Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.


© 1999 by CRC Press LLC
useful in tribology since you are often dealing with rough, inhomogeneous surfaces. We show a sample
SAM map in Figure 3.19.
Depth profiling is the process of sputter-eroding a sample by bombarding the surface with ions while
simultaneously obtaining AES or other spectra. This enables one to obtain the composition of reaction-
formed or deposited films on a surface as a function of sputter time or depth. Consequently, AES has
many applications for studying tribological and other surfaces. Some examples will be given in subsequent
sections.
3.5.2 X-Ray Photoelectron Spectroscopy
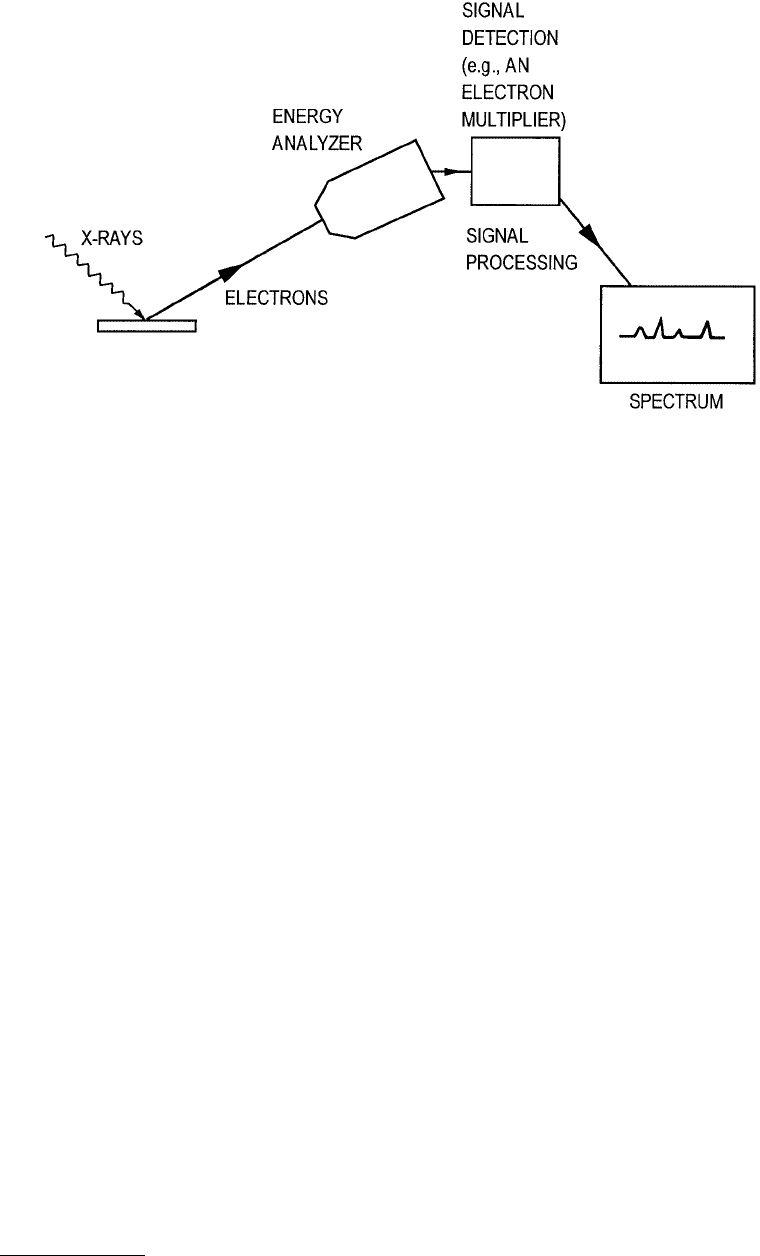
The physical processes involved in XPS are diagrammed in Figure 3.20. XPS is a simpler process than
AES. An X-ray photon ionizes the inner level of an atom and in this case the emitted electron from the
ionization is itself detected, as opposed to AES where several levels are involved in the final electron
production. The dispersion and detection methods are similar to AES.
FIGURE 3.19 Example scanning Auger microscopy results. Sample is silicon carbide fiber-reinforced titanium
aluminide matrix composite. Single element images as labeled, with higher concentrations represented as brighter
regions. (Courtesy of Darwin Boyd).
FIGURE 3.20 XPS transition diagram for an atom. (From Ferrante, J. (1993), in Surface Diagnostics in Tribology
(K. Miyoshi and Y. W. Chung, eds.), World Scientific, Singapore. With permission.)
© 1999 by CRC Press LLC
Monochromatic, incoming X-ray photons are generated from an elemental target such as magnesium
or aluminum. Measurement of the energy distribution of emitted electrons from the sample permits the
identification of the ionized levels by the simple relation
(3.15)
Since the final energy is measured and the X-ray energy is known, one can determine the binding
energy and consequently the material. AES peaks are also present in the XPS spectrum. AES peaks can
be distinguished from the fact that the energies of the Auger electrons are fixed because they depend on
a difference in energy levels, whereas the XPS electron energies depend on the energy of the incident
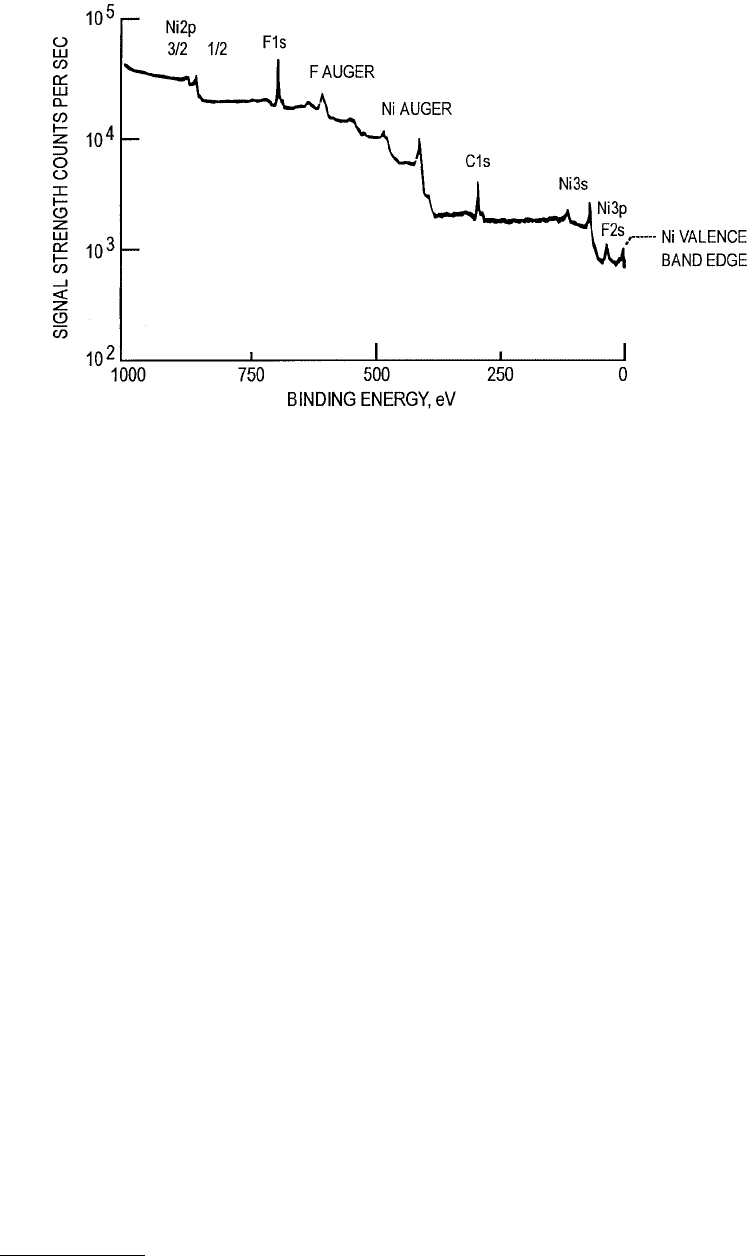
X ray. A sample XPS spectrum is shown in Figure 3.21 and a schematic diagram of the apparatus is shown
in Figure 3.22.
XPS can perform chemical as well as elemental analysis. As stated earlier, when an element is in a
compound, there is a shift in energy levels relative to the unreacted element. Unlike AES, where energy
level differences are detected, a chemical reaction results in an energy shift of the element XPS peak. For
example, the iron peak from Fe
2
O
3
is shifted by nearly 4 eV from an elemental iron peak. Not only are
the shifts simpler to interpret, but it is easier to detect peaks directly (as opposed to AES derivative mode
measurements) since the signal-to-background in XPS is greater than in AES. In addition, the mode of
operation in the dispersion step typically enables higher resolution. The surface sensitivity of XPS is
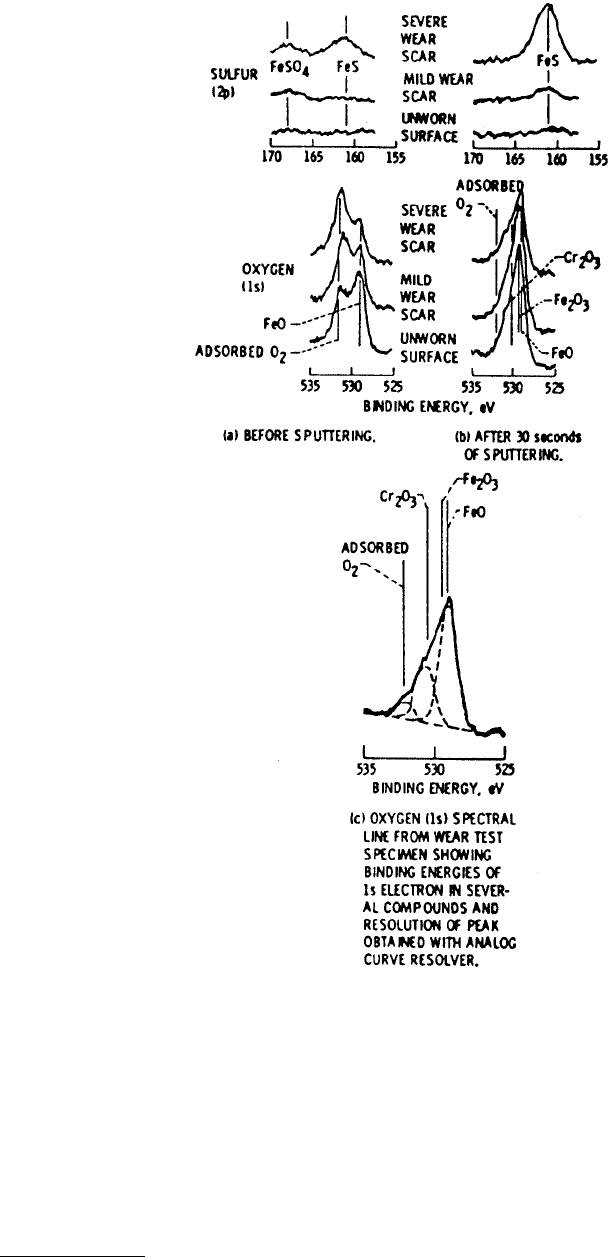
similar to AES because the energies of the emitted electrons are similar. Figure 3.23 shows some examples
of oxygen and sulfur peak shifts resulting from reactions with iron and chromium for wear scars on a
steel pin run with dibenzyl-disulfide as the lubricant additive (Wheeler, 1978).
3.5.3 Secondary Ion Mass Spectroscopy
The physical process involved in SIMS differs from both AES and XPS in that both the excitation source
and detected quantity are ions. Rather than illuminate the sample surface with either electrons (AES) or
photons (XPS), ions are used to bombard the sample surface and knock off (sputter) surface particles.
The dispersion phase analyzes the emitted particle masses, instead of energy analyzing the emitted
electrons as in AES or XPS. Although using sputtering implies an erosion of the sample surface, a
FIGURE 3.21 Example XPS spectrum. (From Ferrante, J. (1993), in Surface Diagnostics in Tribology (K. Miyoshi
and Y. W. Chung, eds.), World Scientific, Singapore. With permission.)
EEE
final xray binding energy
=−
© 1999 by CRC Press LLC
compensating advantage for SIMS is extreme sensitivity. Under advantageous conditions, as few as
10
12
atoms per cm
3
(ppb) have been detected (Gnaser, 1997), with more typical sensitivities for most
elements in the ppm range (Wilson et al., 1989). A comprehensive discussion of the SIMS technique has
been published by Benninghoven et al. (1987).
The SIMS technique typically used in surface studies gives partial monolayer sensitivity using small
incident ion currents (“static” SIMS). Higher ion beam currents, often rastered, give species information
as a function of sputter depth (“dynamic” SIMS or SIMS depth profiling). SIMS instrumentation can be
roughly categorized by the type of ion detector used, e.g., quadrupole, magnetic sector, or time-of-flight,
with their inherent differences in sensitivity and lateral and mass resolution. As well, the incident angle,
energy, and type (e.g., noble gas, cesium, or oxygen) of the primary ion sputtering beam employed can
greatly affect the magnitude and character of the secondary ion yield.
SIMS has several complexities. SIMS only detects secondary ions, rather than all of the sputtered
species, which can lead to difficulty in quantification. Large molecules on the surface such as hydrocarbon
lubricants or typical additives can exhibit complex patterns of possible fragments. A knowledge of the
adsorbate and cracking patterns is often needed for interpretation. As well, multiply ionized fragments
or simply different species may overlap in the spectra, having nearly identical charge-to-mass ratios. As
a simple example, carbon monoxide (CO) and diatomic nitrogen (N
2
) overlap, requiring examination
of other mass fragments to distinguish between the two. As with depth profiling for either AES or XPS,
depth resolution “smearing” can occur either due to ion beam mixing of near-surface species or due to
the development of surface topography after long times under the ion beam. Despite these potential
limitations, SIMS should remain the technique of choice for many low detection limit, high surface
sensitivity studies (Zalm, 1995).
3.5.4 Infrared Spectroscopy
IRS is particularly useful in detecting lubricant films on surfaces. It can provide binding and chemical
information for adsorbed large molecules. It has an additional advantage in that it is nondestructive.
Incident electrons in AES can cause desorption and decomposition even for aluminum oxide, and can
be very destructive for polymers. Similarly, the emitted electrons can cause destruction of some films for
both AES and XPS. In IRS, the specimen is illuminated with infrared light of well-defined energy. If the
FIGURE 3.22 Schematic diagram of XPS apparatus. (From Ferrante, J. (1993), in Surface Diagnostics in Tribology
(K. Miyoshi and Y. W. Chung, eds.), World Scientific, Singapore. With permission.)
© 1999 by CRC Press LLC
energy of the incident light corresponds to a transition between vibrational energy levels in the specimen,
the light can be absorbed. When compared to the reference light beam that has not passed through a
sample, the infrared light interacting with the sample will appear at reduced intensity at these vibrational
excitation energies. The dispersion step is similar to dispersion in photon spectroscopy in that a grating
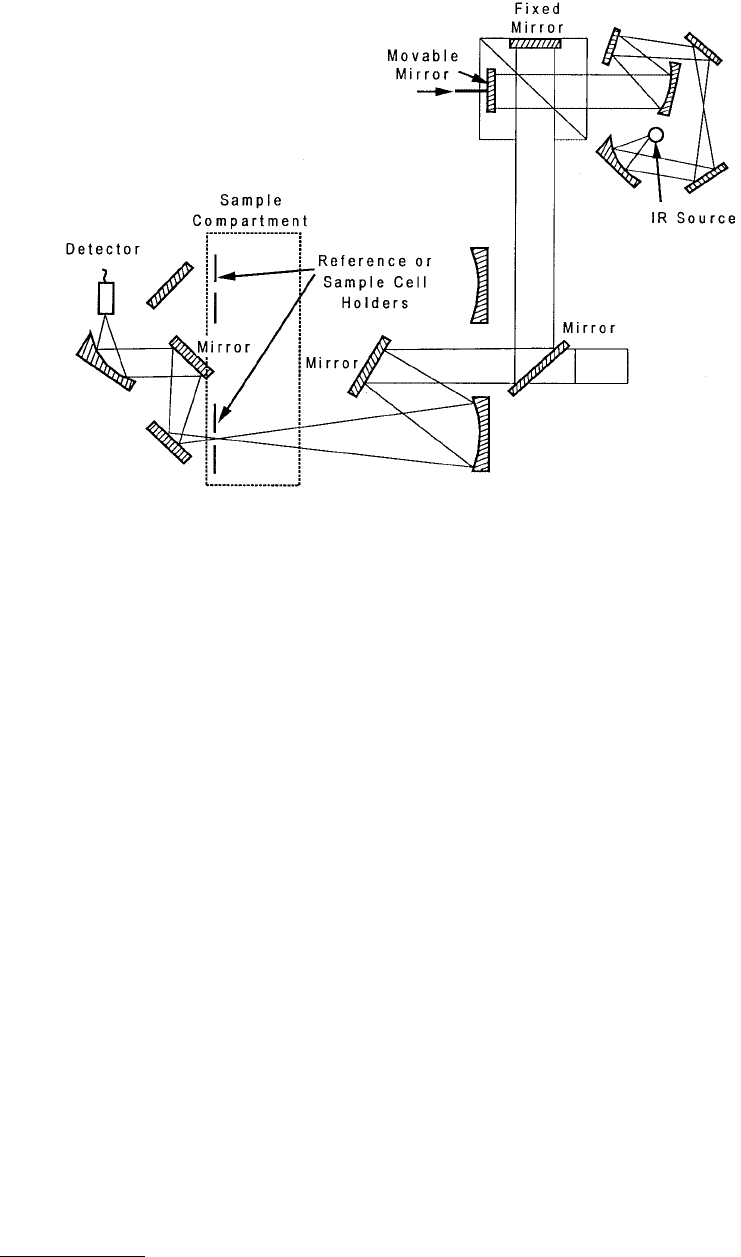
or prism is used to isolate the wavelengths of interest. A variation of IRS which has advantages in sensitivity
and resolution is called Fourier transform infrared spectroscopy (FTIR). In FTIR, the incident beams
are passed through a Michelson interferometer in which one of the paths is modulated by moving a
FIGURE 3.23 Sulfur 2p and oxygen 1s XPS peaks from unworn steel surfaces and wear scars run in mineral oil
with 1% dibenzyl disulfide. (From Wheeler, D. R. (1978), Wear 47, 243–254. With permission.)
© 1999 by CRC Press LLC
mirror. As before, one of the modulated beams is passed through the sample and impinges directly on
the detector, which is a heat-sensitive device. When nonmonochromatic radiation is used, the Fourier
transform of the spatially modulated beam contains all of the information in one signal, as opposed to
the dispersion method where each beam must be analyzed separately. A schematic diagram of the
equipment is shown in Figure 3.24.
IRS is surface sensitive and has been used in a diverse range of analytical science applications (McKelvy
et al., 1996), which we will not address. A primary interest in tribology is the detection of hydrocarbon
or additive films on surfaces. AES and XPS, for contrast, would primarily be useful in detecting elemental
species and are limited to use in ultrahigh vacuum. IRS can be used in air as well as vacuum. The surface
sensitivity of IRS can be enhanced by multiple reflections in the surface films and by using grazing
incidence angles. Orientation of adsorbed molecules can be obtained by examining the polarization
dependence of the spectrum. There are selection rules for what materials can be detected depending on
whether the molecule has a dipole moment and the orientation of the molecule on a metal surface.
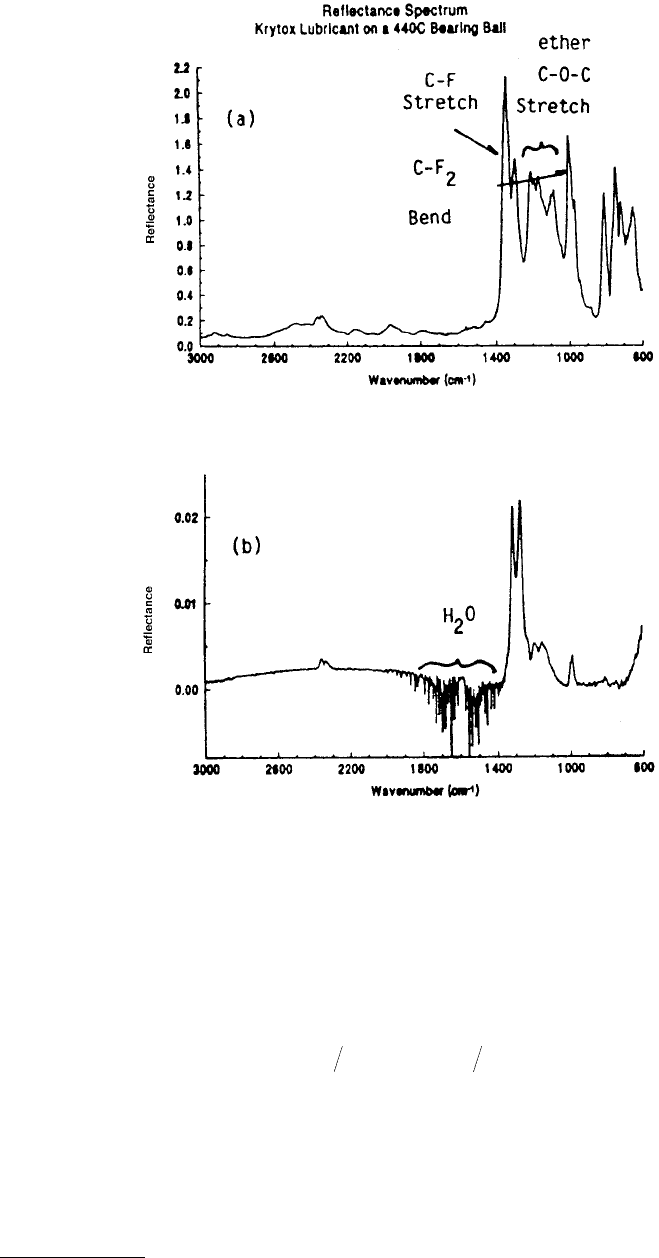
Sample spectra for micron thick and less than 200 Å thick Krytox films on a metal substrate are shown
in Figure 3.25 (Herrera-Fierro, 1993).
3.5.5 Thermal Desorption
We mention briefly at this point another useful tool for examining the behavior of adsorbates on surfaces,
thermal desorption spectroscopy (TDS). We give only a brief description and for a more complete
treatment refer the readers to Zangwill (1988). The methods described so far give little information
concerning binding energies of adsorbates to surfaces, a topic of importance when choosing stable
lubricants or additives. Many species adsorb strongly but do not react chemically, e.g., by forming an
oxide. When the surface is heated they can be removed intact or in some decomposed form. A simple
view would be that the binding energy would resemble the curves presented in Figure 3.12, and the
adsorbates could be removed by giving them sufficient energy to overcome the energy well depth. This
would be accomplished by heating a sample following adsorption and then either observing the surface
FIGURE 3.24 Optical arrangement for a FTIR spectrometer. (From Ferrante, J. (1993), in Surface Diagnostics in
Tribology (K. Miyoshi and Y. W. Chung, eds.), World Scientific, Singapore. With permission.)
© 1999 by CRC Press LLC
coverage via AES or monitoring pressure increases in the vacuum system. There can be a variety of
bonding states depending on the structure of the surface; e.g., at a step edge one would expect a different
binding energy from a surface site. Although the real situation may be quite complex, we describe the
simplest case, a single bonding state, and no decomposition. The rate of desorption can be described by
(Meyers et al., 1996)
(3.16)
where θ is the coverage, ν is the pre-exponential frequency factor, E
d
is the desorption energy, T is the
temperature, and k is the Boltzmann constant. By heating to various temperatures and performing an
Arrhenius plot, one can extract E
d
and determine the strength of bonding to a surface. This technique
will be seen to be useful in studying monolayer and submonolayer adsorbed film effects on friction
between otherwise clean metal surfaces.
FIGURE 3.25 IR spectrum of a krytox film on a 440C bearing showing characteristic absorption corresponding to
certain functional groups: (a) micron-thick film; (b) less than 200-Å-thick film, note the increased sensitivity and
the bend from absorbed water from 1400–2000 cm
–1
. (Courtesy of Herrera-Fierro, 1993).
r d dt E kT
d
== −
()
θθνexp

© 1999 by CRC Press LLC
3.6 Surface Effects in Tribology
In this section we will deal with a number of issues, analyzing evidence first for effects at the atomic
monolayer or submonolayer level in tribology. We will examine monolayer effects both from a funda-
mental standpoint as well as from a more practical viewpoint. This will not be a comprehensive review
of the literature, but in keeping with the objectives of this chapter, again will be didactic in nature. The
references selected, however, will have references to the relevant literature. In this chapter we are not
concerned with the effects of lubricants other than their effects of changing shear strength or adhesion
at the interface. Consequently, we are interested in issues involving boundary lubrication and interfacial
properties (Ferrante and Pepper, 1989; Gellman 1992; Carpick and Salmeron, 1997).
There are a number of issues to address which emphasize the difficulties involved in answering
fundamental questions concerning bonding (Ferrante and Pepper, 1989). One clear difficulty is the fact
that one cannot observe the interface during the interaction. It is necessary to infer what happened at
the interface by examining the states of the surfaces before and after interaction to the extent analytical
techniques can determine. There are often situations where the locus of failure is not the interface, e.g.,
shear or adhesive failure can occur in the bulk of one of the materials rather than at the interface, or
both effects can occur depending on the region in contact. There are uncertainties regarding the mea-
surement of forces, although some recent efforts nicely relate lateral force sensitivity to normal force
sensitivity for a commercial scanning probe microscope (SPM) cantilever beam, i.e., for a single asperity
contact (Ogletree et al., 1996). Clearly, there are elastic effects in any measuring apparatus and in the
materials involved that make measurement of the force distribution at the interface difficult. Materials
can change mechanical properties as a result of the forces applied. For example, such properties as
hardness, ductility, defect formation, plasticity, strain hardening, and creep must be considered. Surface
properties may be altered just by contact with the counterface material (Carpick et al., 1996). Generally,
even the true area of contact is not known in macroscopic studies due to the fact that asperities determine
the contact area on most practical materials.
There is a great deal yet to be learned concerning the basic interfacial properties in tribology. This is
in part due to the complexities involved. As an example of such complexities, in Table 3.1 we show some
results of Buckley and Pepper (1971) for metallic transfer of dissimilar metals in sliding contact performed
using a pin-on-disk apparatus in an ultrahigh vacuum system with AES analysis in the wear track.
Both pin and disk specimens were ion sputter cleaned. As we can see all metals transferred to tungsten,
and cobalt transferred in all cases. However, iron and nickel did not transfer to tantalum, molybdenum,
or niobium. The surprising result is that the softer metals did not transfer to the harder in all cases.
Pepper explained these results in terms of the mechanical properties of the materials. Tungsten which is
the hardest of the materials fits the expected pattern. However, since nickel and iron strain harden, transfer
TABLE 3.1 Metallic Transfer for Dissimilar
Metals in Sliding Contact
Transfer of Metal
Disk Rider from Rider to Disk
Tungsten Iron Yes
Nickel Yes
Cobalt Yes
Tantalum Iron No
Nickel No
Cobalt Yes
Molybdenum Iron No
Nickel No
Cobalt Yes
Niobium Iron No
Nickel No
Cobalt Yes

© 1999 by CRC Press LLC
and deformation are minimized. Cobalt, which has a hexagonal close-packed structure, has easy slip
planes and thus transferred in all cases. Thus, simple explanations based on cohesive and interfacial
energies can be misleading if mechanical properties are not taken into account.
We give a second example by Pepper (1974) demonstrating the care necessary in performing studies
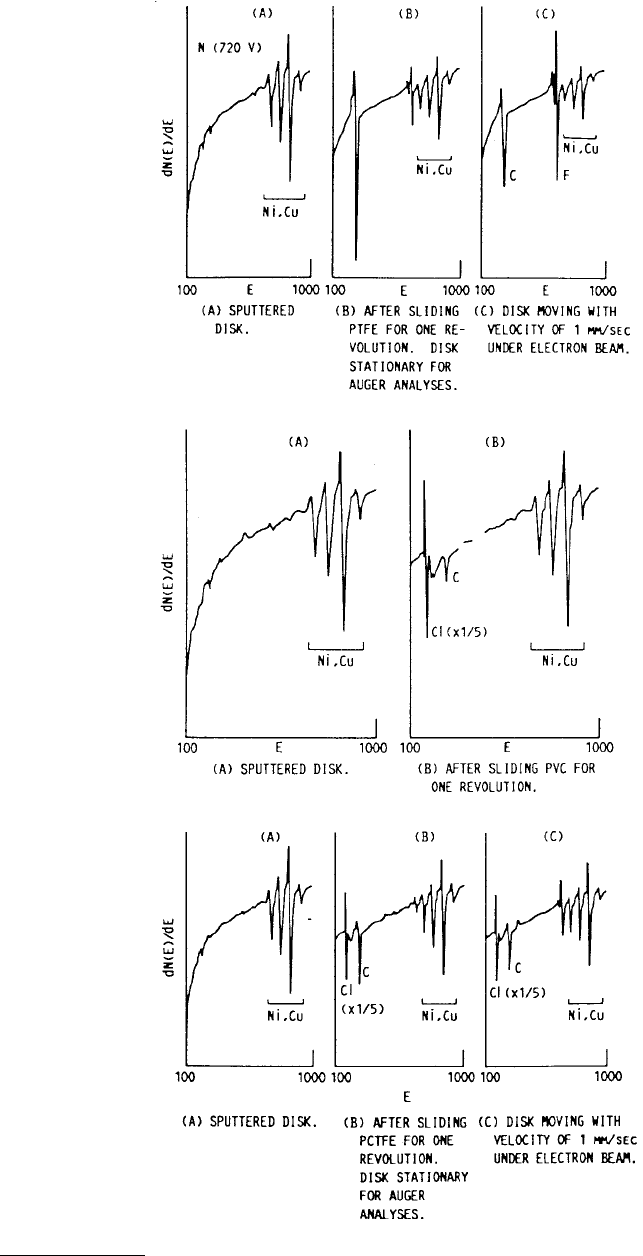
of polymer films transferred from polymer pins sliding on a S-Monel disk in ultrahigh vacuum.
Figure 3.26 shows the AES spectra for polytetrafluoroethylene (PTFE), polyvinyl chloride (PVC) and
polychlorotrifluoroethylene (PCTFE) pins sliding on S-Monel. The PTFE spectrum shows large fluorine
and carbon peaks and large attenuation of the metal peaks (care had to be taken due to electron
bombardment desorption of the fluorine). The friction coefficient was low and smooth suggesting slip.
The combined results indicated that PTFE strands were transferring to the metal surface consistent with
the models of Pooley and Tabor (1972). For PVC the AES spectrum shows a large chlorine peak and
small attenuation of the metal peaks suggesting decomposition and chlorine adsorption rather than
polymer transfer. The friction coefficient, although reduced, remained large and exhibited some stick
slip. For PCTFE the spectrum shows chlorine, carbon, and intermediate attenuation of the metal peaks,
but with stability under electron bombardment suggesting the possibility of both decomposition and
some polymer transfer. The friction coefficient was high with stick slip. Consequently, it is difficult to
anticipate what is happening, again demonstrating the need for more extensive surface characterization.
3.6.1 Monolayer Effects in Adhesion and Friction
The bulk of the discussion in this section will be based on the recent high-quality experiments of Gellman
and collaborators (Gellman, 1992; McFadden and Gellman, 1995a,b), while we acknowledge the pio-
neering contributions of Buckley and the members of his group (Buckley, 1981). Gellman has performed
a number of adhesion and friction experiments on single crystals in contact, both for clean and adsorbate
covered interfaces, namely, Cu(111)–Cu(111) and Ni(100)–Ni(100). The apparatus for the friction and
adhesion experiments is shown in Figure 3.27 (Gellman, 1992).
The contacting crystals had a slight curvature in order to prevent contact at the edges of the samples
where large concentrations of steps were expected, and to ensure point contact. The vacuum system was
also vibration isolated which is very important for reasons to be discussed below. Normally, the experi-
ments were repeated for ten different points. The normals of the crystals were aligned by lasers. The
apparatus provided the ability to sputter clean both contacting surfaces, and LEED and AES could be
performed on the surfaces in order to guarantee the crystallinity and to measure contamination and
determine concentration of adsorbates. The AES measurements were performed with LEED optics in
the old retarding field analyzer mode. Following sputter cleaning, the samples were annealed and analyzed
with LEED in order to verify the crystal structure and with AES to verify cleanliness.
On Cu(111) McFadden and Gellman (1995a) examined the effects of sulfur adsorption, since sulfur
is a common antiwear additive. It is often used along with phosphorous in order to prevent metal to
metal contact when the lubricant breaks down. Both surfaces were dosed with sulfur using hydrogen
sulfide, which decomposes upon heating, desorbing the hydrogen and leaving sulfur behind on the
surface. At saturation, sulfur forms an ordered superlattice which has a × R19 degree LEED
pattern. This corresponds to a close-packed monolayer of sulfide ions (S
2–
) on top of the Cu(111) lattice
with an absolute coverage of 0.43 monolayers relative to the Cu(111) substrate. The results of the adhesion
experiments are shown in Figure 3.28.
The adhesion coefficient, µ
ad
= F
ad
/F
N
, which is the ratio of pull-off force to normal force was found
to be 0.69 ± 0.21. As little as 0.05 monolayers (11% of saturation) gave a substantial percentage reduction.
The saturation value at one monolayer was 0.26 ± 0.07. They also found that there was no dependence
of the adhesion coefficient on contact time, separation time, temperature, or normal force. There would
be no expected dependence on normal force since higher loads would simply increase the contact area,
and the adhesion coefficient, therefore, would be expected to track with the normal force. Buckley (1981)
reported adhesion coefficients much greater than 1.0 for clean single crystals in contact. As mentioned
earlier, it is important to control vibrations in the experiments. Bowden and Tabor (1964) showed that
7
7
© 1999 by CRC Press LLC

© 1999 by CRC Press LLC
when clean metal surfaces were plastically loaded and then translated, the junction grew, i.e., the true
contact area increased until shear occurred. In all probability, the large adhesion coefficients observed
by Buckley were caused by increased contact area from junction growth caused by vibrations. In any
event, there is strong bonding at clean metal interfaces and, as Gellman and others have shown, the
adhesion coefficient can be decreased by submonolayer films.
McFadden and Gellman (1995a) performed a somewhat different experiment on the Cu(111) surface
with regard to static friction measurements. The surfaces were exposed to laboratory atmosphere for
several days. The resulting AES spectrum is shown in Figure 3.29. As can be seen, the primary contam-
inants were sulfur and carbon. Although tenuous, they estimated the coverage to be between 10 and 15 Å
thick. They then executed a number of sputtering cycles followed by annealing with static friction
measurements after each cycle. The results are shown in Figure 3.30. The friction coefficient, µ
s
, increased
with removal of contaminant to their clean surface value of 4.4 ± 1.3. Again these results imply sub-
monolayer effects by these contaminants.
Wheeler (1976) earlier performed static friction experiments with both chlorine and oxygen adsorbed
on polycrystalline surfaces of copper, iron, and steel. The measurements were performed with a pin-on-
disk apparatus, with AES used to monitor coverage. The results of these studies are shown in Figure 3.31.
Wheeler found that there were no differences between the effects of chlorine and oxygen on any of the
surfaces if adsorbate coverage was taken into account. Adsorption at partial monolayer coverages reduced
the coefficient of friction in all cases. Wheeler found that he could get a good correlation with a junction
growth model where the surfaces were partially covered during translation. The values of the coefficient
were of comparable magnitude to those of Gellman.
3.6.2 Atomic Effects Due to Adsorption of Hydrocarbons
Our primary objective in this and following sections is to explore the evidence for atomic effects on
friction. Again, we start by referring to recent publications by Gellman et al. (Gellman, 1992; McFadden
and Gellman, 1995a,b; Meyers et al., 1996) describing a number of friction experiments performed with
hydrocarbon adsorbates. First, we present the effects of ethanol adsorption on a sulfur-covered Ni(100)
surface. The sulfur was adsorbed as previously described and produced a c 2 × 2 structure on the nickel
surface. The sulfur overlayer was used since stable results could not be obtained with ethanol alone,
which Gellman found desorbed at very low temperatures. There were a number of different friction states
depending on ethanol coverage, shown in Figure 3.32 (Gellman, 1992).
These are the classic behaviors observed in friction (Buckley, 1981). The first shown is “slip,” where
once a certain transverse force is applied there is simple sliding. The second is “stick-slip,” where rebonding
occurs and the simple slip process is repeated between rebonding events, with poor definition of a friction
coefficient. This behavior probably results from junction growth with the adsorbate only effective in
certain regions, as observed by Wheeler (1976). Finally, “stick” is where the surfaces weld and it is similar
to shearing a bulk solid. The results of these studies are shown in Figure 3.33 where the key indicates
differences in behavior.
Unlike the results for Cu(111) the sulfur coating did not reduce µ
s
and gave stick behavior. Partial
coverages of ethanol gave erratic behavior typically exhibiting stick-slip. Finally at one monolayer the
ethanol effectively lubricated the surface giving the desired slip as well as low adhesion. Consequently, a
monolayer film can effectively lubricate. Similar behavior was observed for 2,2,2-trifluoroethanol
adsorbed on clean Cu(111) surfaces (McFadden and Gellman, 1995b). Again stick-slip was observed for
coverages less than a monolayer and slip for coverages greater than or equal to one monolayer. Similarly,
high adhesion was observed for low coverages and low adhesion for higher coverages.
FIGURE 3.26 AES spectra from an S-Monel disk with transfer films from different polymers. (From Pepper, S. V.
(1974), J. Appl. Phys. 45, 2947–2956. With permission.)
