides are structural elements in the cell walls of bacteria and plants. In fact,
cellulose, the main constituent of plant cell walls, is one of the most abundant organic compounds in the biosphere.
Fourth, carbohydrates are linked to many proteins and lipids, where they play key roles in mediating interactions among
cells and interactions between cells and other elements in the cellular environment.
A key related property of carbohydrates in their role as mediators of cellular interactions is the tremendous structural
diversity possible within this class of molecules. Carbohydrates are built from monosaccharides, small molecules that
typically contain from three to nine carbon atoms and vary in size and in the stereochemical configuration at one or more
carbon centers. These monosaccharides may be linked together to form a large variety of oligosaccharide structures. The
unraveling of these oligosaccharide structures, the discovery of their placement at specific sites within proteins, and the
determination of their function are tremendous challenges in the field of proteomics.
I. The Molecular Design of Life 11. Carbohydrates
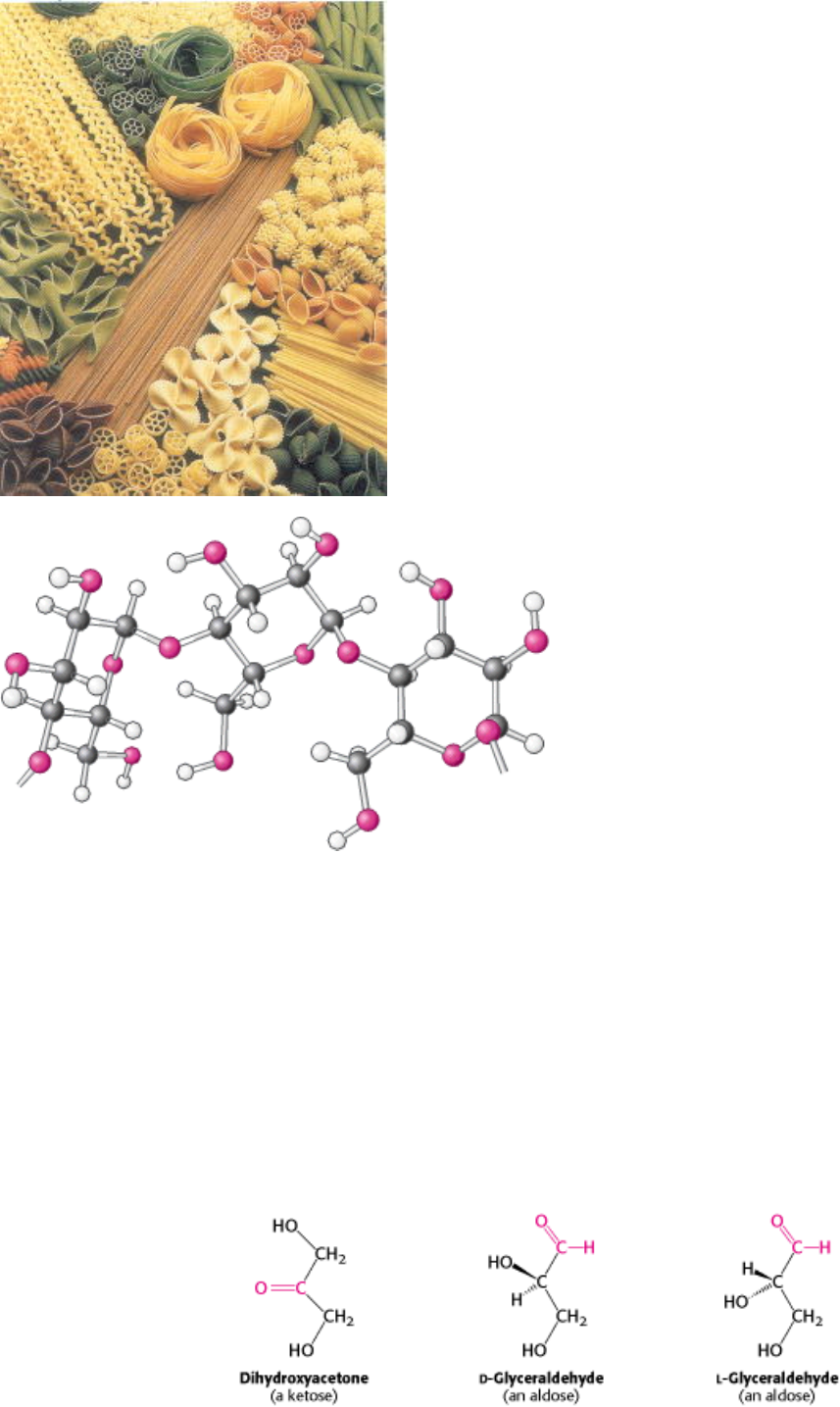
Carbohydrates in food are important sources of energy. Starch, found in plant-derived food such as pasta, consists of
chains of linked glucose molecules. These chains are broken down into individual glucose molecules for eventual use in
generation of ATP and building blocks for other molecules. [(Left) Superstock.]
I. The Molecular Design of Life 11. Carbohydrates
11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Monosaccharides, the simplest carbohydrates, are aldehydes or ketones that have two or more hydroxyl groups; the
empirical formula of many is (C-H
2
O)n, literally a "carbon hydrate." Monosaccharides are important fuel molecules as
well as building blocks for nucleic acids. The smallest monosaccharides, for which n = 3, are dihydroxyacetone and
d-
and l-glyceraldehyde.
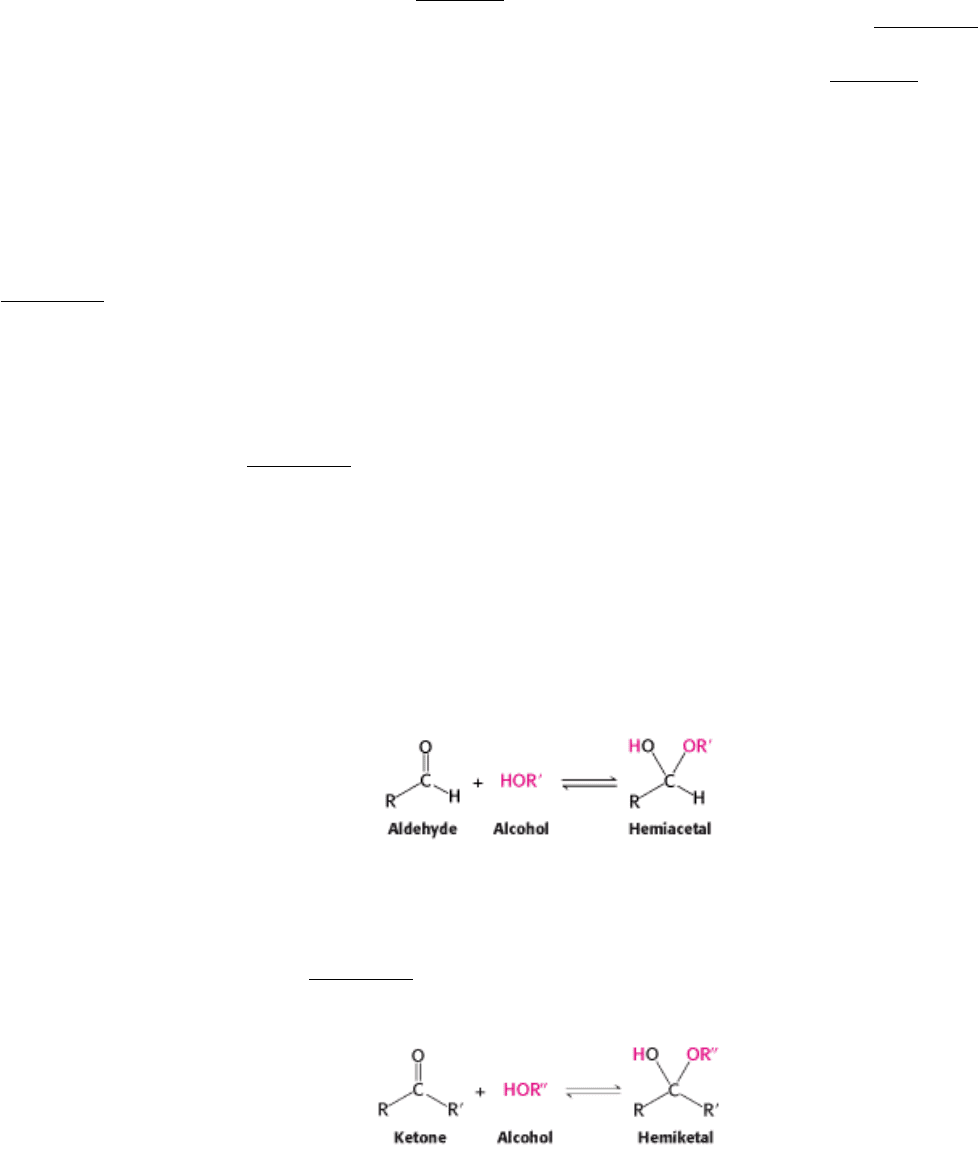
They are referred to as trioses (tri- for 3). Dihydroxyacetone is called a ketose because it contains a keto group, whereas
glyceraldehyde is called an aldose because it contains an aldehyde group. Glyceraldehyde has a single asymmetric
carbon and, thus, there are two stereoisomers of this sugar. d-Glyceraldehyde and l-glyceraldehyde are enantiomers, or
mirror images of each other. As mentioned in Chapter 3, the prefixes d and l designate the absolute configuration.
Monosaccharides and other sugars will often be represented in this book by Fischer projections (Figure 11.1). Recall
that, in a Fischer projection of a molecule, atoms joined to an asymmetric carbon atom by horizontal bonds are in front
of the plane of the page, and those joined by vertical bonds are behind (see the Appendix in Chapter 1). Fischer
projections are useful for depicting carbohydrate structures because they provide clear and simple views of the
stereochemistry at each carbon center.
Simple monosaccharides with four, five, six, and seven carbon atoms are called tetroses, pentoses, hexoses, and
heptoses, respectively. Because these molecules have multiple asymmetric carbons, they exist as diastereoisomers,
isomers that are not mirror images of each other, as well as enantiomers. In regard to these monosaccharides, the
symbols
d and l designate the absolute configuration of the asymmetric carbon farthest from the aldehyde or keto group.
Figure 11.2 shows the common d-aldose sugars. d-Ribose, the carbohydrate component of RNA, is a five-carbon aldose.
d-Glucose, d-mannose, and d-galactose are abundant six-carbon aldoses. Note that d-glucose and d-mannose differ in
configuration only at C-2. Sugars differing in configuration at a single asymmetric center are called epimers. Thus, d-
glucose and d-mannose are epimeric at C-2; d-glucose and d-galactose are epimeric at C-4.
Dihydroxyacetone is the simplest ketose. The stereochemical relation between
d-ketoses containing as many as six
carbon atoms are shown in Figure 11.3. Note that ketoses have one fewer asymmetric center than do aldoses with the
same number of carbons. d-Fructose is the most abundant ketohexose.
11.1.1. Pentoses and Hexoses Cyclize to Form Furanose and Pyranose Rings
The predominant forms of ribose, glucose, fructose, and many other sugars in solution are not open chains. Rather, the
open-chain forms of these sugars cyclize into rings. In general, an aldehyde can react with an alcohol to form a
hemiacetal.
For an aldohexose such as glucose, the C-1 aldehyde in the open-chain form of glucose reacts with the C-5 hydroxyl
group to form an intramolecular hemiacetal. The resulting cyclic hemiacetal, a six-membered ring, is called pyranose
because of its similarity to pyran (Figure 11.4). Similarly, a ketone can react with an alcohol to form a hemiketal.
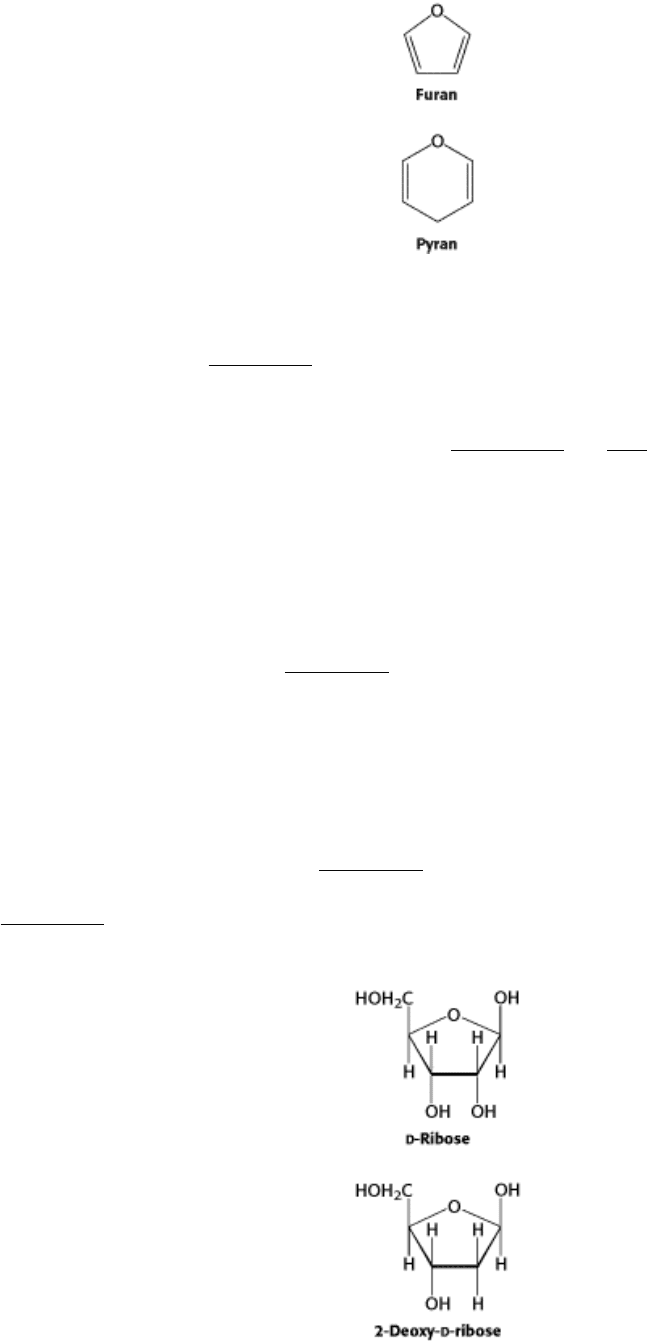
The C-2 keto group in the open-chain form of a ketohexose, such as fructose, can form an intramolecular hemiketal by
reacting with either the C-6 hydroxyl group to form a six-membered cyclic hemiketal or the C-5 hydroxyl group to form
a five-membered cyclic hemiketal (Figure 11.5). The five-membered ring is called a furanose because of its similarity to
furan.
The depictions of glucopyranose and fructofuranose shown in Figures 11.4 and 11.5 are Haworth projections. In such
projections, the carbon atoms in the ring are not explicitly shown. The approximate plane of the ring is perpendicular to
the plane of the paper, with the heavy line on the ring projecting toward the reader. Like Fischer projections, Haworth
projections allow easy depiction of the stereochemistry of sugars. We will return to a more structurally realistic view of
the conformations of cyclic monosaccharides shortly.
An additional asymmetric center is created when a cyclic hemiacetal is formed. In glucose, C-1, the carbonyl carbon
atom in the open-chain form, becomes an asymmetric center. Thus, two ring structures can be formed: α -
d-
glucopyranose and β -d-glucopyranose (see Figure 11.4). For d sugars drawn as Haworth projections, the designation α
means that the hydroxyl group attached to C-1 is below the plane of the ring; β means that it is above the plane of the
ring. The C-1 carbon atom is called the anomeric carbon atom, and the α and β forms are called anomers. An
equilibrium mixture of glucose contains approximately one-third α anomer, two-thirds β anomer, and <1% of the open-
chain form.
The same nomenclature applies to the furanose ring form of fructose, except that α and β refer to the hydroxyl groups
attached to C-2, the anomeric carbon atom (see Figure 11.5). Fructose forms both pyranose and furanose rings. The
pyranose form predominates in fructose free in solution, and the furanose form predominates in many fructose
derivatives (Figure 11.6). Pentoses such as d-ribose and 2-deoxy-d-ribose form furanose rings, as we have seen in the
structure of these units in RNA and DNA.
11.1.2. Conformation of Pyranose and Furanose Rings
The six-membered pyranose ring is not planar, because of the tetrahedral geometry of its saturated carbon atoms.
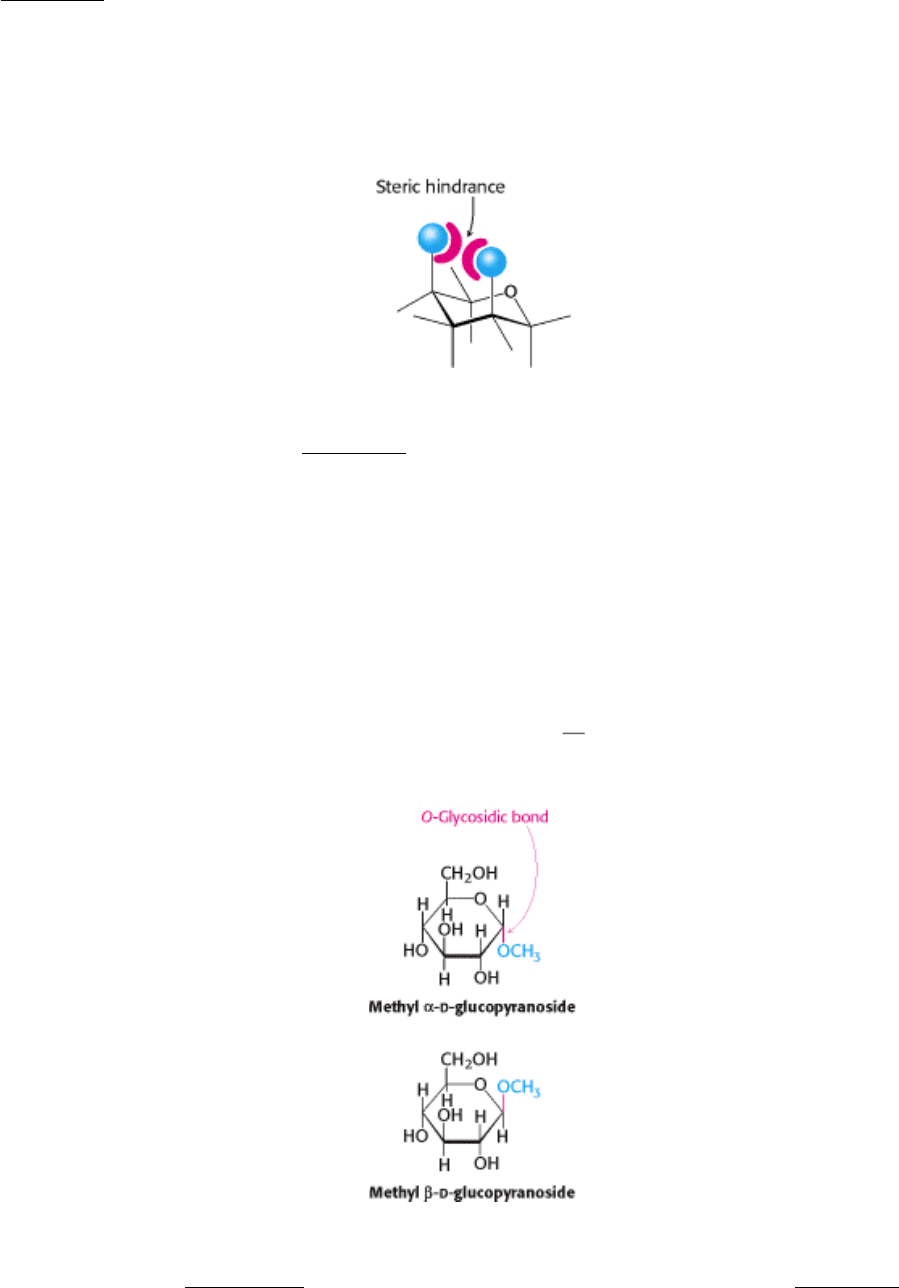
Instead, pyranose rings adopt two classes of conformations, termed chair and boat because of the resemblance to these
objects (Figure 11.7). In the chair form, the substituents on the ring carbon atoms have two orientations: axial and
equatorial. Axial bonds are nearly perpendicular to the average plane of the ring, whereas equatorial bonds are nearly
parallel to this plane. Axial substituents sterically hinder each other if they emerge on the same side of the ring (e.g., 1,3-
diaxial groups). In contrast, equatorial substituents are less crowded. The chair form of β - d -glucopyranose
predominates because all axial positions are occupied by hydrogen atoms. The bulkier -OH and -CH
2
OH groups emerge
at the less-hindered periphery. The boat form of glucose is disfavored because it is quite sterically hindered.
Furanose rings, like pyranose rings, are not planar. They can be puckered so that four atoms are nearly coplanar and the
fifth is about 0.5 Å away from this plane (Figure 11.8). This conformation is called an envelope form because the
structure resembles an opened envelope with the back flap raised. In the ribose moiety of most biomolecules, either C-2
or C-3 is out of the plane on the same side as C-5. These conformations are called C
2
-endo and C
3
-endo, respectively.
11.1.3. Monosaccharides Are Joined to Alcohols and Amines Through Glycosidic Bonds
Monosaccharides can be modified by reaction with alcohols and amines to form adducts. For example,
d-glucose will
react with methanol in an acid-catalyzed process: the anomeric carbon atom reacts with the hydroxyl group of methanol
to form two products, methyl α -
d-glucopyranoside and methyl β -d-glucopyranoside. These two glucopyranosides differ
in the configuration at the anomeric carbon atom. The new bond formed between the anomeric carbon atom of glucose
and the hydroxyl oxygen atom of methanol is called a glycosidic bond specifically, an O-glycosidic bond. The
anomeric carbon atom of a sugar can be linked to the nitrogen atom of an amine to form an N-glycosidic bond.
Indeed, we have previously encountered such reaction products; nu-cleosides are adducts between sugars such as ribose
and amines such as adenine (Section 5.1.1). Some other important modified sugars are shown in Figure 11.9.
Compounds such as methyl glucopyranoside show differences in reactivity from that of the parent monosaccharide. For
example, unmodified glucose reacts with oxidizing agents such as cupric ion (Cu
2+
) because the open-chain form has a
free aldehyde group that is readily oxidized.
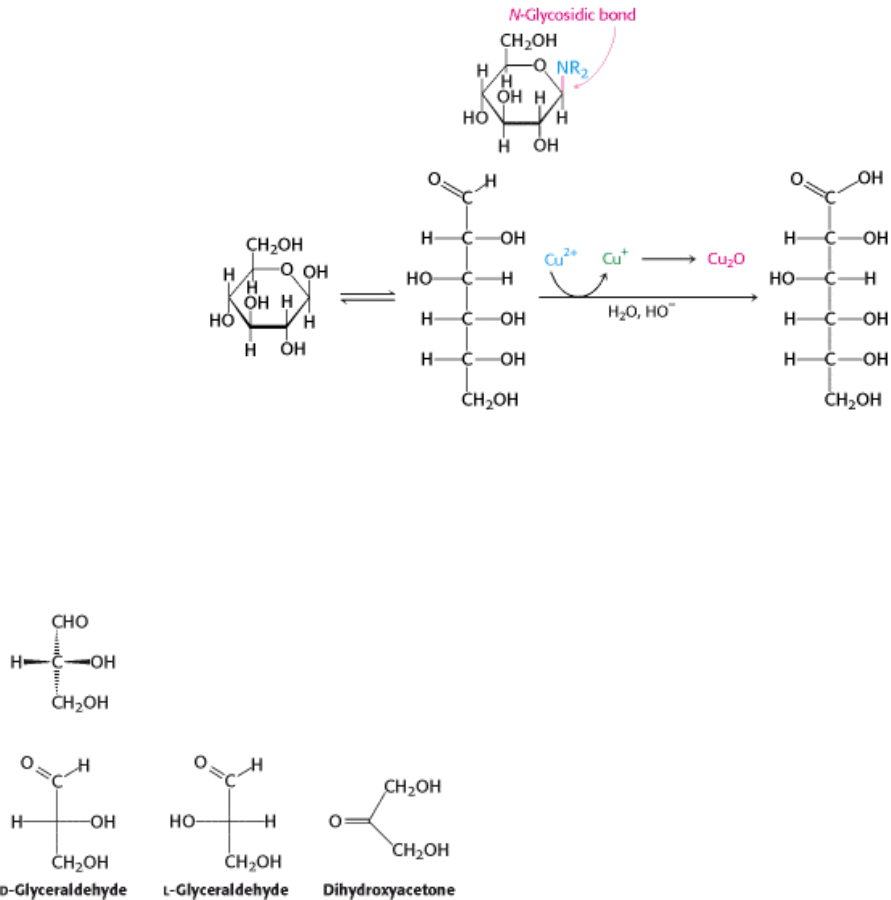
Glycosides such as methyl glucopyranoside do not react, because they are not readily interconverted with a form that
includes a free aldehyde group. Solutions of cupric ion (known as Fehling's solution) provide a simple test for sugars
such as glucose. Sugars that react are called reducing sugars; those that do not are called nonreducing sugars.
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
Figure 11.1. Fischer Projections of Trioses. The top structure reveals the stereochemical relations assumed for Fischer
projections.
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
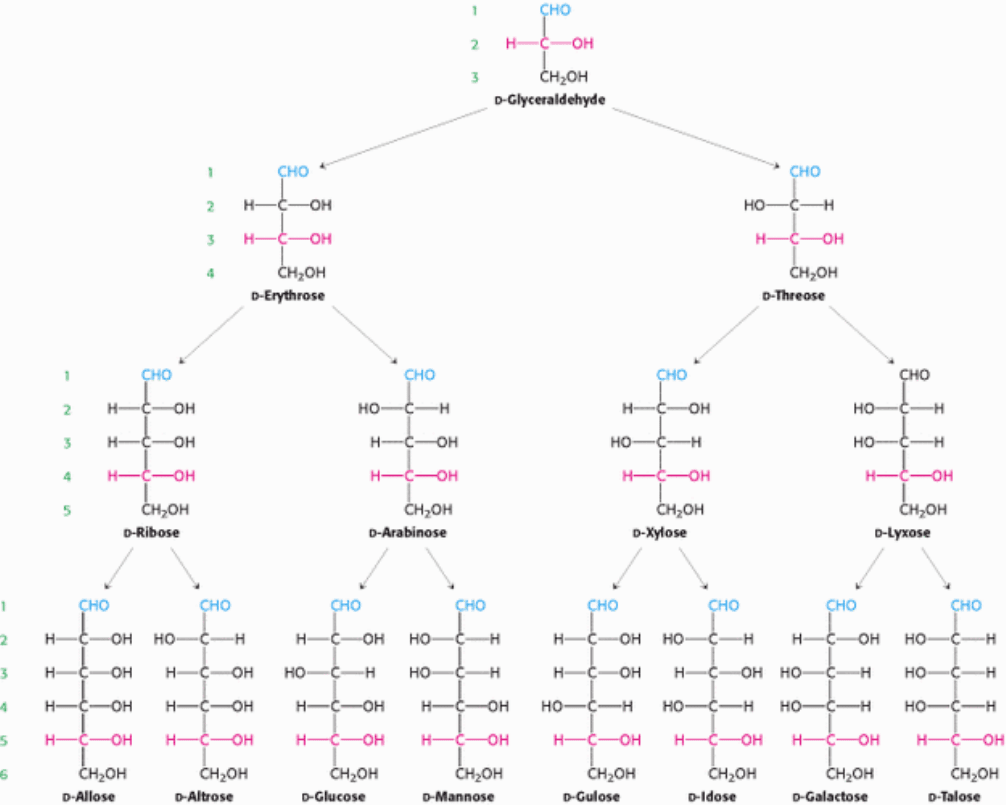
Figure 11.2.
d-Aldoses containing three, four, five, and six carbon atoms. d-Aldoses contain an aldehyde group
(shown in blue) and have the absolute configuration of d-glyceraldehyde at the asymmetric center (shown in red) farthest
from the aldehyde group. The numbers indicate the standard designations for each carbon atom.
I. The Molecular Design of Life 11. Carbohydrates 11.1. Monosaccharides Are Aldehydes or Ketones with Multiple Hydroxyl Groups
