Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.

© 1999 by CRC Press LLC
Finally, we will examine the relationship of tribological experiments to these more fundamental
atomistic considerations. The primary goals of this section will be to again provide sources for further
study of tribological experiments and to raise critical issues concerning the relationship between basic
surface properties with regard to tribology and the ability of certain classes of experiments to reveal the
underlying interactions. We will attempt to avoid overlapping the material that we present with that
presented by other authors in this publication. This chapter cannot be a complete treatment of the physics
of surfaces due to space limitations. We recommend an excellent text by Zangwill (1988) for a more
thorough treatment. Instead, we concentrate on techniques and issues of importance to tribology on the
nanoscale.
3.2 Geometry of Surfaces
We will now discuss simply from a geometric standpoint what occurs when you create two surfaces by
dividing a solid along a given plane. We limit the discussion to single crystals, since the same arguments
apply to polycrystalline samples except for the existence of many grains, each of which could be described
by a corresponding argument. This discussion will start by introducing the standard notation for describ-
ing crystals given in many solid-state texts (Ashcroft and Mermin, 1976; Kittel, 1986). It is meant to be
didactic in nature and because of length limitations will not attempt to be comprehensive. To establish
notation and concepts we will limit our discussion to two of the possible Bravais lattices, face-centered
cubic (fcc) and body-centered cubic (bcc), which are the structures often found in metals. The unit cells,
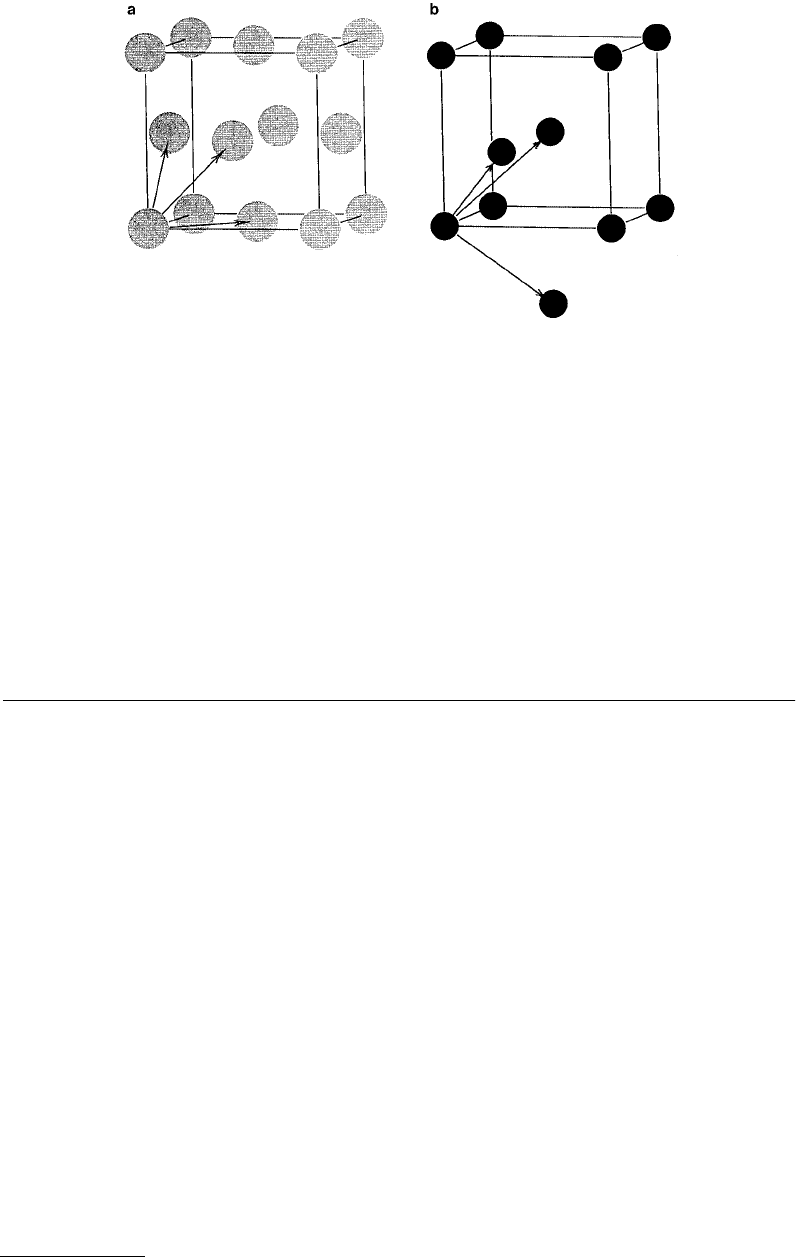
i.e., the structures which most easily display the symmetries of the crystals, are shown in Figure 3.1. The
other descriptions that are frequently used are the primitive cells, which show the simplest structures
that can be repeated to create a given structure. In Figure 3.1 we also show the primitive cell basis vectors,
which can be used to generate the entire structure by the relation
(3.1)
where
n
1
,
n
2
, and
n
3
are integers, and
→
a
1
,
→
a
2
, and
→
a
3
are the unit basis vectors.
Since we are interested in describing surface properties, we want to present the standard nomenclature
for specifying a surface. The algebraic description of a surface is usually given in terms of a vector normal
to the surface. This is conveniently accomplished in terms of vectors that arise naturally in solids, namely,
the reciprocal lattice vectors of the Bravais lattice (Ashcroft and Mermin, 1976; Kittel, 1986). This is
FIGURE 3.1
(a) Unit cube of fcc crystal structure with primative cell basis vectors indicated. (b) Unit cube of bcc
crystal structure, with primative cell basis vectors indicated.
r
rr r
Rnana na=+ +
12233
© 1999 by CRC Press LLC
convenient since these vectors are used to describe the band structure and diffraction effects in the solid.
They are usually given in the form
(3.2)
where
h, k,
and
l
are integers. The reciprocal lattice vectors are related to the basis vectors of the direct
lattice by
(3.3)
where a cyclic permutation of
i, j, k
are used in the definition. Typically, parentheses are used in the
definition of the plane, e.g., (
h,k,l
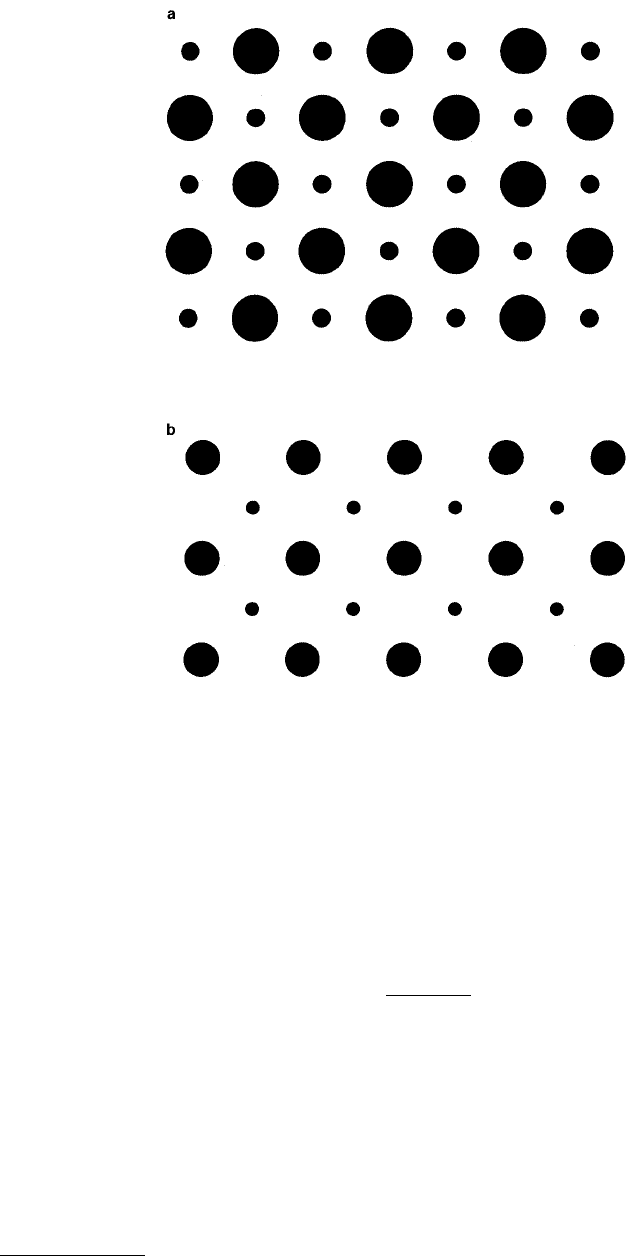
). The (100) planes for fcc and bcc lattices are shown in Figure 3.2
where dots are used to show the location of the atoms in the next plane down.
This provides the simplest description of the surface in terms of terminating the bulk. There is a rather
nice NASA publication by Bacigalupi (1964) which gives diagrams of many surfaces and subsurface
structures for fcc, bcc, and diamond lattices, in addition to a great deal of other useful information such
FIGURE 3.2
Projection of cubic face (100) plane for (a) fcc and (b) bcc crystal structures. In both cases, smaller
dots represent atomic positions in the next layer below the surface.
r
rrr
Khbkb lb=++
123
r
rr
rr r
b
aa
aa a
i
jk
=π
×
×
()
2
12 3
© 1999 by CRC Press LLC
as surface density and interplanar spacings. A modern reprinting of this NASA publication is called for.
In many cases, this simple description is not adequate since the surface can reconstruct. The two most
prominent cases of surface reconstruction are the Au(110) surface (Good and Banerjea, 1992) for metals
and the Si(111) surface (Zangwill, 1988) for semiconductors. In addition, adsorbates often form structures
with symmetries different from the substrate, with the classic example the adsorption of oxygen on
W(110) (Zangwill, 1988). Wood (1963) in a classic publication gives the nomenclature for describing
such structures. In Figure 3.3 we show an example of 2
×
2 structure, where the terminology describes
a surface that has a layer with twice the spacings of the substrate. There are many other possibilities, such
as structures rotated with respect to the substrate and centered differently from the substrate. These are
also defined by Wood (1963).
The next consideration is that the interplanar spacing can vary, and slight shifts in atomic positions
can occur several planes from the free surface. A recent paper by Bozzolo et al. (1994) presents the results
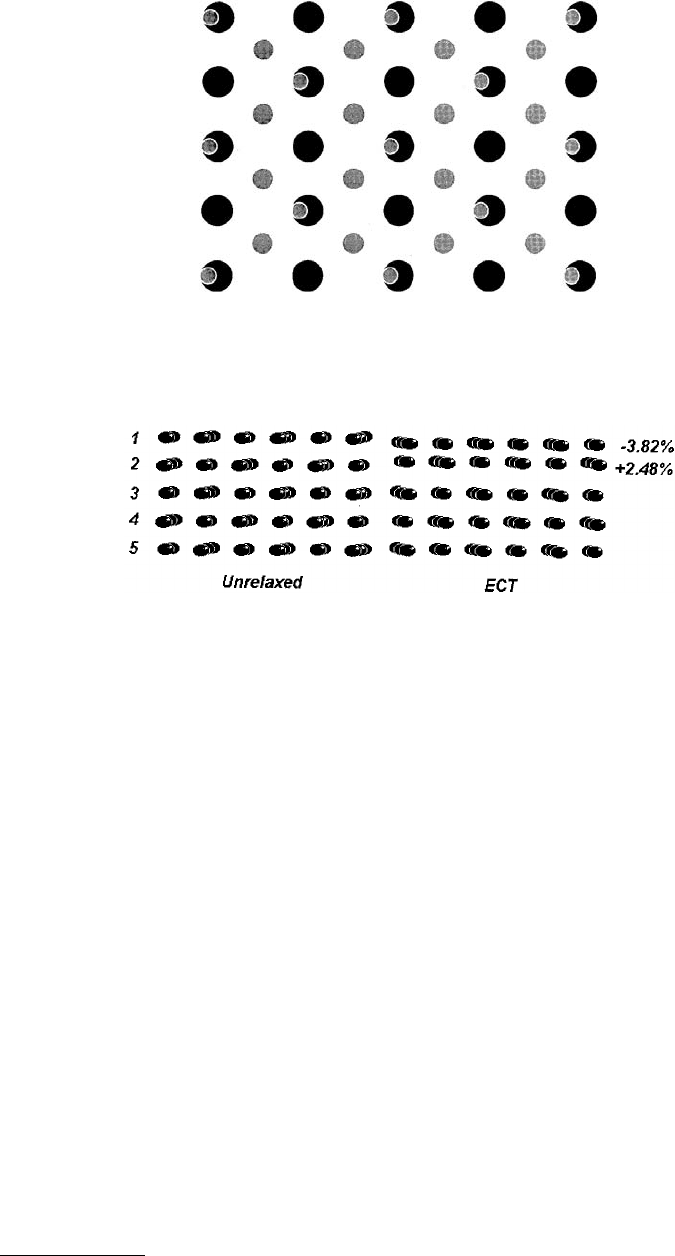
for a large number of metallic systems and serves as a good review of available publications. Figure 3.4
shows some typical results for Ni(100). The percent change given represents the deviation from the
equilibrium interplanar spacing. The drawing in Figure 3.4 exaggerates these typically small differences
to elucidate the behavior. Typically, this pattern of alternating contraction and expansion diminishing as
the bulk is approached is found in most metals. It can be understood in a simple manner (Bozzolo et al.,
1994). The energy for the bulk metal is a minimum at the bulk metallic density. The formation of the
surface represents a loss of electron density because of the missing neighbors for the surface atoms.
Therefore, this loss of electron density can be partially offset by a contraction of the interplanar spacing
between the first two layers. This construction causes an electron density increase between layers 2 and
3, and thus the energy is lowered by a slight increase in the interplanar spacing. There are some exceptions
FIGURE 3.3
Representation of fcc (110) face with an additional “2
×
2” layer, in which the species above the surface
atoms have twice the spacing of the surface. Atomic positions in the next layer below the surface are presented by
smaller dots.
FIGURE 3.4
Side view of nickel (100) surface. On the left, the atoms are positioned as if still within a bulk fcc
lattice (“unrelaxed”). On the right, the surface planes have been moved to minimize system energy. The percent
change in lattice spacing is indicated, with the spacing in the image exaggerated to illustrate the effect. (From Bozzolo,
G. et al. (1994),
Surf. Sci.
315, 204–214. With permission.)

© 1999 by CRC Press LLC
to this behavior where the interplanar spacing increases between the first two layers due to bonding
effects (Needs, 1987; Feibelman, 1992). However, the pattern shown in Figure 3.4 is the usual behavior
for most metallic surfaces. There can be similar changes in position within the planes; however, these
are usually small effects (Rodriguez et al., 1993; Foiles, 1987). In Figure 3.5, we show a side view of a
gold (110) surface (Good and Banerjea, 1992). Figure 3.5a shows the unreconstructed surface and Figure
3.5b shows a side view of the (2
×
1) missing row reconstruction. Such behavior indicates the complexity
that can arise even for metal surfaces and the danger of using ideas which are too simplistic, since more
details of the bonding interactions are needed in this case and those of Needs (1987) and Feibelman
(1992).
Crystal surfaces encountered typically are not perfectly oriented nor atomically flat. Even “on-axis”
(i.e., within a fraction of a degree) single-crystal low-index faces exhibit some density of crystallographic
steps. For a gold (111) face tilted one half degree toward the (011) direction, evenly spaced single atomic
height steps would be only 27 nm apart. Other surface-breaking crystal defects such as screw and edge
dislocations may also be present, in addition to whatever surface scratches, grooves, and other polishing
damage which remain in a typical single-crystal surface. Surface steps and step kinks would be expected
to show greater reactivity than low-index surface planes. During either deposition or erosion of metal
surfaces, one expects incorporation into or loss from the crystal lattice preferentially at step edges. More
generally on simple metal surfaces, lone atoms on a low-index crystal face are expected to be most mobile
(i.e., have the lowest activation energy to move). Atoms at steps would be somewhat more tightly bound,
and atoms making up a low-index face would be least likely to move. High-index crystal faces can often
be thought of as an ordered collection of steps on a low-index face. When surface species and even
interfaces become mobile, consolidation of steps may be observed. Alternating strips of two low-index
crystal faces can then develop from one high-index crystal plane, with lower total surface energy but
with a rougher, faceted topography. Much theoretical and experimental work has been done over the last
decade on nonequilibrium as well as equilibrium surface morphology (e.g., Redfield and Zangwill, 1992;
Vlachos et al., 1993; Conrad and Engel, 1994; Bartelt et al., 1994; Williams, 1994; Kaxiras, 1996).
Semiconductors and insulators generally behave differently. Unlike most metals for which the electron
gas to some degree can be considered to behave like a fluid, semiconductors have strong directional
bonding. Consequently, the loss of neighbors leaves dangling bonds which are satisfied in ultrahigh
vacuum by reconstruction of the surface. The classic example of this is the silicon (111) 7
×
7 structure,
where rebonding and the creation of surface states gives a complex structure. Until STM provided real-
space images of this reconstruction (Binnig et al., 1983) much speculation surrounded this surface.
Zangwill (1988) shows both the terminated bulk structure of Si(111) and the relaxed 7
×
7 structure. It
is clear that viewing a surface as a simple terminated bulk can lead to severely erroneous conclusions.
The relevance to tribology is clear since the nature of chemical reactions between surfaces, lubricants,
and additives can be greatly affected by such radical surface alterations.
There are other surface chemical state phenomena, even in ultrahigh vacuum, just as important as the
structural and bonding states of the clean surface. Surface segregation often occurs to metal surfaces and
interfaces (Faulkner, 1996, and other reviews cited therein). For example, trace quantities of sulfur often
segregate to iron and steel surfaces or to grain boundaries in polycrystalline samples (Jennings et al.,
1988). This can greatly affect results since sulfur, known to be a strong poisoning contaminant in catalysis,
can affect interfacial bond strength. Sulfur is often a component in many lubricants. For alloys similar
geometric surface reconstructions occur (Kobistek et al., 1994). Again, alloy surface composition can vary
dramatically from the bulk, with segregation causing one of the elements to be the only component on
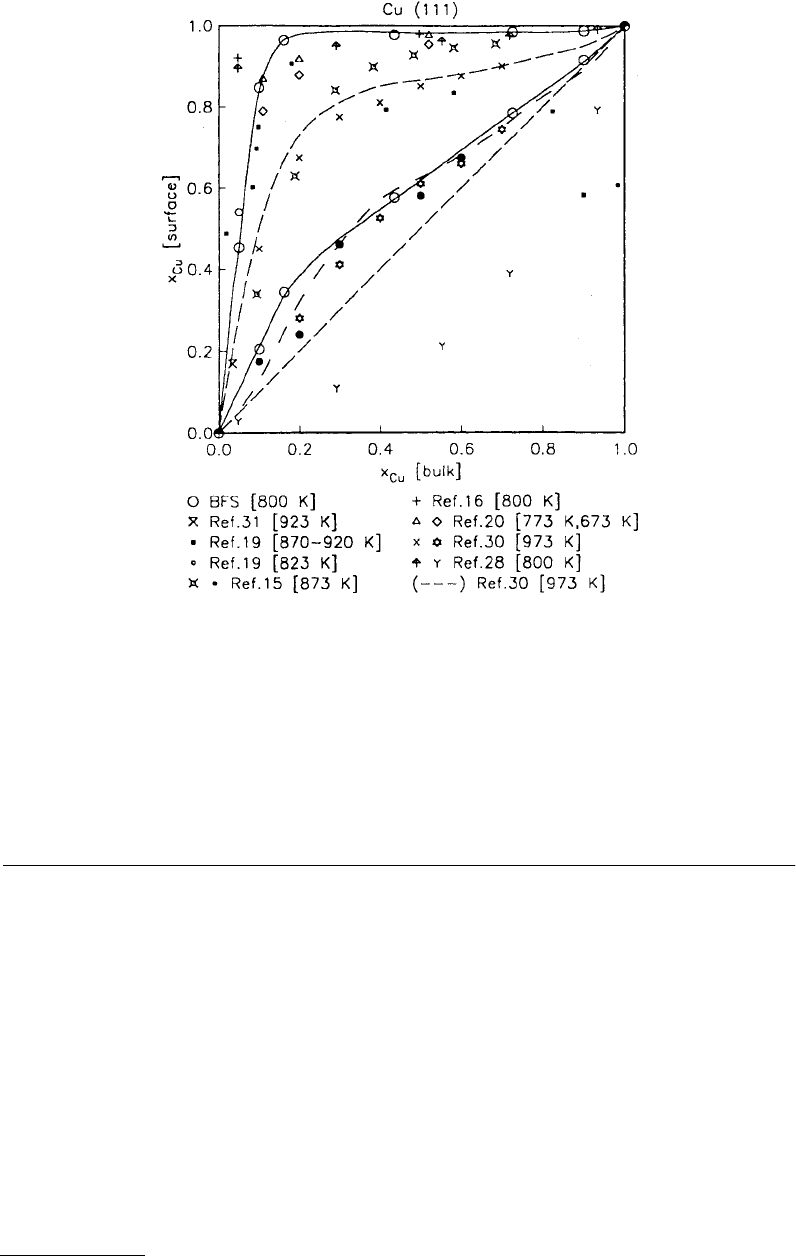
a surface. In Figure 3.6 we show the surface composition for a CuNi alloy as a function of bulk composition
with both a large number of experimental results and some theoretical predictions for the composition
FIGURE 3.5
Side view of gold (110) surface: (a) unrecon-
structed; (b) 1
×
2 missing row surface reconstruction.
(From Good, B. S. and Banerjea, A. (1992),
Mater. Res. Soc.
Symp. Proc.,
278, 211–216. With permission.)
© 1999 by CRC Press LLC
(Good et al., 1993). In addition, nascent surfaces typically react with the ambient, giving monolayer films
and oxidation even in ultrahigh vacuum, producing even more pronounced surface composition effects.
In conclusion, we see that even in the most simple circumstances, i.e., single-crystal surfaces, the situation
can be very complicated.
3.3 Theoretical Considerations
3.3.1 Surface Theory
We have shown how the formation of a surface can affect geometry. We now present some aspects of the
energetics of surfaces from first-principles considerations. For a long time, calculations of the electronic
structure and energetics of the surface had proven to be a difficult task. The nature of theoretical
approximations and the need for high-speed computers limited the problem to some fairly simple
approaches (Ashcroft and Mermin, 1976). The advent of better approximations for the many body effects,
namely, for exchange and correlation, and the improvements in computers have changed this situation
in the not too distant past. One aspect of the improvements was density functional theory and the use
of the local density approximation (LDA) (Kohn and Sham, 1965; Lundqvist and March, 1983). Diffi-
culties arise because in the creation of the surface, periodicity in the direction perpendicular to the surface
is lost. Periodicity simplifies many problems in solid-state theory by limiting the calculation to a single
unit cell with periodic boundary conditions. With a surface present the wave vector perpendicular to the
surface,
→
k
⊥
, is not periodic, although the wave vector parallel to the surface,
→
k
, still is.
FIGURE 3.6
Copper (111) surface composition vs. copper-nickel alloy bulk composition: comparison between the
experimental and theoretical results for the first and second planes. (See Good et al., 1993, and references therein.)
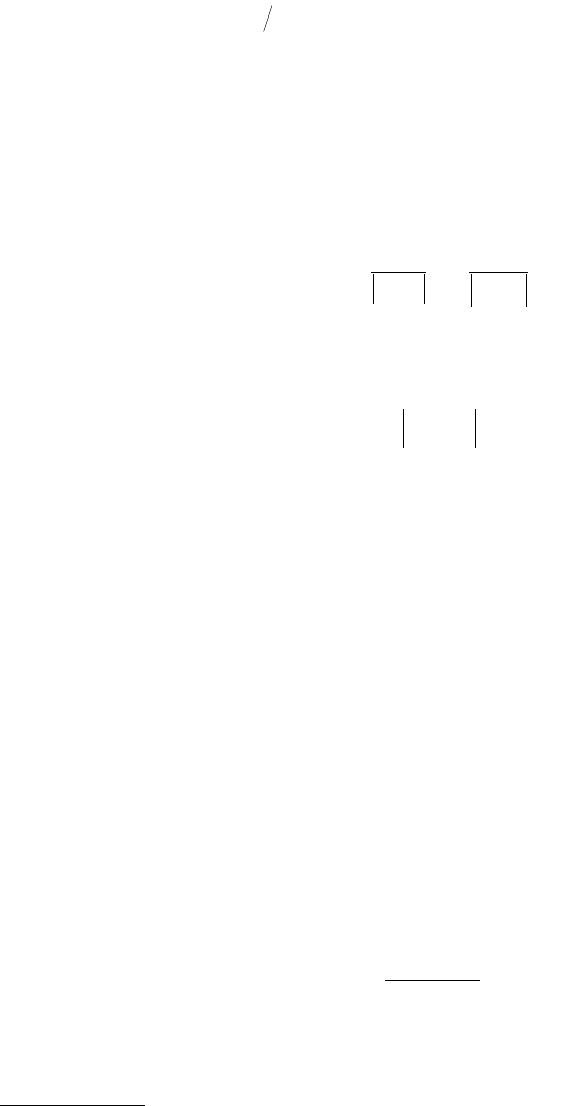
© 1999 by CRC Press LLC
The process usually proceeds by solving the one-electron Kohn–Sham equations (Kohn and Sham,
1965; Lundqvist and March, 1983), where a given electron is treated as though it is in the mean field of
all of the other electrons. The LDA represents the mean field in terms of the local electron density at a
given location. The Kohn–Sham equations are written in the form (using atomic units where the constants
appearing in the Schroedinger equation along with the electron charge and the speed of light,
=
m
e
=
e = c
= 1).
(3.4)
where
Ψ
i
and
ι
are the one-electron wave function and energy, respectively, and
(3.5)
where
V
xc
[
ρ
(
→
r
)] is the exchange and correlation potential,
ρ
(
→
r
) is the electron density (the brackets
indicate that it is a functional of the density), and
Φ
(
→
r
) is the electrostatic potential given by
(3.6)
in which the first term is the electron–electron interaction and the second term is the electron–ion
interaction,
Z
j
is the ion charge, and the electron density is given by
(3.7)
where occ refers to occupied states. The calculation proceeds by using some representation for the wave
functions such as the linear muffin tin orbital approximation (LMTO), and iterating self-consistently.
Self-consistency is obtained when either the output density or potential agree to within some specified
criterion with the input. These calculations are not generally performed for the semi-infinite solid.
Instead, they are performed for slabs of increasing thickness to the point where the interior atoms have
essentially bulk properties. Usually, five planes are sufficient to give the surface properties. The values of
ι
(
→
k
) give the surface band structure and surface states, localized electronic states created because of
the presence of the surface.
The second piece of information given is the total energy in terms of the electron density, as obtained
from density functional theory. This is represented schematically by the expression
(3.8)
where
E
ke
is the kinetic energy contribution to the energy,
E
es
is the electrostatic contribution,
E
xc
is the
exchange correlation contribution, and the brackets indicate that the energy is a functional of the density.
Thus, the energy is an extremum of the correct density. Determining the surface energy accurately from
such calculations can be quite difficult since the surface energy, or indeed any of the energies of various
structures of interest, are obtained as the difference of big numbers. For example, for the surface the
energy would be given by
(3.9)
where
a
is the distance between the surfaces (a = 0 to get the surface energy) and
A
is the cross-sectional
area.
−∇+
()
[]
()
=
() ( )
12
2
Vr k r k k r
iii
r
r
r
rr
r
ΨΨ
,,
Vr r V r
xc
rr r
()
=
()
+
()
[]
Φρ
Φ
rr
r
rr r
r
rdr
r
rr
Z
rR
j
j
j
()
=
′
()
−
′
−∑
−
∫
ρ
ρ
r
r
r
rkr
i
()
=
()
ΣΨ
occ
,
2
EE E Eρρρρ
[]
=
[]
+
[]
+
[]
ke es xc
E
Ea E
A
surface
=
()
−∞
()
2

© 1999 by CRC Press LLC
The initial and classic solutions of the Kohn–Sham equations for surfaces and interfaces were accom-
plished by Lang and Kohn (1970) for the free surface and Ferrante and Smith for interfaces (Ferrante
and Smith, 1985; Smith and Ferrante, 1986). The calculations were simplified by using the jellium model
to represent the ionic charge. In the jellium model the ionic charge is smeared into a uniform distribution.
Both sets of authors introduced the effects of discreteness on the ionic contribution through perturbation
theory for the electron–ion interaction and through lattice sums for the ion–ion interaction. The jellium
model is only expected to give reasonable results for the densest packed planes of simple metals.
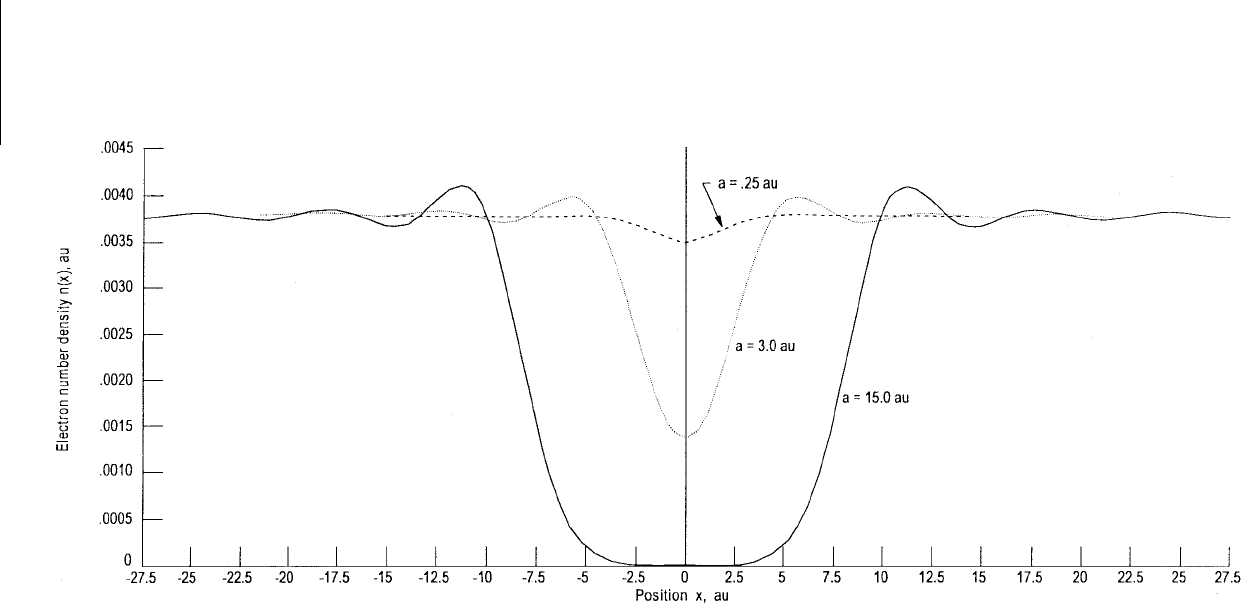
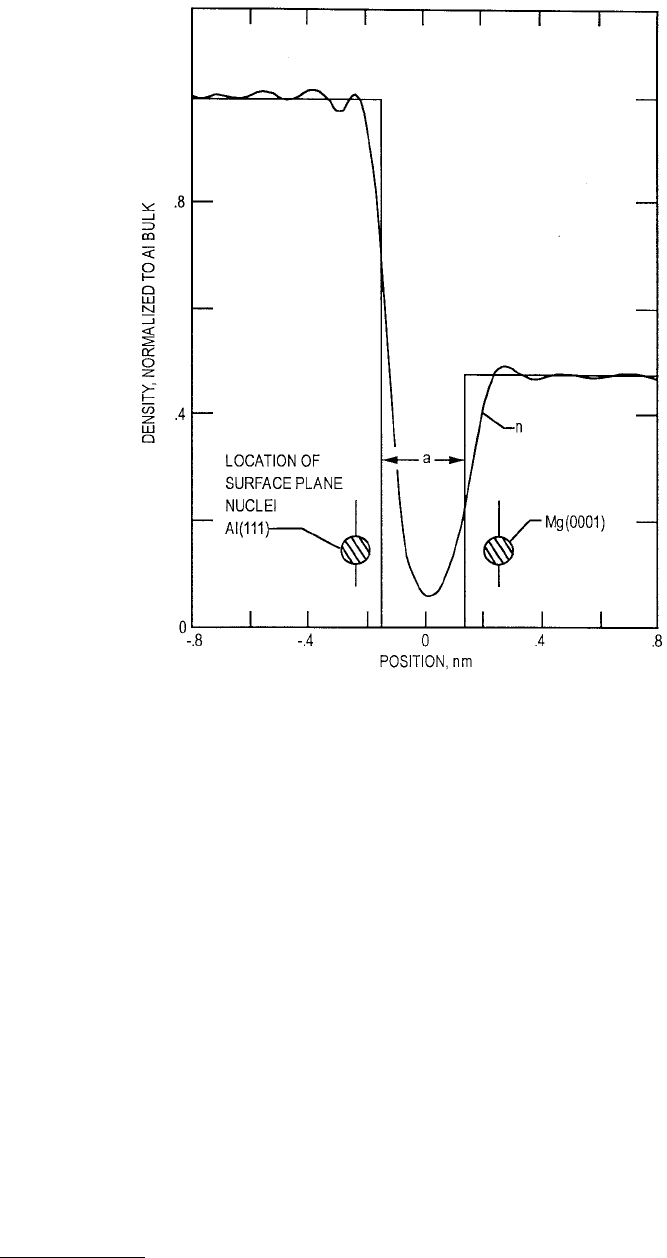
In Figures 3.7 and 3.8 we show the electron distribution at a jellium surface for Na and for an
Al(111)–Mg(0001) interface (Ferrante and Smith, 1985) that is separated a small distance. In Figure 3.7
we can see the characteristic decay of the electron density away from the surface. In Figure 3.8 we see
the change in electron density in going from one material to another. This characteristic tailing is an
indication of the reactivity of the metal surface.
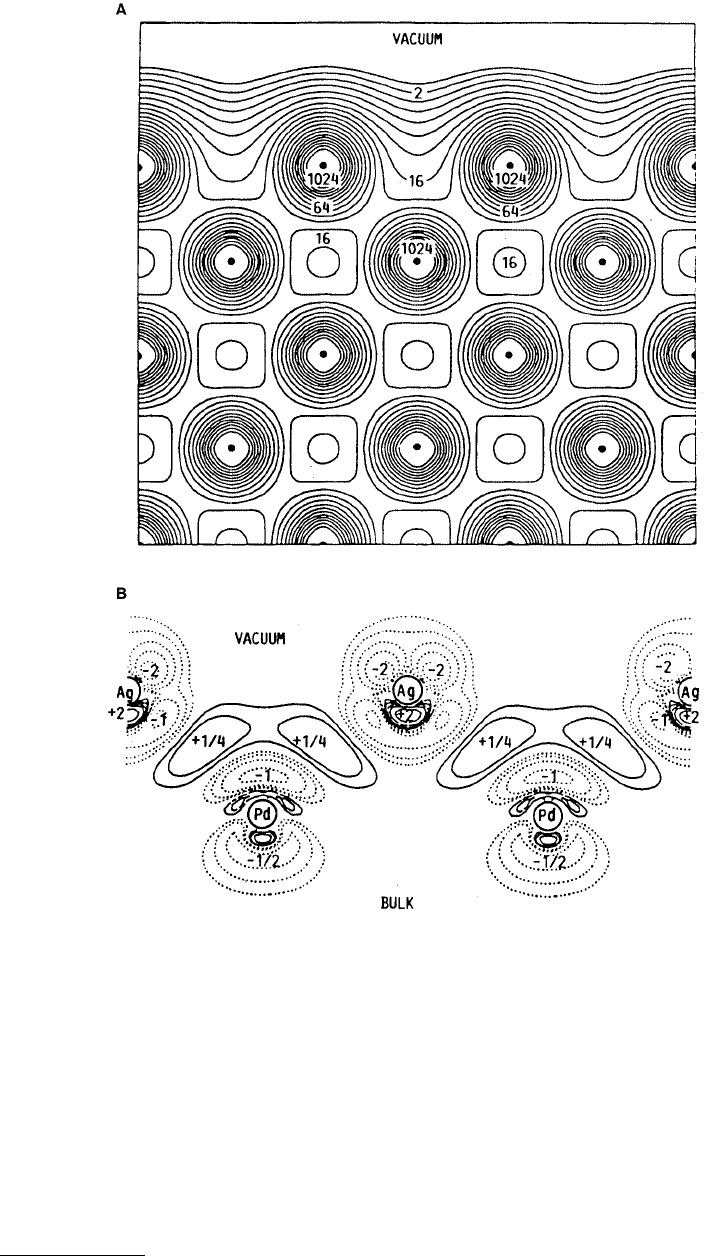
In Figure 3.9 we show the electron distribution for a nickel (100) surface for the fully three-dimensional
calculations performed by Arlinghouse et al. (1980) and that for a silver layer adsorbed on a palladium
(100) interface (Smith and Ferrante, 1985) using self-consistent localized orbitals (SCLO) for approxi-
mations to the wave functions. First, we note that for the Ni surface we see there is a smoothing of the
surface density characteristic of metals. For the adsorption we can see that there are localized charge
transfers and bonding effects indicating that it is necessary to perform three-dimensional calculations in
order to determine bonding effects. Hong et al. (1995) have also examined metal–ceramic interfaces and
the effects of impurities at the interface on the interfacial strength.
In Figure 3.10 we schematically show the results of determining the interfacial energies as a function
of separation between the surfaces with the energy in Figure 3.10a and the derivative curves giving the
interfacial strength. In Figure 3.11 we show Ferrante and Smith’s results for a number of interfaces of
jellium metals (Ferrante and Smith, 1985; Smith and Ferrante, 1986; Banerjea et al., 1991). Rose et al.
(1981, 1983) found that these curves would scale onto one universal curve and indeed that this result
applied to many other bonding situations including results of fully three-dimensional calculations. We
show the scaled curves from Figure 3.11 in Figure 3.12. Somewhat surprisingly because of large charge
transfer, Hong et al. (1995) found that this same behavior is applicable to metal–ceramic interfaces. Finnis
(1996) gives a review of metal–ceramic interface theory.
The complexities that we described earlier with regard to surface relaxations and complex structures
can also be treated now by modern theoretical techniques. Often in these cases it is necessary to use
“supercells” (Lambrecht and Segall, 1989). Since these structures are extended, it would require many
atoms to represent a defect. Instead, in order to model a defect and take advantage of the simplicities of
periodicities, a cell is created selected at a size which will mimic the main energetics of the defects. In
conclusion, we can see that theoretical techniques have advanced substantially and are continuing to do
so. They have and will shed light on many problems of interest experimentally.
3.3.2 Friction Fundamentals
Friction, as commonly used, refers to a force resisting sliding. It is of obvious importance since it is the
energy loss mechanism in sliding processes. In spite of its importance, after many centuries friction
surprisingly has still avoided a complete physical explanation. An excellent history of the subject is given
in a text by Dowson (1979). In this section we will outline some of the basic observations and give some
recent relevant references treating the subject at the atomic level, in keeping with the theme of this chapter,
and since the topic is much too complicated to treat in such a small space.
There are two basic issues, the nature of the friction force and the energy dissipation mechanism.
There are several commonplace observations, often considered general rules, regarding the friction force
as outlined in the classic discussions of the subject by Bowden and Tabor (1964):
1. The friction force does not depend on the apparent area of contact.
2. The friction force is proportional to the normal load.
3. The kinetic friction force does not depend on the velocity and is less than the static friction force.
© 1999 by CRC Press LLC
FIGURE 3.7
Electron density at a jellium surface vs. position for a Na(011)–Na(011) contact for separations of 0.25, 3.0, and 15.0 au. (From Ferrante, J. and Smith, J. R.
(1985),
Phys. Rev. B
31, 3427–3434. With permission.)
© 1999 by CRC Press LLC
Historically, Coulomb (Bowden and Tabor, 1964; Dowson, 1979), realizing that surfaces were not
ideally flat and were formed by asperities (a hill-and-valley structure), proposed that interlocking asper-
ities could be a source of the friction force. This model has many limitations. For example, if we picture
a perfectly sinusoidal interface there is no energy dissipation mechanism, since once the top of the first
asperity is attained the system will slide down the other side, thus needing no additional force once set
in motion. Bowden and Tabor (1964), recognizing the existence of interfacial forces, proposed another
mechanism based on adhesion at interfaces. Again, recognizing the existence of asperities, they propose
that adhesion occurs at asperity surfaces and that shearing occurs on translational motion. This model
explains a number of effects such as the disparity between true area of contact and apparent area of
contact and the tracking of friction force with load, since the asperities and thus the true area of contact
change with asperity deformation (load). The actual arguments are more complex than indicated here
and require reading of the primary text for completeness. These considerations also emphasize the basic
topic of this chapter, i.e., the important effect of the state of the surface and interface on the friction
process. Clearly, adsorbates, the differences of materials in contact, and lubricants greatly affect the
interaction.
We now proceed to briefly outline some models of both the friction force and frictional energy
dissipation. As addressed elsewhere in this book, there have recently been a number of attempts to model
theoretically the friction interaction at the atomic level. The general approaches have involved assuming
a two-body interaction potential at an interface, which in some cases may only be one dimensional, and
FIGURE 3.8
Electron number density
n
and jellium ion charge density for an aluminum (111)–magnesium (0001)
interface. (From Ferrante, J. and Smith, J. R. (1985),
Phys. Rev. B
31, 3427–3434. With permission.)
© 1999 by CRC Press LLC
allowing the particles to interact across an interface, allowing motion of internal degrees of freedom in
either one or both surfaces. Hirano and Shinjo (1990) examine a quasi-static model where one solid is
constrained to be rigid and the second is allowed to adapt to the structure of the first, interacting through
a two-body potential as translation occurs. No energy dissipation mechanism is included. They conclude
that two processes occur, atomic locking where the readjusting atoms change their positions during
sliding, and dynamic locking where the configuration of the surface changes abruptly due to the dynamic
process if the interatomic potential is stronger than a threshold value. The latter process they conclude
is unlikely to happen in real systems. They also conclude that the adhesive force is not related to the
FIGURE 3.9
(a) Electronic charge density contours at a nickel (100) surface. (From Arlinghaus, F. J. et al. (1980),
Phys. Rev. B
21, 2055–2059. With permission.) (b) Charge transfer of the palladium [100] slab upon silver adsorption.
(From Smith, J. R. and Ferrante, J. (1985),
Mater. Sci. Forum,
4, 21–38. With permission.)
