Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.


© 1999 by CRC Press LLC
between them, r. The proportionality factor is 1/4 πεε
0
= 8.99 × 10
9
Nm
2
/C, where ε
o
is the permittivity
constant factor which has the value 8.85 × 10
–12
C
2
/Nm
2
, and ε is the relative permittivity for the medium
across which the force acts.
(5.4)
If, as an illustration, we set q
1
= q
2
= 1.6 × 10
–19
C, the charge of one electron, and solve for the force
when two electrons are an atomic distance 0.2 nm apart, we find that the resulting force is about 6 nN.
This is a magnitude that can be detected with most AFMs. One must also remember that free charges
induce surface charge on nearby surfaces that acts as an image charge buried within the material. Image
charges always carry the opposite sign of the original charge. In this case, the force relationship becomes
(5.5)
with ε
m
and ε
s
representing the permittivities of the medium and sample, respectively. For metals, where
the permittivities are infinite, the term in parentheses approaches one. Because of the high permittivity
of liquids, if the system is immersed in a liquid, the force is drastically reduced and can even be repulsive
(i.e., positive), depending on the relative values of ε
m
and ε
s
.
5.3.2.2 Dipoles
Molecules can have positive and negative ends to them, due to one atom or atomic group having a
stronger electronegativity than the others. Dipoles have associated electric fields, and these electric fields
interact with other charges and dipoles. The magnitude of a dipole moment of a molecule or an atomic
bond is p = ql, where charges ±q lie a distance l apart. Ubiquitous water’s dipole moment is 6.18 ×
10
–30
Cm, which is a modestly high value, exceeded in general only by strongly ionic pairs such as NaCl.
It is interesting that the interaction potential of a dipole with a charge, another dipole, or a polarizable
atom or molecule is related to whether the dipole is fixed or free to rotate. The functional dependence
upon distance changes. Although the force between two dipoles can be quite weak, collective effects may
be large enough to be measurable using SPM techniques. Typically, one integrates the force or potential
over the volumes where the charges, molecules, or dipoles are located. Once again, this changes the
functional dependence of the force law. For example, the interaction potential between a fixed dipole
and a polar molecule is proportional to 1/r
6
. If the fixed dipole finds itself in front of a semi-infinite half-
space of polar molecules (a flat sample), the interaction potential is then a function of 1/r
3
. The derivations
may be found in Israelachvili (1992).
5.3.2.3 Polarizability
All atoms and molecules are polarizable. The effect originates from the charged nature of atoms. In an
electric field, the positively charged nucleus moves slightly in the direction of the field, and the electrons
against it, until the force exerted by the electric field is balanced by the internal restoring forces of the atom
or molecule. This is similar to the dipole moment p = ql, but it is an induced, rather than permanent,
dipole moment. The relation among the induced dipole moment µ, the electric field E, and the polarizability
α is simply µ = αE. Polarizabilities are of the order of 10
–40
C
2
m
2
/J. Because electric field strengths and
functional dependencies on distance depend on whether the source of the field is a dipole or charge, the
interaction potentials between two individual atoms or molecules exhibit either 1/r
4
or 1/r
6
proportionalities.
5.3.2.4 Applied Electrostatic Fields
An easy way in which the experimentalist can actively control an SPM measurement is to apply a voltage
between the tip and sample, forming a capacitor between them. The energy stored in such an electric
field is equal to W = –½CV
2
, where C is the tip–sample capacitance and V the applied voltage. The
F
qq
r
=
π
12
0
2
4 εε
.
F
qq
r
m
sm
sm
=
−
π
−
+
12
0
2
4 εε
εε
εε
,

© 1999 by CRC Press LLC
capacitance of two parallel plates separated by a distance δ is ε
0
A/δ, where A is the area of the plates.
Therefore, the work per unit area for two planes is ϖ(δ)
planes
= –½ε
0
V/δ, and by using the Derjaquin
approximation above (Equation 5.3), we arrive at
(5.6)
The presence of a dielectric material with dielectric constant ε of thickness b/2 upon each electrode (tip
and sample) will modify the equation to
(5.7)
Try taking the limits b → 0 and b → δ in order to check this latter result with the former one. A typical
value for the force at contact (δ ≈ 0.2 nm) with an applied voltage of 10 V and tip radius of 10 nm is
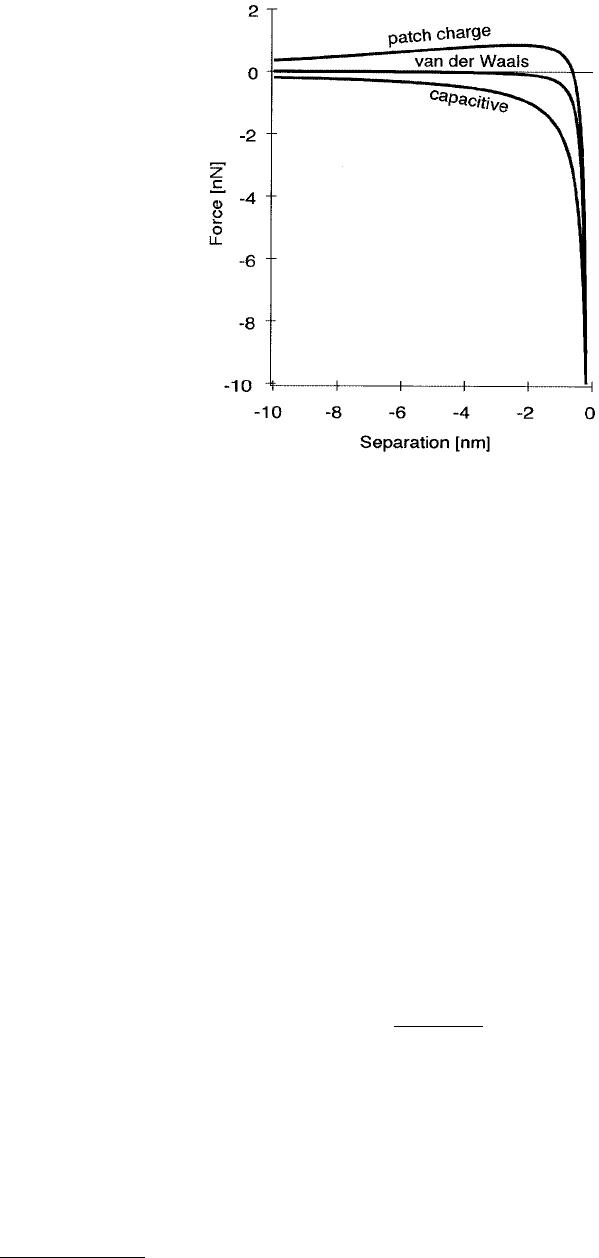
–14 nN. The functional dependence on δ for a capacitive interaction is plotted in Figure 5.6.
5.3.2.5 Innate Electrostatic Fields
If there is no voltage applied between two conductors that form a capacitor, there may still be an
electrostatic field between them. If the work functions Φ
i
(nominally, the potential difference between
the Fermi level and the vacuum level) of the two materials are not equal, when electrically connected
the Fermi levels equilibrate, and the voltage difference is Φ
1
– Φ
2
. The value can be of the order of a volt,
giving rise to a nonnegligible force. An offset voltage may be applied to compensate this contact potential
difference.
Even when the contact potential difference has been removed, the system may continue to be affected
by innate electrostatic fields due to work function anisotrophies. The work function is very sensitive to
perturbations at a surface. Surface preparation, uneven distribution of adsorbates, crystallographic ori-
entation, and the presence of surface steps, hillocks, pits, or defects can all influence the work function
and make its value change with position on the surface.
Let us now perform a short gedanken experiment. We assume a metallic material, the surface of which
incorporates a patch of adsorbates having work function Φ
A
and the rest of the surface, bare, having
work function Φ
B
. We know that energy must be conserved, and that everywhere inside the metal, the
electron has a mean energy equal to the Fermi level. An electron taken out of the material via the patch
and put back into it through the bare surface will not conserve energy (Φ
A
– Φ
B
≠ 0) unless there exist
electric fields external to the material. The electric fields emanate from the adsorbate patches, which can
be modeled as dipole sheets. The fields are strong enough to affect electron trajectories in field emission
microscopy, and they induce surface charge in the sample. The resulting tip–sample forces are known as
patch charge forces.
The calculation of patch charge forces is complex, first, because of the nontrivial nature of the electric
field associated with the dipole sheet, and second because the distribution of image charges induced in
nearby bodies is heavily dependent on their geometry. Nonetheless, the resulting force is notable not
only in that it can initially be repulsive, as in Figure 5.6, or attractive, but also because induced image
charges are always of the opposite sign, and therefore the force always turns attractive as the distance
between tip and sample is reduced (Section 5.3.2.1). The normal component of the electric field E
z
along
the central axis of a dipole disk with dipole moment per unit area µ and diameter ρ is
(5.8)
F
RV
δ
ε
δ
()
=−
π
sphere–flat
0
2
.
F
RV
b
δ
εε
εδ ε
()
=−
π
+−
()
[]
sphere–flat
0
2
1
.
E
z
z
z
z
=±
µ
+
−
+
()
2
1
0
22
2
22
32
ε
ρ
ρ
.
© 1999 by CRC Press LLC
In the limits of either an infinitely large disk or at an infinite distance from the patch, E
z
→ 0. The
magnitude of the electric field is maximum at about ρ ≈ z. If we assume that there is one extra electron
on the tip, then the force felt by that electron 10 nm from a patch on the sample of diameter ρ = 10 nm
and dipole moment per unit area µ = 1.6 × 10
–8
C/m will be F
z
= qE
z
= ±5 nN, and its image force will
be –2 pN. As the tip is lowered onto the sample, the image force grows and the charge-patch field force
will drop. There will also be image charges built up in the tip from the patch field, which is attractive to
the patch.
5.3.3 Electrodynamic Forces
5.3.3.1 The Dispersion Force
Now we need to consider oscillating electrons. The position of an electron about the nucleus of an atom
is not fixed with time; it oscillates, generating a fluctuating dipole field. The field interacts with nearby
atoms, inducing the appropriate instantaneous dipole moments in them that are always attractive. This
is known as the dispersion force because the frequencies correspond to those of visible and ultraviolet
light, which the fluctuations disperse. The London equation gives the dispersion potential W(r) between
two nonpolar molecules a distance r apart:
(5.9)
The electrons orbit the nuclei at a frequency ν, h is the Planck constant, and α is the polarizability of
the atom. The dispersion force acts between all materials, because all atoms have fluctuating electrons.
For two atoms with α/4πε
0
= 10
–30
m
3
, hv = 10
–18
J, and interatomic distance of 2 × 10
–10
m, W = –1.2
× 10
–20
J. This is three times more energy than the thermal energy kT at 300 K, so the dispersion force
may not be ignored at room temperature. The London constant 3hνα
2
/4 (4πε
0
)
2
for Ar–Ar is approxi-
FIGURE 5.6 General behavior of electrostatic and electrodynamic forces as a function of tip–sample separation δ.
Capacitive forces approximately follow a 1/δ force, with van der Waals forces following 1/δ
2
. Patch charge forces can
first be repulsive before becoming attractive.
Wr
h
r
()
=−
π
()
3
44
2
0
2
6
να
ε
.

© 1999 by CRC Press LLC
mately 50 × 10
–79
Jm
6
, whereas for CCl
4
–CCl
4
it is about 1500 × 10
–79
Jm
6
, the difference being largely
due to the sixfold change in polarizability of the molecular pairs.
The electronic motion is correlated at visible and ultraviolet frequencies, which are of the order of
10
15
Hz. During one period of the fluctuation frequency, the electromagnetic field travels 300 nm.
Correlated electron motion does not occur if upon leaving one atom, the field travels to another atom
and returns to the original one to find that the motion is out of phase. Therefore, at distances greater
than approximately 100 nm, the dispersion potential for two atoms drops off at a rate of 1/r
7
, instead of
1/r
6
. This is known as the retardation effect. Integrated over the volume of a flat surface and an approaching
spherical tip, the distance dependence for nonretarded dispersion forces become 1/δ
2
; that for retarded
dispersion forces is 1/δ
3
.
5.3.3.2 van der Waals Forces
There is not just one van der Waals force, but rather there are van der Waals forces. The terminology
van der Waals forces encompasses three forces of different origin. The dominant contribution is the
dispersion, or London force, due to the nonzero instantaneous dipole moments of all atoms and molecules,
as described in the previous section. The second contribution is the Keesom force, which originates from
the attraction between rotating permanent dipoles. The interaction between rotating permanent dipoles
and the polarizability of all atoms and molecules generates the third contribution, the Debye force. The
interaction potential between atoms or molecules of each force is a function of 1/r
6
. The dispersion force
is the most important component of van der Waals forces because all materials are polarizable, whereas
for Keesom and Debye forces, there must be permanent dipoles present.
The Hamaker constant, A, reflects the strength of the van der Waals interaction for two bodies 1 and
2 in medium 3, with permittivities ε
i
and indexes of refraction n
i
. The first term includes Keesom and
Debye interactions, the second the London interaction.
(5.10)
Inspection of this equation reveals that for identical materials across any medium (ε
1
= ε
2
and n
1
=
n
2
), A is positive (attractive), whereas if ε
3
and n
3
are intermediate to ε
1
, n
1
and ε
2
, n
2
, A is negative
(repulsive). If ε
3
and n
3
equal the permittivity and index of refraction of either of the two bodies, A
vanishes. For ε
3
= n
3
= 1 (air or vacuum), A is always positive. In other words, van der Waals forces can
be attractive, repulsive, or zero. The judicious choice of the medium in which an SPM experiment is
carried out helps control the van der Waals forces between tip and sample.
The form of the van der Waals interaction potential for two flat surfaces is W(δ)
planes
= –A/12πδ
2
. Using
Derjaguin’s equation from Section 5.3.1, we find that the force between a sphere and a flat is F(δ)
sphere–flat
=
–AR/6δ
2
, as seen in Figure 5.6. A typical value for A is 10
–19
J; in air or vacuum a force at contact of the
order of –4 nN would be expected for a 10 nm tip radius. In water, A is drastically reduced because of
the high permittivity of water (≈80).
5.3.4 Electromagnetic Forces
There are three classes of magnetism — diamagnetism, paramagnetism, and ferromagnetism. In paramag-
netic and diamagnetic materials, electron spins are randomly oriented due to thermal fluctuations,
yielding no net permanent magnetic moment for the material. However, in ferromagnetic materials, a
strong quantum effect called exchange coupling causes the spins to align, giving the ferromagnet a
permanent magnetic moment.
Spins will respond to an external magnetic field. In all materials, the change in electron orbital moment
is opposite to the external field, giving a repulsive force. (This is an atomic analog of Lenz’s law, which
AkT
hv
nnnn
nn nn nn nn
=
−
+
−
+
+
−
()
−
()
+
()
+
()
+
()
++
()
3
4
3
82
13
13
23
23
1
2
3
2
2
2
3
2
1
2
3
2
12
2
2
3
2
12
1
2
3
2
12
2
2
3
2
12
εε
εε
εε
εε
.

© 1999 by CRC Press LLC
says that it takes work to move a magnet toward a current loop.) Materials in which this is the only effect
are diamagnets. The weak diamagnetic interaction can easily be overridden. In paramagnetism, the
electron spins in atoms when magnetic moments line up in the same direction as the external field,
yielding a net attraction. Paramagnetism is stronger than diamagnetism, but weaker than ferromagnetism
by several orders of magnitude.
The force on a magnetic dipole with magnetic moment
→
m is F = ∇
→
m ·
→
B, where
→
B is the magnetic
flux density. For constant magnetic moment
→
m, the force depends on the gradient of the flux density.
In magnetic force microscopy, if the tip is ferromagnetic, and the sample is paramagnetic or diamagnetic,
then the diverging field from the tip interacts with the induced magnetic moment of the sample. If the
sample is ferromagnetic, then a para- or diamagnetic tip would detect magnetic force only when the tip
is over part of the sample where the field has a gradient, for example, at the edge of a magnetic domain.
If both sample and tip are ferromagnets, then a magnetic force of some magnitude and direction will be
detected over all of the sample.
The interpretation of magnetic force microscope images is not simple, and requires considerable
theoretical effort to understand the data. They depend on the magnetic structure of both tip and sample.
The orientation of the tip with respect to the sample determines its sensitivity to the in-plane or
perpendicular components of the gradient of the field. Topography may contribute to the overall “mag-
netic” image. The functional dependence on tip–sample distance will vary with induction effects. (Para-
magnetism and diamagnetism are induced magnetic effects.) The field from a ferromagnetic tip may
alter the domain structure of the sample. Yet another complication is that of magnetic surface charge
which gives rise to an apparent double image of thin domains.
5.3.5 Forces in and Due to Liquids
The high permittivities of liquids have a strong affect on the strength of the force interaction between
tip and sample. Equations 5.5, 5.7, and 5.10 all explicitly state the influence of the permittivity on the
force magnitude. Also, there are other effects due to the presence of liquids. They are the double-layer,
charge regulation, hydration, structural, and capillary forces.
5.3.5.1 Double-Layer, Charge Regulation, Hydration, and Structural Forces
When immersed in a polar liquid such as water, surface charge on the tip and sample may be induced
by the fluid. This may occur by either ionization or dissociation of the surface species, or by adsorption
of ions from solution. To maintain electrical neutrality, ions of the opposite charge gather near the surface
of the tip and sample to form a diffuse electrical double layer. If the tip and sample are pushed together,
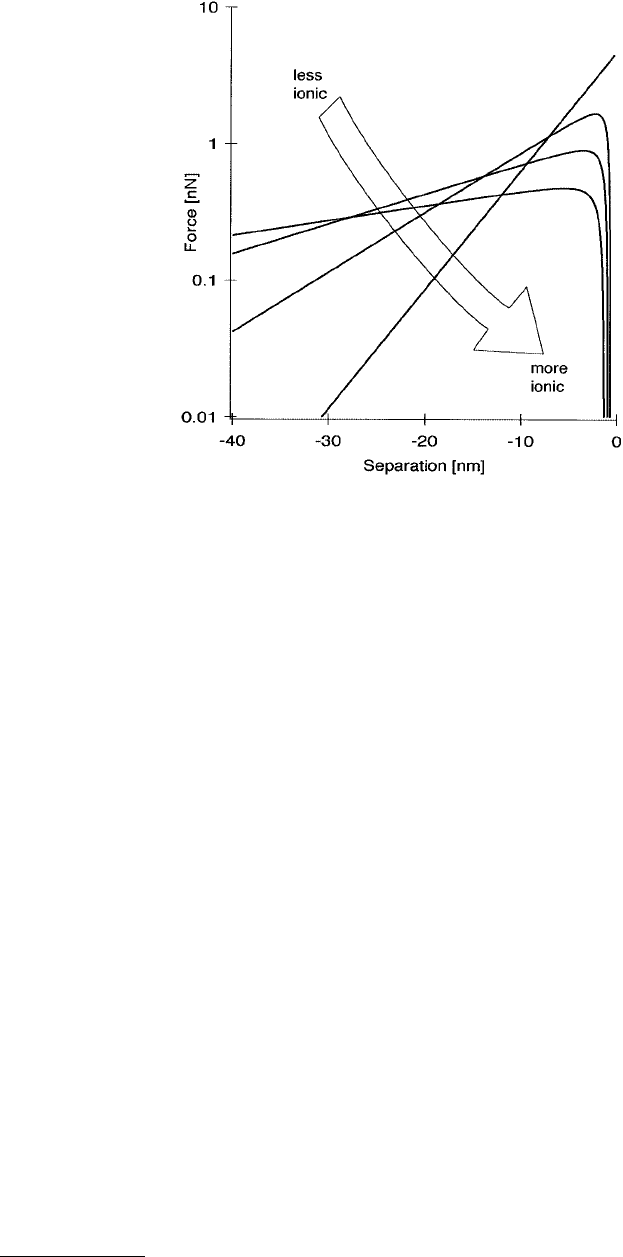
a strong, long-ranged repulsive force is observed because of the overlap of the electrical double layers
(Figure 5.7). This does not necessarily remain repulsive all the way to contact, however. Charge
regulation — the readsorption of counterions onto the surface, reducing the surface charge density —
diminishes the repulsive force in concert with attractive van der Waals forces. Acting to keep the force
repulsive is the hydration force, which comes from the repellent interaction between hydrated ions bound
to the tip and sample surfaces. Whether attractive or repulsive forces dominate near contact depends on
the specific materials and medium involved.
In nonpolar liquids, much more subtle effects due to molecular ordering or structure at the liquid–solid
interface can be detected because of the low surface tensions and thereby reduced tip–sample force
interactions when nonpolar fluids are present. Specifically, when tip and sample are within ten or fewer
molecular diameters of each other, it is possible to observe oscillations between attractive and repulsive
forces that display a period equal to the molecular diameter. Almost a standard experiment using a surface
forces apparatus, it is less reliable in SPM setups, probably because of the small curvature radius at the
end of the cantilever tip.
5.3.5.2 The Capillary Force
If a thin wetting film (that is, no droplets, but rather a uniform layer) of water or another liquid covers
the tip and sample, one would expect a force–distance relationship as presented in Figure 5.8. The process
© 1999 by CRC Press LLC
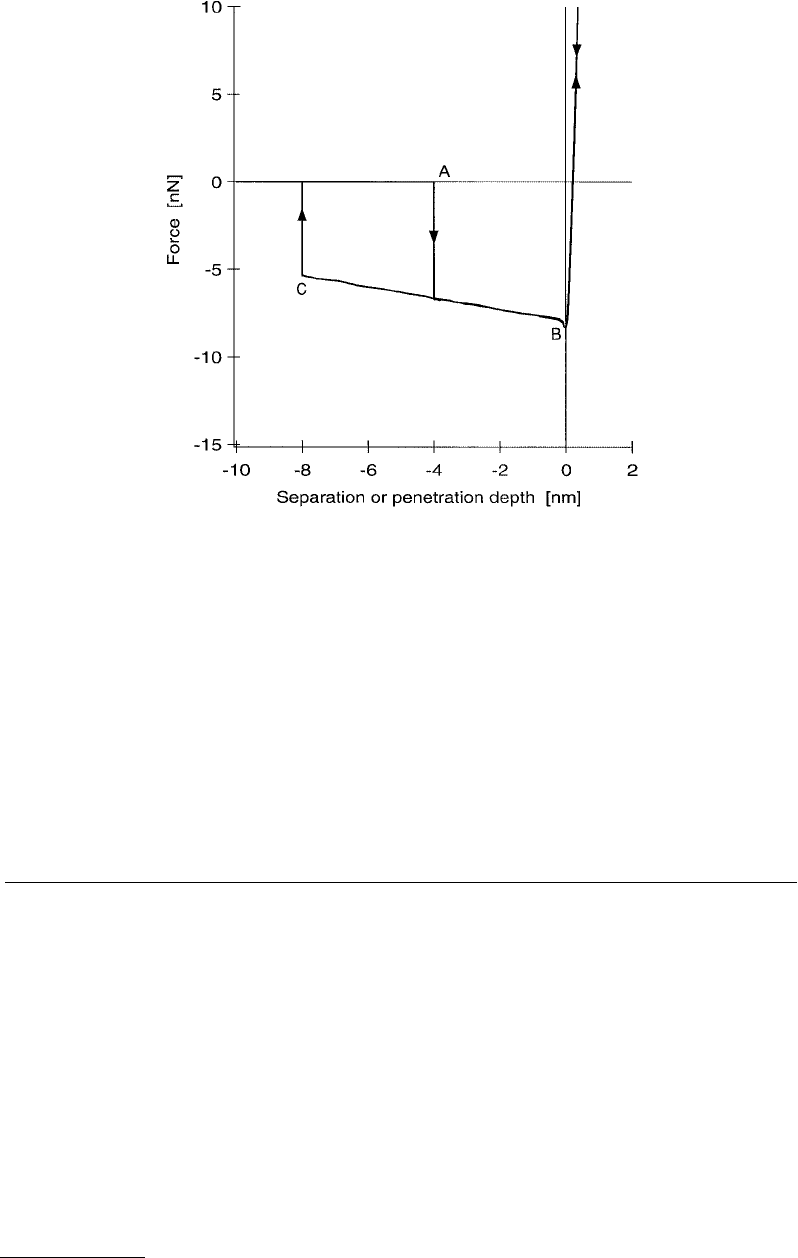
by which the peculiar shape is formed in the following. The tip approaches the sample, and when the
liquids touch, the system is driven to minimize the fluid surface area. The liquids suddenly draw the tip
toward the sample (marked “A” in the figure). Although the work of adhesion between the tip and sample
is generally modified by the presence of the fluid, in the displayed case there remains enough tip–sample
attraction such that the force becomes more negative until tip–sample contact is achieved. Upon retrac-
tion, the force curve shows extreme hysteresis. This corresponds to the elongation of a liquid bridge, or
capillary, between the tip and sample. If the microscope were stable enough, the liquid bridge would thin
until the waist of the capillary were of atomic dimensions, but usually mechanical or thermal vibrations
cause it to break, generating the second instability labeled “C” in the figure.
The overall magnitude of the capillary force can be large enough to obscure van der Waals forces. The
liquid films surely make a large contribution to any damping measurements that are performed. The
permittivities of the films must be considered if any attempt is made to quantify capacitive or other
electrical forces. (See Equations 5.7 and 5.10.) Any SPM imaging mode — contact, noncontact, or
intermittent contact — is subject to the effect of the capillary force via the change in work of adhesion,
the modified contact stiffness due to the presence of the liquid films, or the viscous properties of the
films. Experiments where quantitative results are desired should be performed in dry nitrogen, in
liquids, or in vacuum to temper the strong capillary influence on the data.
5.3.6 Overview
Figures 5.6 through 5.8 compare the distance dependencies of the forces discussed in this section. One
can see that the shapes of the curves can be quite different. The shape of the curve, plus the magnitude
of the force, is an aid to the interpretation of the data. But remember that the shape will be accurately
revealed only if a stiff cantilever is used. Of course, more than one surface force may be operative, in
which case the data present more of a challenge.
FIGURE 5.7 Typical shapes of force curves taken in liquids. The logarithmic dependence on distance is characteristic
of the double-layer force. The higher the concentration of ions in the solution, the steeper the curve. Charge regulation
and van der Waals forces can overcome the repulsive interactions at small separations, whereas the most ionic solution
remains repulsive all the way to contact because of the hydration force.
© 1999 by CRC Press LLC
We remind the reader that the discussion above has been limited to the situation where the tip and
sample may be modeled as a sphere and a flat. This may or may not apply well to a given experiment.
In Section 5.1, we stated that surface forces predominate at small enough scales. The order of magnitude
of the forces from the examples given in this section is approximately 10
–8
N. We know that this is enough
force to attract an AFM tip to a sample. What radius spherical particles would stick to a surface if they
had the density of water? Of plutonium?
5.4 Adhesive Forces
One of the first questions that comes to mind upon viewing a hysteretic force curve is “What is the origin
of the hysteresis?” Or, in other words, “Why is adhesion greater than attraction?” We shall see in this
section that the chemical and mechanical properties of the surface and near-surface region of the tip and
sample determine the adhesive behavior. A compliant tip–sample system of large curvature radius and
large mutual attraction is more likely to be hysteretic than a stiff system with small curvature radius and
negligible mutual attraction. Also, materials are not elastic for pressures approaching infinity; at high
enough loads all materials permanently deform, and hysteresis results.
We first cover anelasticity, where energy is dissipated as a function of relative tip–sample velocity. There
is no permanent damage. We then treat elastic continuum contact mechanics, where no permanent
deformation occurs and the tip and sample are assumed to be continuous media. The origins of adhesion
hysteresis will be examined in this limit. Subsequently, the role of surface forces in generating permanent
deformation will be introduced. This becomes important at the nanoscale. At the macroscale, permanent
deformation occurs only when force is purposefully applied. Then the central ideas of molecular dynamics
simulations, where the atomistic nature of material is incorporated into the calculations, will be presented.
FIGURE 5.8 Capillary force as a function of separation or penetration depth. As the tip approaches the sample,
the fluid layers touch, and the tip is suddenly accelerated into the fluid by surface tension (A). The attractive force
increases until tip–sample contact is established at B. Upon withdrawal, there is a lot of hysteresis in the curve due
to the extension of a liquid bridge between the tip and sample. Typically, a mechanical vibration causes the film to
break at a finite bridge diameter (C).

© 1999 by CRC Press LLC
5.4.1 Anelasticity
Taking the resonant behavior of a cantilever as an example, we can ask ourselves why its resonant peak
is not infinitely sharp. It is because of the presence of the dashpot in the model for the system, visible
in Figure 5.1, and represented in Equation 5.1 as β
c
. Energy is absorbed by the dashpot. The dashpot is
not easily related to a specific physical process, but a result is the generation of heat. As seen in
Equation 5.1, the dissipative force is a function of the effective mass m*, β
c
, and
·
d, the velocity. Anelasticity
is usually studied by means of modulation techniques, where the phase lag between excitation and
response is monitored as a function of frequency or temperature. For velocities (frequencies) approaching
zero, this term may be ignored. Because force curve acquisition should be performed under equilibrium
(quasi-static) conditions, we shall not focus on anelastic behavior.
5.4.2 Adhesion Hysteresis in Elastic Continuum Contact Mechanics
The work of adhesion must be distinguished from adhesion which must be distinguished from adhe-
sion hysteresis. The work of adhesion is the energy per unit area required to separate two flat surfaces
in vacuum from contact to infinity. Adhesion, as we use the word here, is the maximum force needed
to separate two bodies. If one of the bodies is curved and indented into the second, then extra force is
required to separate them. In force curve analysis, the maximum negative force upon separation of the
tip and sample is often referred to as the pull-off force. Unfortunately, measuring the pull-off force does
not directly give the work of adhesion. The pull-off force is a function of the local curvature radius, the
equilibrium interatomic distance, the reduced elastic modulus of the tip and sample, as well as the work
of adhesion. Details are forthcoming in Section 5.4.2.6.
If the maximum observed force upon tip–sample approach (the pull-on force) in the absence of
cantilever instabilities is the same as the pull-off force, there is no adhesion hysteresis. Adhesion hysteresis
is the energy difference between loading and unloading, and is proportional to the difference between
pull-on and pull-off forces. The energy lost is presumed to be transformed into heat via the generation
of phonons. Recent publications indicate that friction and adhesion hysteresis are strongly linked, much
more so than friction and adhesion.
Although there are many different sources of adhesion hysteresis including plasticity, chemical bonding,
and molecular entanglement, its fundamental behavior can be understood from classical contact mechan-
ical theories. In classical continuum mechanics, no atomistic structure to the materials is evident. Five
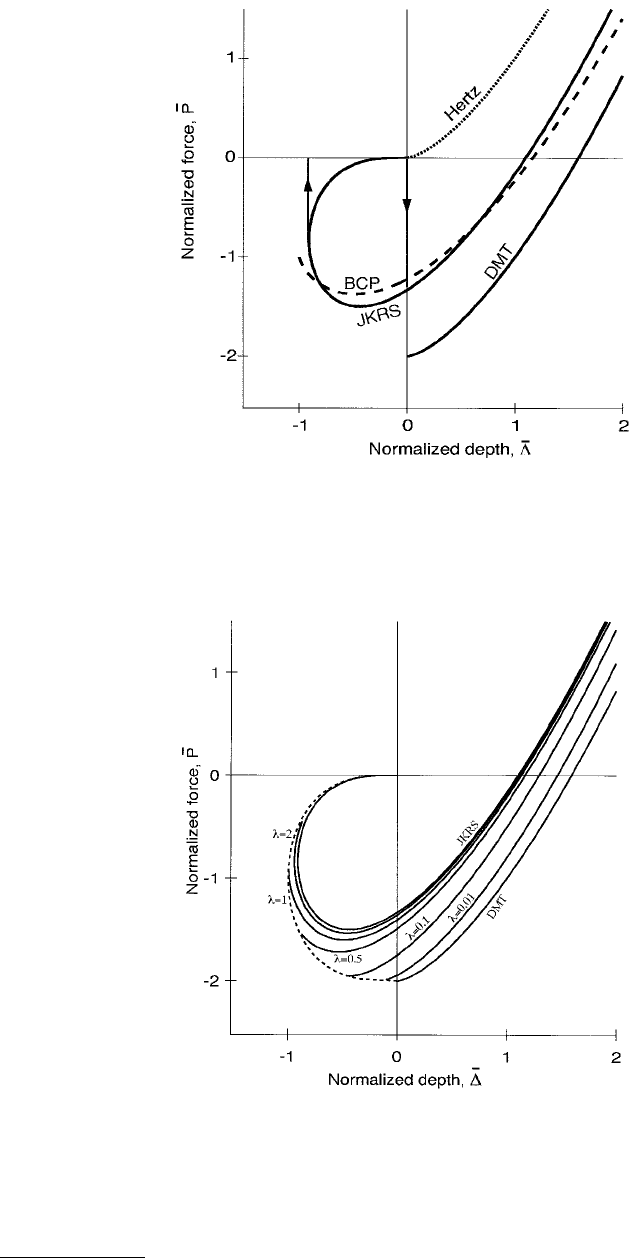
theories are summarized below. The first three behave nonhysteretically; that is, the loading curve is the
same as the unloading curve, but the second two may. Before proceeding, we define some notation
common to all five. The five approaches are discussed in increasing order of sophistication.
5.4.2.1 Notation
The Poisson ratio ν and the Young’s modulus E are needed to write about the reduced elastic modulus K
for the tip–sample system
Other variables used in this section are ξ
0
the interatomic equilibrium distance at the tip–sample interface,
a the radius of the contact area between tip and sample, ϖ the work of adhesion evaluated at contact,
1/R = 1/R
tip
+ 1/R
sample
the reduced tip–sample curvature, P the load, and δ the penetration depth of the
tip into the sample. The load P for various contact mechanical theories is plotted as a function of δ in
Figures 5.9 and 5.10.
13
4
11
2
KE E
=
−
+
−
νν
tip
tip
sample
2
sample
.
© 1999 by CRC Press LLC
FIGURE 5.9 Normalized force plotted as a function of normalized penetration depth for Hertz, DMT, BCP, and
JKRS. The normalization factors are given in Equations 5.18. Hertz, DMT, BCP are not hysteretic, whereas JKRS is.
Even a hypothetical infinitely stiff cantilever, indicated by the vertical lines with arrows, would detect adhesion
hysteresis for the JKRS case.
FIGURE 5.10 Maugis contact mechanics, where normalized force is displayed as a function of normalized pene-
tration depth, using the notation of Equations 5.18. Low λ values approach DMT mechanics, and high λ values
JKRS. Adhesion hysteresis appears for λ > 0.94.
© 1999 by CRC Press LLC
5.4.2.2 Hertzian Mechanics
The Hertzian theory goes back over a hundred years to 1888. Neither surface forces nor adhesion hysteresis
are assumed (ϖ → 0). The relationship between the load P and the system parameters is
(5.11)
and between the contact radius a and the indentation depth δ,
(5.12)
One can see from the above two equations that P is a function of δ
3/2
. In the limit of high loads or
low surface forces, Hertzian mechanics can be applied to SPM experiments. But since an SPM experiment
is typically run under conditions where the cantilever is near its free equilibrium position in the presence
of nonnegligible attractive forces, one of the four following approaches should be more applicable.
5.4.2.3 DMT Mechanics
DMT (Derjaguin, Muller, Toporov) equations date back to 1975. They apply to rigid systems, low
adhesion, and small radii of curvature, but may underestimate the true contact area. They account for
long-ranged attraction around the periphery of the contact area, but constrain the tip–sample geometry
to remain Hertzian. The equations take the following form:
(5.13)
In other words, DMT is Hertz with an offset due to surface forces. There is no hysteresis between loading
and unloading. At δ = a = 0, P = –2πRϖ. When one tries to match attractive forces that exist up to the
point of contact at δ = 0, the slope at zero is not continuous. The next theory attempts to rectify this
unphysical behavior.
5.4.2.4 BCP Mechanics
The BCP (Burnham, Colton, Pollock) semiempirical approach was formulated in 1991, after several years
of force curve study revealed that existing possibilities (Hertz, DMT, and JKRS) did not match the
experimental SPM data. No surface forces are incorporated into Hertzian mechanics, DMT mechanics
predict a sharp discontinuity in slope at contact, and JKRS mechanics predict no detectable attractive
forces before contact. Experimentally, with stiff cantilevers, one observes long-ranged attractive forces
well before contact, and then a gradual transition between negative and positive slopes in the curves.
BCP behavior is representative of many combinations of tip–sample materials.
(5.14)
P
Ka
R
=
3
,
δ=
a
R
2
.
P
Ka
R
R
a
R
=−π
=
3
2
2 ϖ
δ
,
.
P
Ka
R
Ka
R
a
R
R
K
=−
π
−π
=−
π
33
222
2
13
3
2
ϖ
ϖ
δ
ϖ
,
.
