Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.


© 1999 by CRC Press LLC
15.2.2 Lattice Structures and Structural Defects
Crystalline as well as amorphous (disordered) materials are regularly used in MST. One important
material in micromechanics of the latter type is glass. Another important material is silicon dioxide, SiO
2
,
which is used in crystalline form (quartz) as well as in amorphous form (for instance, low-temperature
oxide, LTO). Also silicon and most other relevant materials can be grown in both forms. From a
mechanical-strength viewpoint, an amorphous structure is sometimes preferred, due to the lack of active
slip planes for dislocation movement in such structures. In general, however, the strength performance
is more related to the distribution and geometry of microscopic flaws in the material, especially surface
flaws.
It would lead too far in the present context to define all crystalline lattice structures of interest in MST.
For this reason we will confine ourselves to very brief descriptions of two important lattice types: the
diamond lattice type found in crystalline silicon and the zinc blend (ZnS) lattice type found in III-V
semiconductors.
The
diamond structure
is one of the simplest and most symmetric lattice types, and is found in Si and
Ge, for example. It consists of two face-centered cubic (fcc) lattices which are inserted into each other
in such a manner that they are shifted relative to each other by one quarter of a cube edge along all three
principal axes. Each atom is surrounded by four other atoms in a tetragonal configuration.
The
zinc blende structure
is found in III-V compounds such as GaAs, InP, and InSb. It is identical with
the diamond structure apart from the fact that one of the two overlapping fcc lattices consists entirely
of the type III element (e.g., Ga) and the other entirely of the type V element (e.g., As). Every atom of
one kind is tetragonally surrounded by four atoms of the other kind, and crystallographic planes of any
chosen orientation are periodically arranged in parallel pairs consisting of one III-type and one V-type
atomic plane (in some orientations the parallel planes of a pair coincide).
Common crystal defects are
point defects
such as vacancies (one atom is missing), substitutionals (one
atom is replaced by an impurity atom), or interstitials (one atom is “squeezed in” between the ordinary
atoms). Other frequent crystal imperfections are
line defects
, such as dislocations, and more
complex
defects
, such as stacking faults or twins. All types of lattice defects affect the mechanical properties of a
crystal to a greater or lesser extent, but dislocations are the most detrimental of the lattice defects from
a mechanical-strength viewpoint due to their extremely high mobility (when a critical load limit,
the
yield limit,
has been exceeded).
Beyond the basic crystalline lattice structure (and the various types of lattice defects that may be
present in it), a number of
superstructures
can be of major importance to the mechanical behavior. The
grain structure of a polycrystalline material is one superstructure influencing the hardness and the yield
limit of the material, and precipitates of impurities, alloying substances, or intermediary phases are other
examples. The size and shape distribution of geometric flaws, for instance, voids or cracks in the micron
or submicron range, is of crucial importance to the fracture strength of a brittle material. These super-
structures will be discussed in further detail in following sections.
Foreign atoms
of dopants, or contaminants such as oxygen, nitrogen, and carbon, commonly occur in
semiconductor materials, and are of great importance to their electronic properties (Hirsch, 1983;
TABLE 15.1
Melting Points (°C) of a Number
of Semiconductors and Other Materials
Si 1412 Nylon 137–150
Ge 937 Teflon 290
SiC 2537 Stainless steel 1400–1500
BN 4487 Al
2
O
3
2050
AlAs 1737 TiC 3100
GaAs 1238 HfC 3890
GaP 1467 SiO
2
1610
InP 1070 Glass ~700
InSb 536
© 1999 by CRC Press LLC
Sumino, 1983a). At “normal” levels of doping or contamination in electronic components, the influence
of such impurities on the mechanical behavior is fairly limited, however. For extreme doping levels, some
influence on the plasticity behavior can be observed, especially at elevated temperatures, as will be
exemplified later on.
15.3 Elasticity Properties
15.3.1 Isotropic Elasticity
For small deformations at room temperature most metals and ceramics (including conventional semi-
conductors) display a linear elastic behavior, i.e., they obey Hooke’s law, Equation 15.4, for the relation
between applied normal stress (
σ
) and resulting normal strain (
ε
). The corresponding relationship
between shear stress (
τ
) and shear strain (
γ
) is given by
(15.5)
where
G
is the
shear modulus
of the material. The Young’s modulus and the shear modulus are anisotropic
in crystalline materials. For fine-grained polycrystalline materials, however, isotropic (averaged)
E
and
G
values are sometimes sufficient.
When a linear-elastic material is subjected to a uniaxial strain (relative elongation)
ε
= (
L
–
L
o
)/
L
o
, its
cross-sectional dimension will diminish by a relative contraction
ε
c
= (
d
o
–
d
)/
d
o
. The ratio of these two
strains is a materials constant called the
Poisson’ s ratio
:
(15.6)
In isotropic media the elastic parameters are related by
(15.7)
The relative
volume change
caused by a uniaxial stress
σ
is given by
(15.8)
where
K
is the
compressibility
:
(15.9)
The
bulk modulus
is defined as the inverse value of the compressibility:
(15.10)
Multilayer structures consisting of different materials are frequent within micromechanics. For such
layered composites the Young’s moduli in the lateral and the transverse directions can be calculated from
(15.11)
τγ= G ,
νεε=
c
.
GE=+
()
[]
21 ν .
∆VV E K
o
=−
()
=12 3νσ σ ,
KE=−
()
31 2ν .
BKE== −
()
[]
1312ν .
EfE
nn
n
N
=
=
∑
1
,

© 1999 by CRC Press LLC
(15.12)
where E
||n
and E
⊥
n
are the Young’s moduli of the constituent materials in the two directions, and
f
n
are
the relative thickness fractions (= relative volume fractions) of the layers. Equations 15.11 and 15.12 are
applicable to stress-free multilayer structures built up of layers of individual thicknesses of ~100 nm or
more. In some superlattice structures the existence of a “supermodulus effect” has been suggested, i.e.,
the composite
E
values are supposed to radically deviate from the values predicted by conventional elastic
theories for multilayer structures or for homogeneous alloys. The existence of this effect is at present
under debate, and no physical model for it has been generally accepted as yet.
15.3.2 Anisotropic Elasticity
In single-crystalline materials the anisotropic elasticity is described by the elastic stiffness constants
C
ij
(i,j = 1, 2, … 6) or, alternatively, by the elastic compliance constants
S
ij
. These matrices are symmetric,
and in cubic crystals their number of elements is reduced by symmetry considerations to three indepen-
dent constants: C
11
, C
12
, and C
44
(or S
11
, S
12
, and S
44
). Table 15.2 gives typical room-temperature values
in gigapascals of these constants for a number of materials (Simmons and Wang, 1971).
The stiffness and compliance constants of cubic crystals are related by
(15.13)
(15.14)
(15.15)
Anisotropic values of E and ν can be calculated from these elastic constants. The Young’s modulus in the
crystallographic direction 〈lmn〉 is given by
(15.16)
TABLE 15.2 Values of Elastic Stiffness
Constants of a Number of Semiconductors
at 300 K (in units of GPa)
C
11
C
12
C
44
Si 165.78 63.94 79.62
Ge 129.11 48.58 67.04
GaAs 118.80 53.80 59.40
InP 102.20 57.60 46.00
InAs 83.29 45.26 39.59
Diamond 1076.4 125.2 577.4
The values are results published by different
workers, as compiled by Simmons et al.
(1971). The values for diamond were pub-
lished by van Enckevort (1994).
1
1
EfE
nn
n
N
⊥⊥
=
=
∑
,
CC SS
11 12 11 12
1
−=−
()
−
,
CCSS
11 12 11 12
1
22+=+
()
−
,
CS
44 44
1
=
−
.
12 2
11 11 12 44 1
ES S S S k=− −−
()
,
© 1999 by CRC Press LLC
where:
(15.17)
and the directional cosines are normalized according to
(15.18)
For a longitudinal stress in the direction 〈lmn〉, resulting in a transverse strain in a perpendicular direction
〈ijk〉, the Poisson ratio is given by Brantley (1973):
(15.19)
where E is the Young’s modulus of the 〈lmn〉 direction given by Equation 15.16, and k
2
is given by
(15.20)
Orthonormality conditions, supplementing Equation 15.18, are
(15.21)
(15.22)
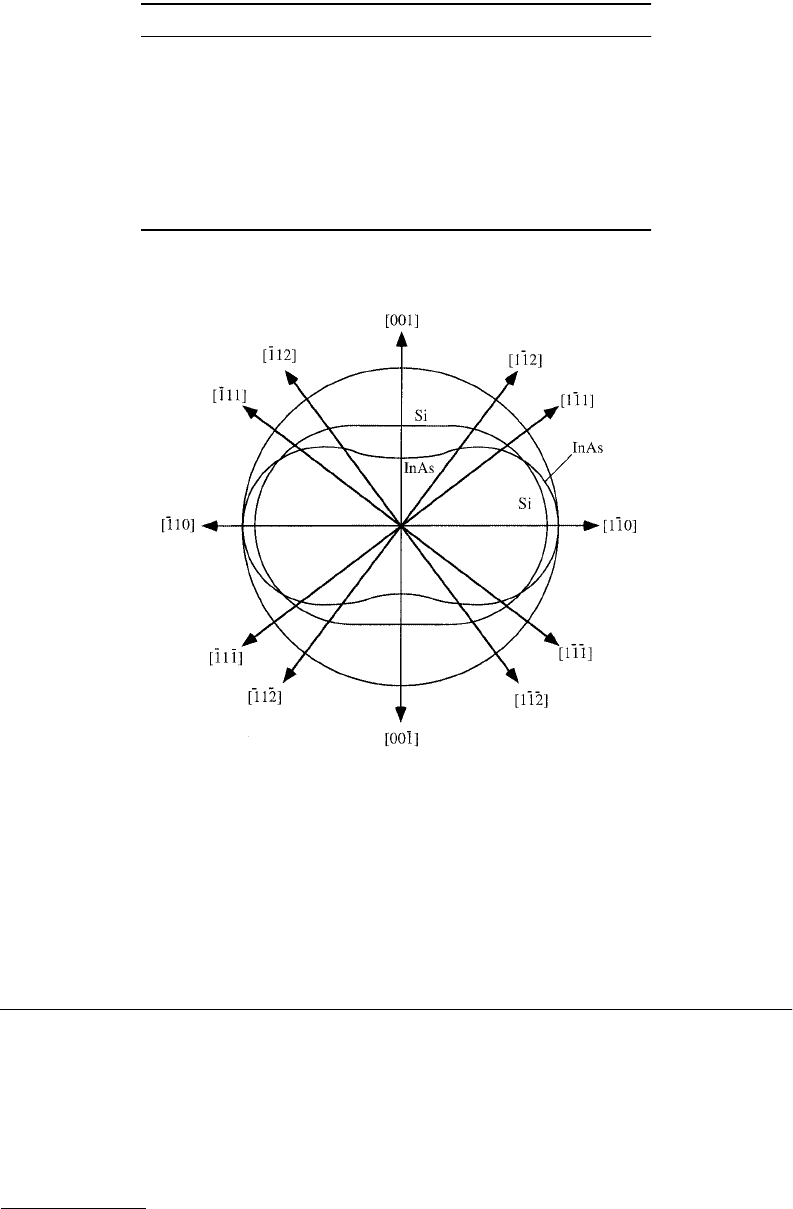
To illustrate the strong anisotropy of Young’s modulus and the Poisson ratio in crystalline semiconductors,
room-temperature values in various directions have been calculated and listed in Tables 15.3 and 15.4
for a few materials. The anisotropy of the Poisson ratio in a couple of semiconducting materials is
graphically illustrated by Figure 15.2. For the case of a hexagonal crystal structure, Thokala and
Chaudhuri (1995) calculated the Young’s modulus and the Poisson ratio for 6H–SiC, Al
2
O
3
, and AlN.
It is sometimes desirable to calculate the elastic properties of a randomly polycrystalline, but macro-
scopically isotropic, aggregate from the anisotropic single-crystal elastic constants. Theories for such
aggregate properties exist, and Simmons and Wang (1971) have tabulated so-called Voigt and Reuss
averages for a large number of crystalline materials. In polycrystalline thin films the grain structure is
TABLE 15.3 Young’s Moduli E at 300 K
for Various Directions (in units of GPa)
Directions
<100> <110> <111> Poly
Si 130.2 169.2 187.9 163
Ge 102.5 137.5 155.2 132
GaAs 85.3 121.4 141.3 116
InP 60.7 93.4 113.9 89
InAs 51.4 79.3 96.7 76
Diamond 1050.3 1163.6 1207.0 1141
The polycrystalline results are mean values of
the Hashin and Shtrikman bounds, as calculated
by Simmons et al. (1971).
klm mn nl
1
222
=
()
+
()
+
()
,
lmn
222
1++=.
ν=− + − −
()
[]
SSSS kE
12 11 12 44 2
2,
kil jm kn
2
222
=
()
+
()
+
()
.
ijk
222
1++= and
il jm kn++=0.
© 1999 by CRC Press LLC
often strongly textured, and theories or expressions for elastic averaging over such morphologies also
exist (Brantley, 1973; Guckel et al., 1988; Maier-Schneider, 1995a). The resulting Young’s modulus of a
textured polycrystalline film can deviate up to 10% from a nontextured polycrystalline film.
15.4 Internal Stresses
The presence or nonpresence of internal stresses in a layered structure can be of great importance to the
mechanical behavior of the component, so for this reason some aspects of internal stresses will be
summarily discussed also in the present context. For instance, internal stresses can cause loss of adhesion
between the film and the substrate and, consequently, lead to delamination failure of the composite.
They can have a beneficial or detrimental effect on the fracture properties of the structure, by inhibiting
or promoting crack propagation in film or substrate (Johansson et al., 1989). Furthermore, various
TABLE 15.4 Poisson Ratios ν at 300 K for Tension along [lmn]
and Contraction in the Perpendicular <ijk> Direction
System Si Ge GaAs InP InAs Diamond
[100]<010> 0.278 0.273 0.312 0.360 0.352 0.104
[100]<011> 0.278 0.273 0.312 0.360 0.352 0.104
[110]<001> 0.362 0.367 0.443 0.555 0.543 0.115
[110]<1
–
10> 0.062 0.026 0.021 0.015 0.001 0.008
[110]<1
–
11> 0.162 0.139 0.162 0.195 0.182 0.044
[111]<1
–
10> 0.180 0.157 0.188 0.238 0.222 0.045
[110]<1
–
12> 0.262 0.253 0.303 0.375 0.362 0.079
[111]<112
–
> 0.180 0.157 0.188 0.238 0.222 0.045
Poly 0.222 0.208 0.243 0.294 0.283 0.070
The poly values have been calculated by the method indicated in Table 15.3.
FIGURE 15.2 Illustration of the anisotropy of the Poisson ratio ν for a normalized (hypothetical) case of 100%
elastic straining along the [110] axis in Si and InAs. The outer contour illustrates a circular cross section of an
unstrained rod (a {110} plane), and the two inner contours illustrate cross sections in hypothetical states of 100%
elastic strain.
© 1999 by CRC Press LLC
mechanisms of relaxation of internal stresses can have rather a drastic influence on the morphology of
ductile films (Smith et al., 1991). Internal stresses in layered structures are of two fundamentally different
origins: thermal stresses caused by thermal mismatch between two adhering layers and intrinsic, or
microstructural, stresses generated during the deposition process.
15.4.1 Thermal Film Stress
One of the most important parameters in the generation of thermal stresses is the linear coefficient of
thermal expansion (α), or, to be more precise, the difference in α for two adhering layers. The materials
parameter α is defined as the relative elongation of a body per degree temperature rise:
(15.23)
which can be expressed as
(15.24)
where ε
therm
is the thermal strain and ∆T is the difference between the initial and the final temperatures.
In cubic (isometric) single crystals, as well as in amorphous or polycrystalline materials, α is nearly
isotropic. In noncubic (anisometric) single crystals, α can be strongly anisotropic. In certain extreme
cases, e.g., Al
2
TiO
5
, LiAlSi
2
O
6
, and LiAlSiO
4
, α is positive in one direction and negative in a perpendicular
direction. This anisotropy can be utilized in micromechanical structures to control the spatial dimensions
by temperature variation. Some selected α values are found in Table 15.5.
A thermal stress is generated when the thermal expansion (or contraction) of one layer is prevented
by external forces of constraint, for instance, by adjacent layers with differing α values or differing
temperatures. In the case of a uniaxially clamped structure, the thermal stress caused by a temperature
difference ∆T can easily be calculated from Hooke’s law, Equation 15.4, and Equation 15.24:
(15.25)
For a thin film on a thick substrate, we have biaxial stress conditions, and Hooke’s law is
(15.26)
TABLE 15.5 Linear Coefficients of Thermal Expansion α
(in units of 10
–6
K
–1
)
Isometric
Anisometric
(⊥ Axis) ( Axis)
Si 2.4 SiO
2
(quartz) 13.7 7.5
GaAs 6.0 Graphite 1.0 27.0
AlAs 5.2 Al
2
O
3
8.3 9.0
SiC 4.5–5.0 Al
2
TiO
5
–2.6 11.5
Diamond 1.3 LiAlSi
2
O
6
6.5 –2.0
Glass 8.0 LiAlSiO
4
8.2 –17.6
Cu 16.2
α=
1
L
dL
dT
o
,
εα
therm
==
∆
∆
L
L
T
o
,
σεα
therm therm
==EET∆ .
σ
ν
α
therm
=
−
E
T
f
f
1
∆∆,
© 1999 by CRC Press LLC
where E
f
and ν
f
are the Young’s modulus and Poisson ratio of the film material, and ∆α is the difference
in α between film and substrate. It is apparent from Equation 15.26 that thermal stresses are minimized
by low E
f
values as well as by small differences in the expansion coefficient and the temperature. The
latter difference is minimized by high thermal conductivities (k values) of the constituent materials.
In interfaces between differently oriented crystals, for instance, in grain boundaries, thermal stresses
can also be caused by anisotropy effects in E, α, and k. If the thermal stresses are not completely relaxed
upon cooling, which commonly occurs in thin-film deposition, residual thermal stresses will be present
in the structure.
15.4.2 Intrinsic Film Stress
Internal stresses of intrinsic origin, on the other hand, are of a more complex physical nature and cannot
be expressed in terms of fundamental materials parameters. These nonthermal stresses are generated
during the film growth process and strongly depend on which deposition technique is used and on
various process parameters. The magnitude of the intrinsic stresses can be very high, sometimes exceeding
the yield or fracture strengths of the corresponding bulk materials. Many theories to explain these stresses
have been suggested, and a summary is given in a review by Windischmann (1992). Intrinsic tensional
stresses have been attributed to grain boundary formation, to constrained shrinkage of disordered
material buried behind the advancing film surface, and to attractive interatomic forces acting between
detached grains separated by a few atomic distances. Intrinsic compressive stresses, on the other hand,
have been attributed to impurity or working gas incorporation, increased defect density, and to recoil
implantation of film atoms.
The total residual stress in a film after deposition hence can be expressed as a sum of the thermal
residual and the intrinsic stresses:
(15.27)
where nonthermal stresses induced by lattice mismatch at the interface have been included among the
intrinsic stresses.
15.4.3 Substrate and Interface Stresses
Residual stresses in a film will induce balancing stresses of opposite sign in the substrate. Using equilib-
rium relationships for forces and moments, the stress response induced in the substrate surface can be
expressed as
(15.28)
where t
f
and t
s
are the thicknesses of film and substrate, respectively. Hence, in very thick substrates (t
f
t
s
), negligible stress response is induced. Equation 15.28 is derived for low t
f
/t
s
ratios. If this ratio is larger
than 0.01, the error in σ
s
res
becomes noticeable (>5%).
All stresses in film and substrate discussed so far are normal tensile or compressive stresses oriented
parallel to the interface. In a well-adhered film–substrate composite no shear stresses are present in the
inner parts of the interface. Along the edges of a coated region, on the other hand, shear stresses may be
present for moment balance reasons. In thin, layered structures this effect is sometimes manifested by a
visible buckling of the edges. If nonbonded areas exist in the interior part of an interface, shear stresses
may be present along their boundaries and contribute in lowering the mechanical strength of the interface.
σσ σ
res therm intr
=+,
σσ
s
res res
=−4
t
t
f
s
f
,
© 1999 by CRC Press LLC
15.5 Plasticity and Thermomechanical Properties
At room temperature, silicon, quartz, the III-V compounds, and many other materials used in MST
display a linear-elastic response to tensile stresses, all the way to brittle fracture. Hence, the plastic yield
limit is never reached, and plastic deformation or other effects based on dislocation slip will not occur
under tension at room temperature. At elevated temperature, however, or for high compressive loads at
room temperature, many of these materials may reach their yield limits before they reach the fracture
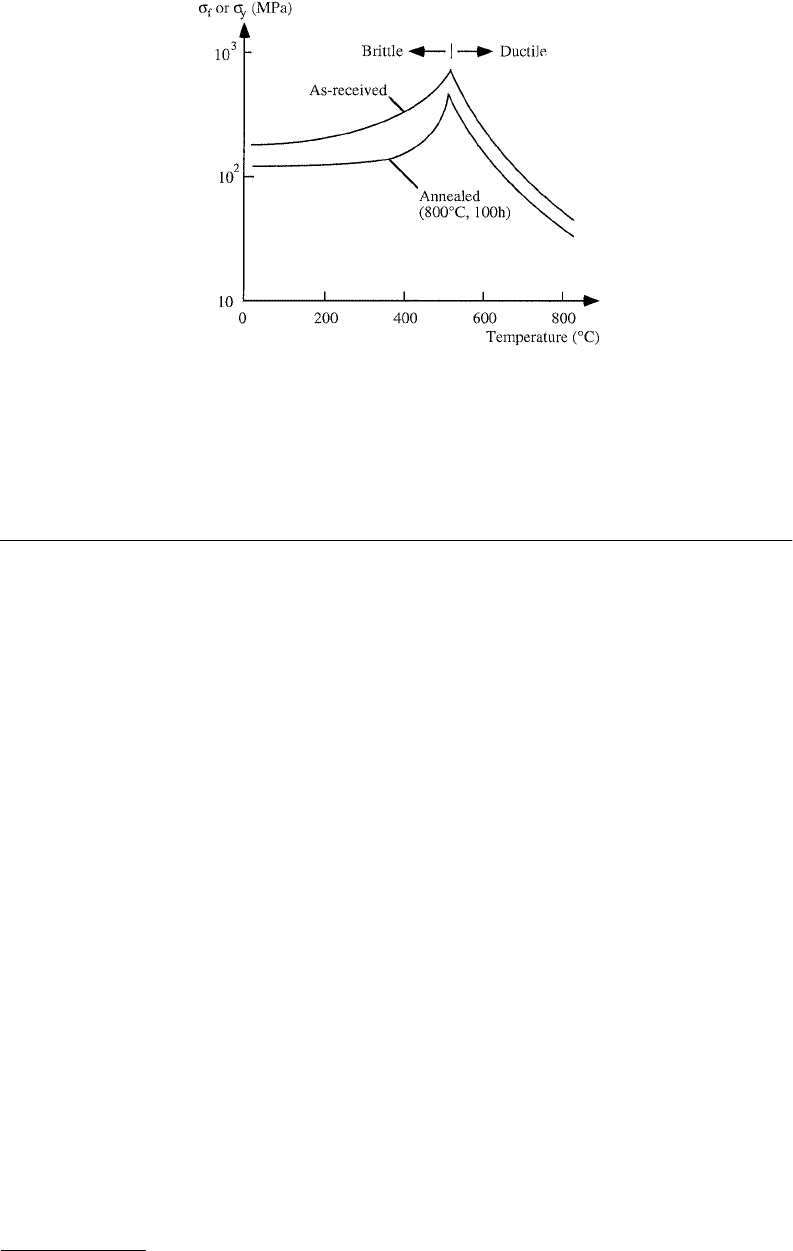
limit, in which case dislocation slip is activated and eventually they will deform plastically. Figure 15.3
illustrates the variation of the fracture limit σ
f
or the yield limit σ
y
of silicon as a function of temperature
(Yasutake et al., 1982a). In most applications plastic yield is undesirable and is, therefore, avoided by
ample dimensioning or by a “safe” materials selection. In some cases, however, the room temperature
yield limit is locally exceeded in high-compressive-stress fields which may be generated internally during
processing of multilayer structures or by, e.g., unintentional microscratches or microindentations. In yet
other cases dislocation slip may be activated by high operating temperatures and induce time-dependent
processes such as creep, aging, or fatigue.
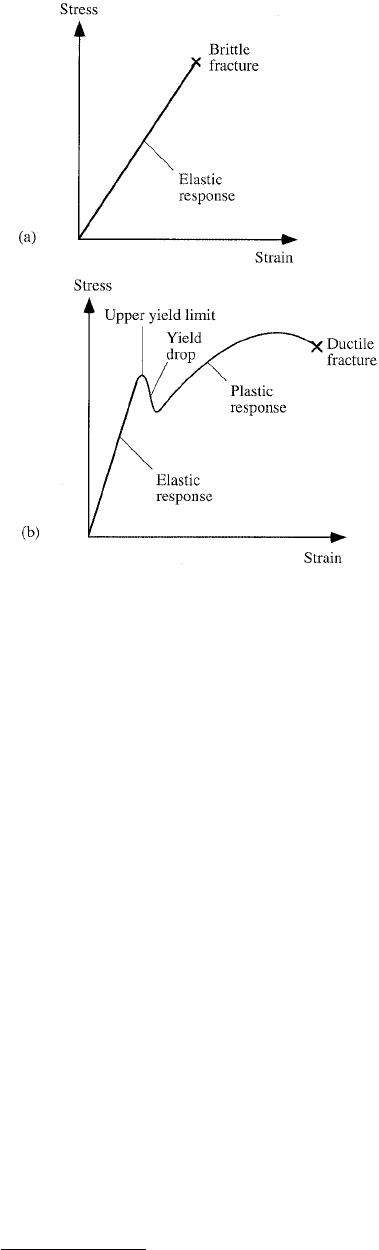
15.5.1 Elastic–Ductile Response
The difference between elastic–brittle response and elastic–ductile response of a material is illustrated by
Figures 15.4a and b. The first diagram illustrates the case when the fracture limit is lower than the critical
load limit for dislocation slip. The second diagram illustrates the opposite case; i.e., the dislocations (if
they exist from the start) are immobile until the critical resolved shear stress is reached in some slip
systems, where dislocation slip and eventually dislocation multiplication are initiated. The resulting plastic
deformation — contrary to elastic deformation — is irreversible upon unloading. The plastic curve
segment in Figure 15.4b is not as steep as the elastic curve segment, but still displays a positive slope
corresponding to a strain hardening effect. This effect is primarily due to the gradually increasing density
of dislocations, which tend to get entangled and obstruct further dislocation slip, hence demanding a
gradual increase of the applied stress in order to maintain the straining process.
FIGURE 15.3 Variation of fracture limit σ
f
and yield limit σ
y
of silicon as a function of temperature. The upper
curve is as-received CZ or FZ silicon (difference negligible), and the lower curve is CZ silicon annealed at 800°C for
100 h. For temperatures below 525
°
C (curves maxima) the curves illustrate the fracture limit σ
f
, above this transition
temperature they illustrate the plastic yield limit σ
y
. (From Yasutake, K. et al., 1982a.)
© 1999 by CRC Press LLC
Much work has been devoted to the study of dislocations and plastic flow in silicon and other
semiconductors. Essential parts of this work are surveyed in well-known articles by Alexander and Haasen
(1968) and by Hirsch (1985). In the plastic interval the dopant and impurity levels play a certain role
(Alexander and Haasen, 1968; Yonenaga and Sumino, 1978, 1984; Sumino et al., 1980, 1983, 1985; Sumino
and Imai, 1983; Imai and Sumino, 1983; Sumino, 1983b; Hirsch, 1985). At high levels of n-doping in Si,
the mobility of the dislocations is increased; i.e., the strain hardening effect is diminished. Impurities
such as oxygen or nitrogen atoms, on the other hand, tend to gather around the dislocations and hamper
their motion; so-called Cottrell atmospheres are formed around the dislocations. This means that higher
loads are required to “tear loose” the dislocations from these atmospheres, implying increased yield limit.
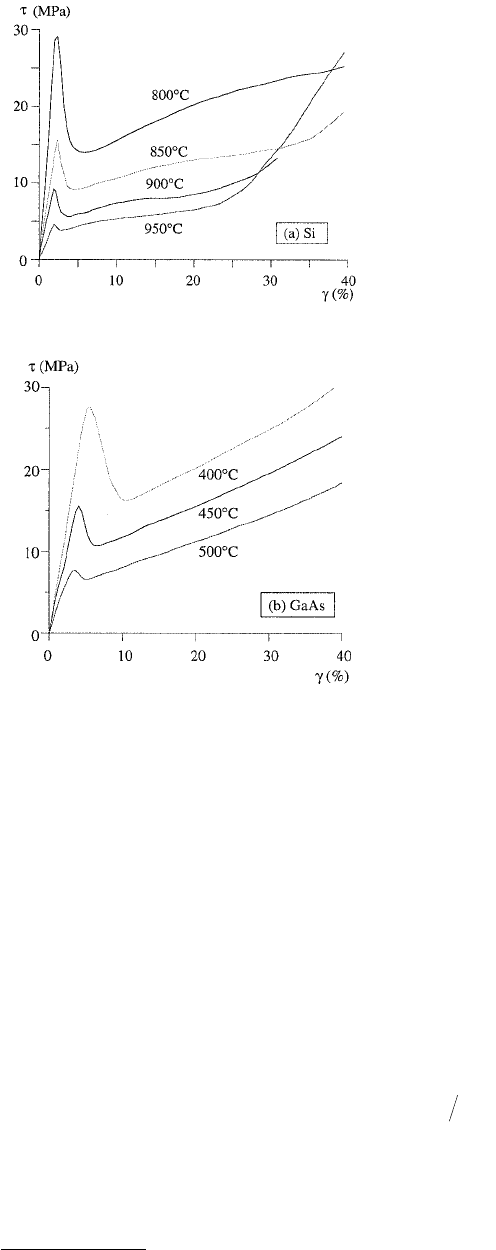
When the dislocations have been torn loose, they regain their high mobility, resulting in a marked yield
drop, see Figures 15.4b and 15.5.
The strain rate
·
ε during plastic deformation of semiconductors has been the subject of many studies
(Alexander and Haasen, 1968; Sumino, 1983a). Its dependency on temperature, effective resolved shear
stress, and dislocation velocity has been investigated in detail. Also the influence of doping levels,
impurities, and growth process (float-zone growth, FZ, or Czochralski growth, CZ) have been studied,
and found to be considerable (Imai and Sumino, 1983; Sumino and Imai, 1983). Micromechanical
elements normally are designed to function below the yield limit, so the strain rate will only be discussed
here in connection with creep.
Microhardness is a complex materials parameter involving several properties of a more fundamental
nature, in particular plasticity properties. From a practical viewpoint the microhardness is a simple and
convenient measure of the susceptibility of a material to contact damage in the micron range (microin-
dentations, microscratches, etc.), and it plays a major role in most models describing tribological pro-
cesses. The microhardness can be measured by several methods, among which Vickers indentation and
Knoop indentation are most commonly used. In both methods a diamond stylus is pressed into the
surface of the body by a given load and at a given loading rate. Upon unloading, the size of the residual
FIGURE 15.4 (a) Linear elastic–brittle response.
(b) Elastic–ductile response.
© 1999 by CRC Press LLC
indentation mark in the surface is measured and related to a characteristic hardness parameter (dimen-
sion: force/area). In the Vickers method the diamond stylus has the shape of a low profile, square pyramid,
whereas the Knoop stylus has a rhombic pyramid shape.
In single-crystalline surfaces, the Vickers hardness (H
v
) is weakly anisotropic (Ericson et al., 1988).
Table 15.6 gives a few characteristic H
v
values for semiconductor surfaces of different crystallographic
orientations.
15.5.2 Time-Dependent Effects
Creep is a thermally activated deformation process which occurs under constant load (below the yield
limit) and during an extended period of time (Alexander and Haasen, 1968). Creep testing of brittle
materials is usually performed under compression. In Figure 15.6 two sets of typical creep curves (strain
vs. time) for Si under various loads and temperatures are displayed. In these curve sets, the points of
maximum creep rate
·
ε
max
(i.e., the points of inflection) obey an exponential type of relationship (Reppich
et al., 1964):
(15.29)
where U is an activation energy and T is the temperature. This general creep behavior of Si is typical
also for Ge and many III-V compounds. Doping has a major influence on
·
ε
max
. Doping of Si with As to
FIGURE 15.5 Resolved shear stress τ vs. shear strain γ
for tensile testing of single crystalline specimens at various
temperatures for (a) Si (Adapted from Yonenaga, I. and
Sumino, K., 1978, Phys. Status Solidi 50, 685.) and (b)
GaAs (Adapted from Sumino, K. et al., 1985, in Proc. 27th
Meeting, 145 Committee of JSPS, p. 91.)
˙
exp ,
max
ετ=−
()
CUkT
n
