Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.


314 13. INTRODUCTORY CHEMISTRY REVffiW
chlorine to give NaCl. There is a complication
that 3d orbitals can be filled with bonding elec-
trons so that an element like sulfur commonly has
two valence {sharing or combining) electrons but
can also have four or six valence electrons; indeed
in the most stable compounds of sulfur, the sulfur
atoms share six electrons as in the case of H2SO4.
This,
of course, also happens in the higher rows.
Fig. 13-1 shows the valence electrons for some
atomic, ionic, and molecular structures; the next
section helps one predict the types of bonds
formed as shown in this figure. Depiction of
electrons in this fashion is credited to G.N. Lewis.
13.2 IONIC AND COVALENT BONDS
The elements of the lower left of
the
periodic
table donate electrons the most easily and are the
electropositive elements. The metallic elements
(element to the lower right and including the
elements of the diagonal formed by Al, Ge, Sb,
and Po) donate electrons easily. When electrons
are donated, the atom remains as a cation such as
Na"^, Ca^"^,
AP"^,
and so on. The elements in the
upper right, not including the inert gases, attract
electrons with high affinity and are the electroneg-
ative elements, including (in order of decreasing
electronegativity) F, O, N, CI, Br, Se, S, and I.
The periodic table of the elements gives values for
electronegativity of the elements (the values are
listed in the lower right of each element's box).
When electrons are accepted, the atom becomes an
anion, such as CI', I", S^', and so on. Chemical
bonds cover a spectrum of equally sharing elec-
trons to forming ion pairs or ionic bonds. Bonds
between the electronegative elements and the
electropositive ions are highly ionic in
nature;
such
compounds when dissolved in water will conduct
an electric current and are described as strong
electrolytes. Some examples of these are NaCl,
CaBrj,
and Na-OH. Bonds between elements of
intermediate electronegativity share the electrons,
forming covalent bonds, and do not disassociate
into individual ions and will not conduct electricity
in water
(nonelectrolytes)
or are weak conductors
(weak electrolytes). Some examples are CH4,
CH3OH, H2S, and CO2. Generally, if the differ-
ence in electronegativity of two elements sharing
electrons is 1.7 or greater, the bond formed
Valence electrons of atomic structures
H • • C •
:
0 : : CI :
Valence electrons of nnolecular structures
2 H' ~* H:H = H-H
2H'+:0:
-^ H:0:H = H-O-H
Valence electrons of ionic compounds
No-
4- :CI : —^ Na"^ + :CI :""
Fig. 13-1. Lewis structures showing the valence
electrons of various structures.
between the two elements behaves in a highly
ionic manner. If the difference is less than 1.0,
the bond behaves in a covalent manner. The
actual amount of ionization in a chemical contain-
ing intermediate bonds depends on many things,
such as the presence and type of solvent.
There are several particularly important
polyatomic ions encountered. For example, the
ammonium cation NH4^ behaves very similarly to
the Na^ ion, except the ammonium ion decom-
poses at pH above 9 and is subject to combustion.
Many polyatomic anions with several atoms of
oxygen have delocalized (that is, spread out over
two or more atoms) negative charges, which
increase the stability of the oxygen anion such as
in the cases of
RCOj-,
COa^", NO{, NO2", SO42-,
and
SOj^'.
Resonance structures are Lewis struc-
tures which differ only in the position of a double
bond and other electrons. The actual structure is
a resonance
hybrid,
which is an average of all of
the resonance structures. Fig. 13-2 shows how
two resonance structures contribute to the carbox-
ylate anion. Experiments indicate that the two C-
O bonds are identical and intermediate in prop-
erties between C-0 and C=0 bonds.
Fig. 13-2. Resonance structures for the carbox-
ylate anion.
IONIC AND COVALENT BONDS 315
Table 13-2. Approximate bond energies for
interatomic bonds.
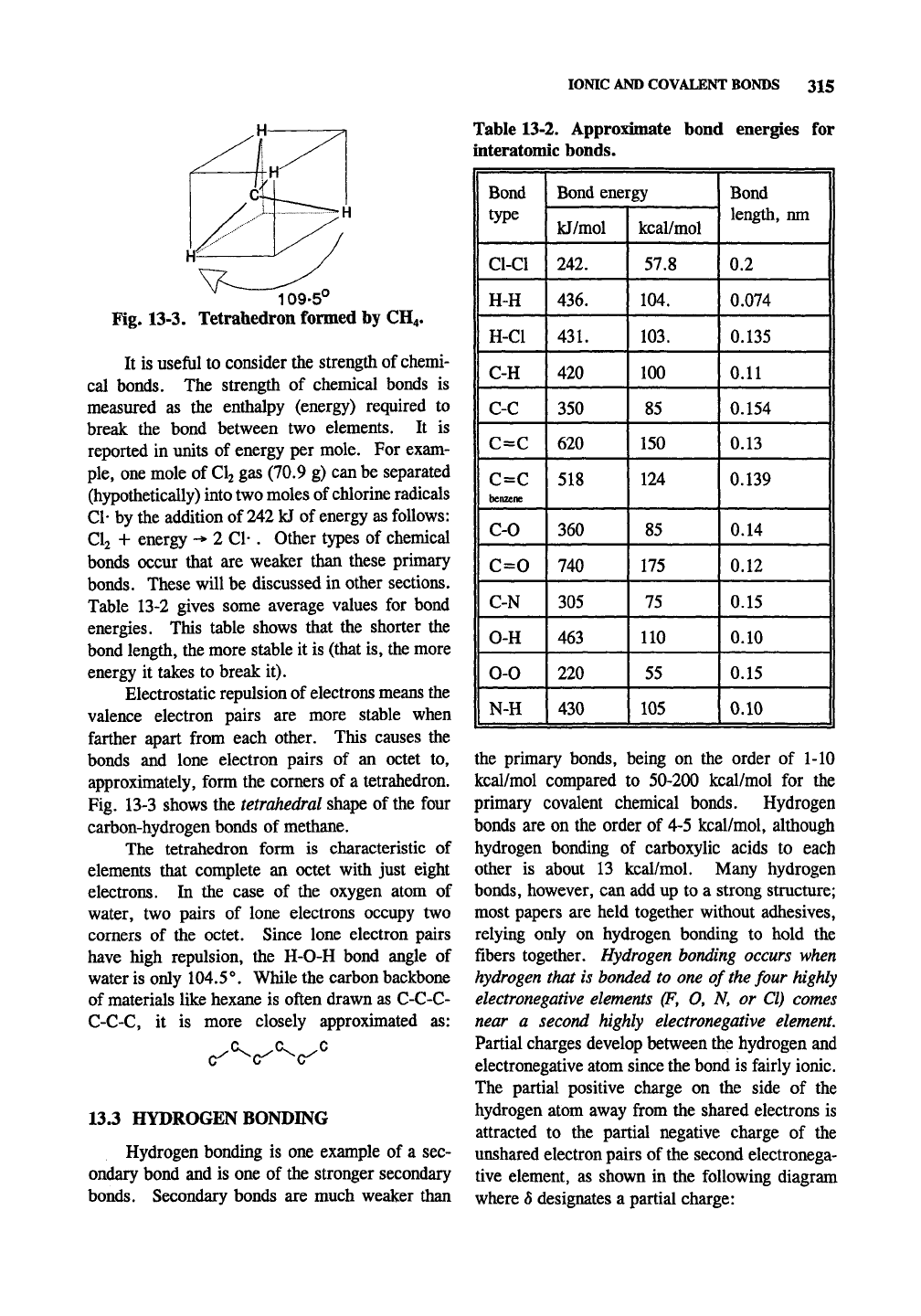
109-5"
Fig. 13-3. Tetrahedron formed by CH4.
It is useful to consider the strength of chemi-
cal bonds. The strength of chemical bonds is
measured as the enthalpy (energy) required to
break the bond between two elements. It is
reported in imits of energy per mole. For exam-
ple,
one mole of
CI2
gas (70.9 g) can be separated
(hypothetically) into two moles of chlorine radicals
CI-
by the addition of
242
kJ of energy as follows:
CI2 + energy -* 2 CI- . Other types of chemical
bonds occur that are weaker than these primary
bonds. These will be discussed in other sections.
Table 13-2 gives some average values for bond
energies. This table shows that the shorter the
bond length, the more stable it is (that is, the more
energy it takes to break it).
Electrostatic repulsion of electrons means the
valence electron pairs are more stable when
farther apart from each other. This causes the
bonds and lone electron pairs of an octet to,
approximately, form the comers of a tetrahedron.
Fig. 13-3 shows the tetrahedral shape of the four
carbon-hydrogen bonds of methane.
The tetrahedron form is characteristic of
elements that complete an octet with just eight
electrons. In the case of the oxygen atom of
water, two pairs of lone electrons occupy two
corners of the octet. Since lone electron pairs
have high repulsion, the H-O-H bond angle of
water is only 104.5°. While the carbon backbone
of materials like hexane is often drawn as C-C-C-
C-C-C, it is more closely approximated as:
13.3 HYDROGEN BONDING
Hydrogen bonding is one example of a sec-
ondary bond and is one of the stronger secondary
bonds. Secondary bonds are much weaker than
Bond
type
1 ci-ci
1
H-"
1
H-ci
C-H
C-C
II
^=^
C=C
1 benzene
1
C-0
|c=o
II
^-N
1
0-H
0-0
II
N-H
Bond energy
kJ/mol
242.
436.
431.
420
350
620
518
360
740
305
463
220
1
430
kcal/mol
57.8
104.
103.
100
85
150
124
85
175
75
110
55
105
Bond
length, nm
0.2
II
0.074
1
0.135
1
0.11
II
0.154
1
0.13
1
0.139
0.14
1
0.12
1
0.15
1
i
010 1
0.15
1
0.10
1
the primary bonds, being on the order of 1-10
kcal/mol compared to 50-200 kcal/mol for the
primary covalent chemical bonds. Hydrogen
bonds are on the order of 4-5 kcal/mol, although
hydrogen bonding of carboxylic acids to each
other is about 13 kcal/mol. Many hydrogen
bonds, however, can add up to a strong structure;
most papers are held together without adhesives,
relying only on hydrogen bonding to hold the
fibers together. Hydrogen bonding occurs when
hydrogen that is bonded to one of the four highly
electronegative elements (F, O, N, or CI) comes
near a second highly electronegative element.
Partial charges develop between the hydrogen and
electronegative atom since the bond is fairly ionic.
The partial positive charge on the side of the
hydrogen atom away from the shared electrons is
attracted to the partial negative charge of the
unshared electron pairs of the second electronega-
tive element, as shown in the following diagram
where 6 designates a partial charge:
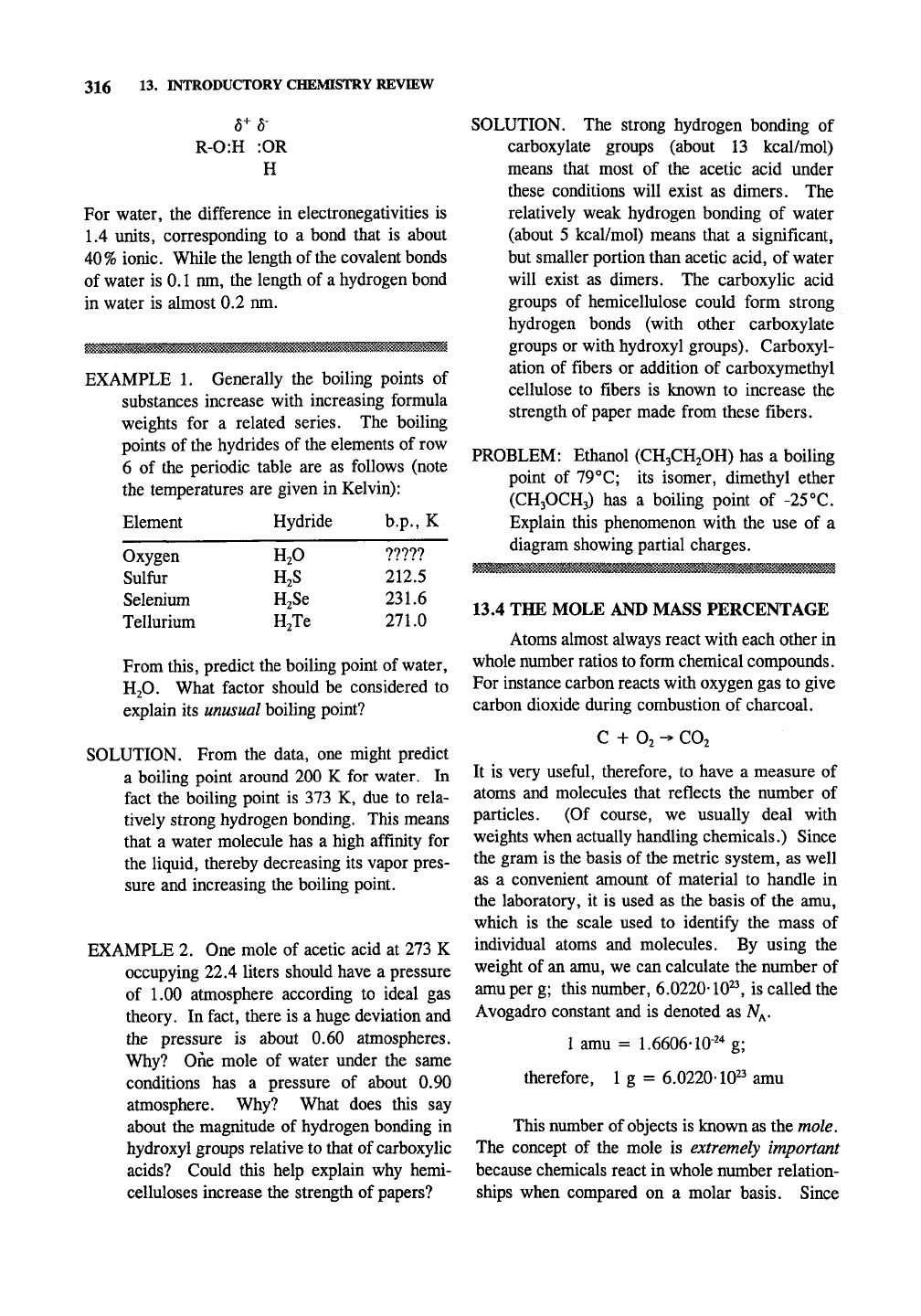
316 13. INTRODUCTORY CHEMISTRY REVIEW
6+6-
R-0:H :OR
H
For water, the difference in electronegativities is
1.4 units, corresponding to a bond that is about
40%
ionic. While the length of the covalent bonds
of water is 0.1 nm, the length of a hydrogen bond
in water is almost 0.2 nm.
EXAMPLE 1. Generally the boiling points of
substances increase with increasing formula
weights for a related series. The boiling
points of the hydrides of the elements of row
6 of the periodic table are as follows (note
the temperatures are given in Kelvin):
Element
Hydride
b.p.,
K
Oxygen
Sulfur
Selenium
Tellurium
HjO
HjS
HjSe
HjTe
?????
212.5
231.6
271.0
From this, predict the boiling point of water,
H2O.
What factor should be considered to
explain its unusual boiling point?
SOLUTION. From the data, one might predict
a boiling point around 200 K for water. In
fact the boiling point is 373 K, due to rela-
tively strong hydrogen bonding. This means
that a water molecule has a high affinity for
the liquid, thereby decreasing its vapor pres-
sure and increasing the boiling point.
EXAMPLE 2. One mole of acetic acid at 273 K
occupying 22.4 liters should have a pressure
of LOO atmosphere according to ideal gas
theory. In fact, there is a huge deviation and
the pressure is about 0.60 atmospheres.
Why? One mole of water under the same
conditions has a pressure of about 0.90
atmosphere. Why? What does this say
about the magnitude of hydrogen bonding in
hydroxyl groups relative to that of carboxylic
acids? Could this help explain why hemi-
celluloses increase the strength of papers?
SOLUTION. The strong hydrogen bonding of
carboxylate groups (about 13 kcal/mol)
means that most of the acetic acid under
these conditions will exist as dimers. The
relatively weak hydrogen bonding of water
(about 5 kcal/mol) means that a significant,
but smaller portion than acetic acid, of water
will exist as dimers. The carboxylic acid
groups of hemicellulose could form strong
hydrogen bonds (with other carboxylate
groups or with hydroxyl groups). Carboxyl-
ation of fibers or addition of carboxymethyl
cellulose to fibers is known to increase the
strength of paper made from these fibers.
PROBLEM: Ethanol (CH3CH2OH) has a boiling
point of 79°C; its isomer, dimethyl ether
(CH3OCH3) has a boiling point of
-25
°C.
Explain this phenomenon with the use of a
diagram showing partial charges.
13.4 THE MOLE AND MASS PERCENTAGE
Atoms almost always react with each other in
whole number ratios to form chemical compounds.
For instance carbon reacts with oxygen gas to give
carbon dioxide during combustion of charcoal.
C +
O2
^ CO2
It is very useful, therefore, to have a measure of
atoms and molecules that reflects the number of
particles. (Of course, we usually deal with
weights when actually handling chemicals.) Since
the gram is the basis of the metric system, as well
as a convenient amount of material to handle in
the laboratory, it is used as the basis of the amu,
which is the scale used to identify the mass of
individual atoms and molecules. By using the
weight of an amu, we can calculate the number of
amu per g; this number, 6.0220-10^^ is called the
Avogadro constant and is denoted as
N^^,
lamu = L6606-10-2^g;
therefore, 1 g = 6.0220-102^ amu
This number of objects is known as the mole.
The concept of the mole is extremely important
because chemicals react in whole number relation-
ships when compared on a molar basis. Since

THE MOLE AND MASS PERCENTAGE 317
carbon is 12.01 amu, one mole of carbon is 12.01
g of carbon. This is often called the gram atomic
weight since it is the atomic weight of a substance
in grams. Similarly, one mole of carbon dioxide
(CO2) is 12.01 + (2 X 16.00) = 44.01 grams. In
the case of molecules with covalent bonds we
speak of gram molecular weights, and in the case
of compounds with ionic bonds we speak of gram
formula weights. The gram molecular weight of
CO2 is 44.01 grams, while the gram formula
weight of NaCl is 58.44 (from 22.99 + 35.45).
It is not so important to remember atomic versus
molecular versus formula weights as it is to under-
stand the concept of the mole, because one can
speak of 1 mole of carbon, carbon dioxide, and
sodium chloride.
The mass percentage of a substance is a
convenient method of expressing its composition.
Each component (such as "A") of the whole may
be expressed as a mass percentage as follows:
(13-1)
mass%A= '"^ of
A
in sample ^^^^^
total mass of sample
EXAMPLE 3. Calculate the formula weight of
Al2(S04)3 and the mass percentages of the
individual elements.
SOLUTION: This compound consists of two
aluminum cations and three sulfate anions.
The formula weight of this material is calcu-
lated as follows:
2 Al is 2 X 27 = 54
3 S is 3 X 32 = 96
12 O is 12 X 16 = 192
342 = formula weight
%A1 = 54/342 X 100% = 15.8%
%S = 96/342 X 100% = 28.1%
%0 = 192/342 X 100%
=56.1%
PROBLEM: Calculate the molecular weight for
glucose and the mass percentage of carbon in
glucose. The formula for glucose is
C6H12O6. Answers: 180 g/mol and 40.0%
carbon.
EXAMPLE 4. Given the (quantitative) chemi-
cal equation below, which is the conversion
of the kraft makeup chemical sodium sulfate
(Na2S04) into the active cooking species
sodium sulfide (NajS) in the recovery boiler,
how many grams of NajS will be produced
from 100 grams of Na2S04?
2C + Na2S04 -* Na2S + 2CO2
SOLUTION: The formula weight of sodium
sulfate is 142 g/mole and that of sodium
sulfide is 78 g/mole.
1 mol Na^SO. 1 mol Na,S
lOOgNa^SO.
X
-^—^
X
-?—
^ ^ * 142 g Na^SO^ 1 mol Na2S04
78 g Na2S
1 mol Na^S
=
54.9gNa2S
PROBLEM: In this example, how many grams
of carbon were reacted? Answer: 16.9 g C.
13.5 EQUIVALENCY, MOLARITY AND
NORMALITY
Molarity
(A/)
is a measure of concentration in
terms of the mole and is moles of a substance per
liter (mol/L) of solution. One M solution of NaCl
would be 58.44 g/L of NaCl. An equivalent is
one mole of reactive species, whether it be an H^
of acid-base reactions, an electron of reduction-
oxidation reactions, or some other unit. In acid-
base reactions the basis of the equivalent is one
mole of hydrogen ions, H"^. For a monoprotic
acid,
which has one ionizable proton, such as
HCl, one equivalent is the same as one mole. For
a diprotic
acid,
which has two ionizable protons,
such as H2SO4 ?^ 2 H+ + S04^-, the equivalent
weight is one-half the molecular weight. One
mole of
H2SO4
is 98 g; one equivalent of
H2SO4
is
49 g. In electron-transfer (oxidation/reduction)
reactions the electron is the basis of the mole.
Potassium permanganate (KMn04) transfers five
electrons in most reactions where it is used. The
molecular weight of KMn04 is 158.0, and the
equivalent weight is 31.6 g/eq. Normality is
analogous to molarity and is concentration ex-
pressed as equivalents per liter. For example,
1
N
318
13.
INTRODUCTORY CHEMISTRY REVIEW
H2SO4 is 49 g/L of H2SO4 in an acid-base reac-
tion. In titrations, the volume is measured in ml.
Note that 1 eq/L = 1 meq/ml and 1 mol/L = 1
mmol/ml; these are useful relationships for
titrations.
EXAMPLE 5. If 17 grams of sodium chloride is
dissolved in water to make 600 ml of solu-
tion, what is the molarity of this solution?
SOLUTION: The formula weight of NaCl is
58.44.
Start with 17 grams NaCl per 0.6 L
and convert this to mol/L:
17 g NaCl 1 mole NaCl
0 X
0.6
H
58.44 g NaCl
=
0.485 mol/^ NaCl
=
0.485 M NaCl
PROBLEM: 2.0 g HCl is dissolved in water to
make 1 L of solution. What is the normality
of this solution? Same question with sulfuric
acid? Answers: 0.0549 iVHCl and 0.0408 iV
H2SO4.
13.6 ACIDS, BASES, AND THE pH SCALE
For the purposes of pulp and paper science it
is useful to use the Bronsted-Lowry theory of acids
and bases. According to this theory, an acid is a
compound that is a proton
(H"*^)
donor and a base
is a compound that is a proton acceptor. There
must be a proton acceptor to accept a donated
proton since protons are not stable in an uncom-
bined form. Thus, an acidic solution has a rela-
tively high concentration of protons (H"^ ions) in
hydrated form, since water is the proton acceptor.
The hydrated proton is known as the hydronium
ion,
HjO^. H^ and HsO"*" are often used inter-
changeably, although H"^ does not exist in water.
Alkaline or basic solutions have very low
concentrations of protons which, in water, neces-
sarily means a high concentration of
OH"
ion (to
be shown in Eq. 13-4). The empirical formula of
acids are often written with leading protons. For
example, CH3CH2OH, C2H4, and C3H8 are not
acids,
whereas HjS, H2SO3, and HC2H3O2 are
acids.
There are many exceptions to this rule.
For example, HC2H3O2 is acetic acid and is often
written as CH3COOH to show that it is a carbox-
ylic acid.
Another rule of
thumb
is that the elements H,
Al,
Ga, Sn, and Pb form oxides that are ampho-
teric, that is, compounds that are both acidic and
basic.
The first two of these elements are particu-
larly relevant to pulp and paper. Elements (not
including the noble gases) to the upper left of
these on the periodic table of the elements form
acidic oxides (for example, SO2 forms the acid
H2SO3
in water), while those elements to the lower
right of these form basic oxides [for example,
CaO forms the base Ca(0H)2 in water].
Examples of proton donors are HCl, which
becomes CI"; HNO3, which becomes NO3"; and
RCOOH, which becomes RCOO". Examples of
proton acceptors other than water are OH" from
bases such as NaOH, which becomes water since
H+ + OH ^ H2O; carboxylate anion RCOO",
which becomes RCOOH; and NH3, which be-
comes
NH4'*'.
A proton donor and the correspond-
ing base formed after the donation of a proton are
called
conjugate
pairs. The compound left after
donating a proton is called a conjugate base since,
in principle, it should be able to accept a proton.
A compound like HCl is highly ionic and com-
pletely dissociates in water to form H"^ (as Yi^O^)
and CI" ions; consequently, it is a very good
hydrogen donor
{strong
acid). On the other hand,
the conjugate base, CI", would not be expected to
have a high affinity for protons. Therefore, the
conjugate
base of a strong acid is a weak base. In
the same manner the conjugate acid of a strong
base is a weak
acid.
For this reason Na"*^ is a
very weak acid because its conjugate base (NaOH)
is a very strong base. The group lA ions of Li to
Fr are very poor bases and act as spectator
ions
in
acid-base reactions, that is, they are ions not
involved in a reaction.
In the case of acetic acid, CH3COOH, there
is partial ionization to form the acetate and proton
ions in solution. This is termed a weak
acid.
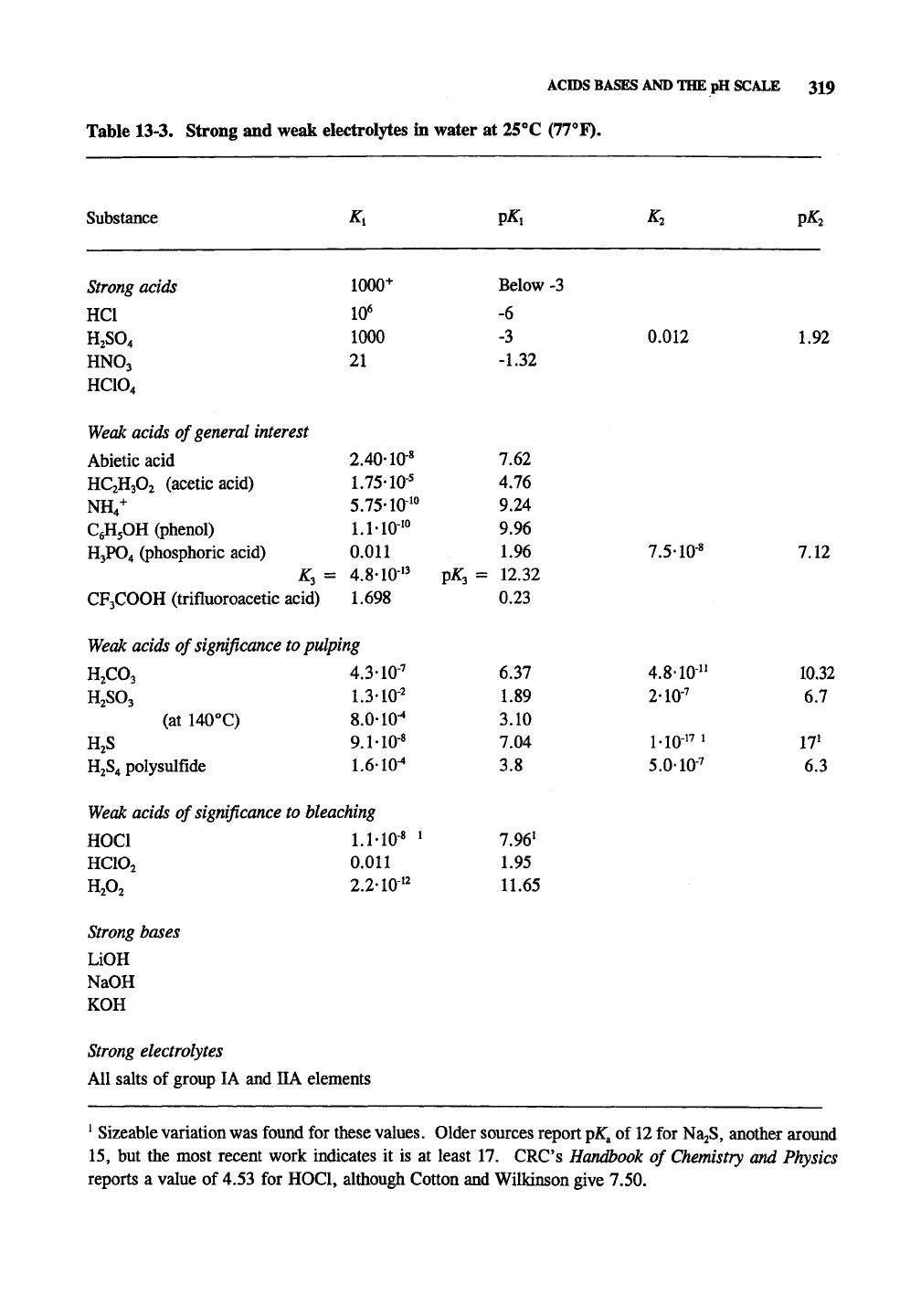
Table 13-3 lists all of the commonly encountered
strong acids, which are completely ionized in
water, and some weak acids with their ionization
constants. The acid
ionization
constant,
K^, is the
equilibrium constant for the degree of ionization.
ACIDS BASES AND THE pH SCALE 319
Table 13-3. Strong and weak electrolytes in water at 25^C (77^F).
Substance
Strong
acids
HCl
H2SO4
HNO3
HCIO4
Weak
acids of general interest
Abietic acid
HC2H3O2 (acetic acid)
NH/
C^HjOH
(phenol)
H3PO4 (phosphoric acid)
CF3COOH (trifluoroacetic
K,=
acid)
K,
1000+
10*
1000
21
2.40-10-«
1.75-10'
5.75-lO"
l.MO-'"
0.011
4.8-10"
1.698
Weak
acids of
significance
to
pulping
H2CO3
H2SO3
(at 140°C)
HjS
H2S4
polysnlfide
Weak
acids of
significance
HOC!
HCIO2
H,0,
4.3-10-'
1.3-10-^
8.010^
9.1-10-'
1.610^
to
bleaching
l.MO-«
•
0.011
2.2-10''
P^i
Below -3
-6
-3
-1.32
7.62
4.76
9.24
9.96
1.96
p^3 = 12.32
0.23
6.37
1.89
3.10
7.04
3.8
7.96'
1.95
11.65
K^
0.012
7.5-10-«
4.8-10"
2-10'
1-10" '
5.0-10-'
VKi
1.92
7.12
10.32
6.7
17'
6.3
Strong
bases
LiOH
NaOH
Strong
electrolytes
All salts of group lA and HA elements
^
Sizeable variation was found for these
values.
Older sources report p^a of 12 for NajS, another around
15,
but the most recent work indicates it is at least 17. CRC's Handbook of Chemistry and Physics
reports a value of 4.53 for HOCl, although Cotton and Wilkinson give 7.50.

320 13. INTRODUCTORY CHEMISTRY REVIEW
In the case of polyprotic acids, they are listed as
ATj,
K2,
K^,,..,
There may be considerable varia-
tion in the reported values of some acid dissocia-
tion
constants!
Since the concentration of
water
is
not included in the definition of the equilibrium
constant (since it is approximately constant in
dilute solutions), using A' as a general term for the
conjugate base (such as Ac for CH3COO", from
acetic acid, HAc) gives the following relationship:
(13-2)
HA
+
H^O^-HjO^+A"
K^ =
[H301[A-]
[HA]
Analogously, the base ionization
constant,
K^, is
the equilibrium constant for the production of OH",
such as from
NH3
(abbreviated as B) to give
NH4'^
(abbreviated as B"*") as shown in the following
equation:
(13-3)
B+HLjO^-BH^+OH-
^ _ [OH-][BH1
' [B]
A base can begin as a negative ion such as HSOj",
in which case BH"^ is H2SO3. These ionization
constants are examples of the law of mass action,
which is explained in more detail in the following
section. Strictly speaking, the
effective
concentra-
tion of ionic species is lower than the actual
concentration. The ratio of effective concentration
to the actual concentration is known as the activity
coefficient of the ion. Generally, as the ionic
strength of
a
solution increases, the activity coeffi-
cient of a given species decreases from unity.
Most pH calculations assume activity coefficients
of
unity.
The activity is the
effective concentration
of
an
ionic species. All equilibrium constants are
correctly stated only in terms of activity. Also,
pH meters and indicators actually measure hydro-
gen ion activity. Fortunately, in dilute solutions
the activity coefficients are approximately equal to
unity, and one can use concentration directly in-
stead of activities to simplify the calculations.
Even concentrated solutions are analyzed (such as
by titration) under relatively dilute conditions, al-
though in pulp and paper mills the pH of concen-
trated solutions is sometimes measured.
Water is both an acid and a base since it can
accept a proton to form Rf>^ or donate a proton
to become OH". The ionization constant for
water, K^, is 1 x 10"^^ at 25°C; p^ is 14.00.
Substituting water as the acid into Eq. (13-2), and
remembering that water as the solvent is not
included in the definition of an equilibriimi con-
stant, one obtains:
[H3O+] [OH] = 10-^^ = K^ (25°C) (13-4)
Lange's Handbook gives the p^ for water as
14.94 at 0\ 13.26 at 50°, 12.26 at 100°, and
11.91 at 130°C, while CRCs Handbook gives
11.64 at 150°C and 11.29 at 200°C.
This means that for pure water at 25°C, the
[H3O+] = [OH] = 10-^. The concentration of
H30"^
in water can vary from above 10^ to less
than
10"^^
molar. Because the acidity can vary by
over 16 orders of magnitude, it becomes conve-
nient to use a logarithmic scale to describe how
acidic or basic a solution is! The pH scale was
devised for that purpose. The pH is the negative
value
of the
base-10 logarithm
of the
concentration
of
hydrogen
ions. The pH of pure water is 7, the
pH of
0.1
M
(10-^
M) HCl is 1, and the pH of
10"
^
M NaOH is 13. By definition the pH scale goes
from 0 to 14, but it is sometimes useful to go
beyond a pH of 0 or 14. For example, one can
(secretly) imagine
10
M HCl as having a pH of
-1.
In general, the p function is quite useful as
will be seen in the next chapter. The p function is
the negative logarithm of a value. For example:
p(x) = - log (x)
We will use the p(OH), the p(^,), and the p(^b)
functions. In water p^a + P^b (of the conjugate
base) = 14; for example, the p^^ of ammonium
ion (4.76) plus the pXj, of ammonia (9.24) equal
14.
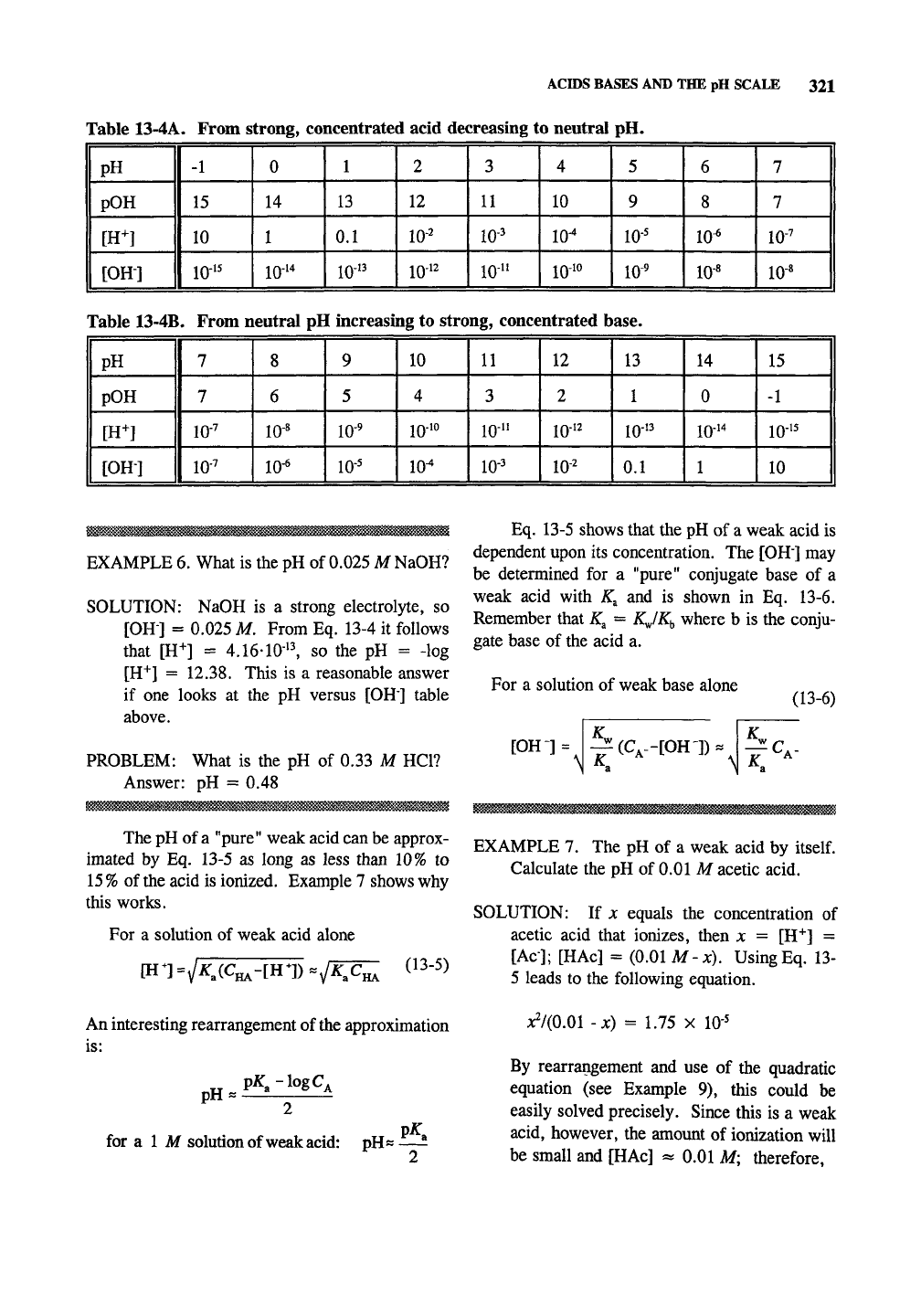
Table 13-4 shows the relationship between
pH, pOH,
[H"^],
and [OH] in dilute, aqueous
solutions.
By taking the logarithm of both sides of Eq.
(13-4) and using the definition of the p function,
we obtain the relationship pH 4- pOH = 14 for
water at 25°C.
ACIDS BASES AND THE pH SCALE 321
Table 13-4A. From strong, concentrated acid decreasing to neutral pH.
|pH
1
pOH
1
^"^^
1
1
[OH]
1
1
-1
15
1
10
1
10-''
0
14
1
10-'*
1
13
0.1
10-'^
2
12
10-^
10-'^
3
11
10-^
10"
4
10
10-"
10-10
5
9
10-'
10-'
6
8
10-*
io-«
7
1
7
10-'
1
io-«
1
Table 13-4B. From neutral pH increasing to strong, concentrated base.
IfpH
1
1
P^H
1
1
^"^^
1 [OH]
1
"^
1
"^
1
10'
1
10-'
8
6
10-'
10-*
9
5
10-'
10-'
10
4
10-10
10-*
11
3
10"
10-'
12
2
10-'^
10-^
13
1
10'^
0.1
14
0
10-'*
1
15
1
-1 1
10"
1
10
1
EXAMPLE 6. What is the pH of
0.025
M NaOH?
SOLUTION: NaOH is a strong electrolyte, so
[OH] = 0.025M. From Eq. 13-4 it follows
that [H+] = 4.16•10-^^ so the pH = -log
[H"*^] = 12.38. This is a reasonable answer
if one looks at the pH versus [OH] table
above.
PROBLEM: What is the pH of 0.33 M HCl?
Answer: pH = 0.48
Eq. 13-5 shows that the pH of a weak acid is
dependent upon its concentration. The [OH] may
be determined for a "pure" conjugate base of a
weak acid with K^ and is shown in Eq. 13-6.
Remember that
K^
= KJK^, where b is the conju-
gate base of the acid a.
For a solution of weak base alone
[0H-]= 5^(C.--[0H-])«
(13-6)
N^a
The pH of
a
"pure" weak acid can be approx-
imated by Eq. 13-5 as long as less than 10% to
15 %
of the acid is ionized. Example 7 shows why
this works.
For a solution of weak acid alone
An interesting rearrangement of
the
approximation
is:
pH«
pJ^^-logC^
for a 1 Af solution of weak
acid:
pH« —-
2
EXAMPLE 7. The pH of a weak acid by
itself.
Calculate the pH of 0.01 M acetic acid.
SOLUTION: If x equals the concentration of
acetic acid that ionizes, then x = [H"^] =
[Ac];
[HAc] = (0.01 M-x). Using Eq. 13-
5 leads to the following equation.
;c2/(0.01 -x) = 1.75 X 10-^
By rearrangement and use of the quadratic
equation (see Example 9), this could be
easily solved precisely. Since this is a weak
acid, however, the amount of ionization will
be small and [HAc] « 0.01 Af; therefore,
322 13. INTRODUCTORY CHEMISTRY REVIEW
jc^
= 1.75 X 10-^ X 0.01;
X = [H+] = 4.18 X 10-"; pH = 3.38
Notice that the assumption that x will have a
negligible effect on [HAc] is approximately
true; otherwise
A:
would have to be recalculat-
ed.
Use of the quadratic equation would
have given a pH of 3.39, which is not appre-
ciably different.
EXAMPLE 8. What is the pH of 0.1 M sodium
acetate?
SOLUTION: Using the approximation in Eq.
13-6 gives:
[OH] = ((IO-^VI.75 X 10-5)(0.1))«'^
[OH] = 7.56 X
10-^;
pH = 8.89
Since the [OH] is small relative to sodium
acetate, the approximation is warranted.
EXAMPLE 9. ^3 for phosphoric acid, H3PO4, is
4.8 X 10-^\ What is the pH of 0.1 M
trisodium phosphate (TSP)?
SOLUTION. Use of Eq. 13-5 with the approxi-
mation that
[H]"^
is small gives:
[OH] = ((10-^V4.8 X
10-^3)(0.1)f^
[OH] = 0.046; Note that the approximation
is not valid since this acid is nearly 50%
ionized!
Therefore, let x = [TSP] that reacts with
H+, so that
X
= [OH] = [CJ. At equilib-
rium, [TSP] = (0.1 Af -
jc).
Using Eq. 13-5
leads to the following equation.
A^ = (0.1 -
Jc)
KJK^ = (0.0208X0.1 -
Jc)
j^
+ 0.0208
X
- 0.00208 = 0
By use of the quadratic equation this is
solved as
A;
= 0.0364 (the negative solution
or root, -0.057, is discarded since concentra-
tions must be positive). This corresponds to
a pH of 12.56, which is very alkaline.
For polyprotic acids the determination of
pH
of
the
intermediate salt, such as NaHSOj, is more
complex since the bisulfite ion can act as a base or
acid. In this case, the [H"^] for a solution of the
salt alone can be shown to be equal to (^1^2)°'^ ^
long as Ki> >K2.
{K^
should be 10* or 10,000
times as large as
K2.)
By taking the log of both
sides this is rewritten as pH = (p^i + pK^/2.
Notice that the concentration of the salt does not
affect the pH; however, this is not a buffer solu-
tion as it represents an equivalence point of a
titration. See the section on pulp liquor analysis
for more details on this point. Therefore, for a
solution of
a
conjugate base which itself is an acid
and
J^i
> > K2:
EXAMPLE 10.
KHSO3?
Determine the pH of 0.1 M
Since the approximation cannot be used, the SOLUTION: Using Eq. 13-7 gives: pH =
quadratic equation is used to get a more exact
answer. A quadratic
equation
is in the form
of
ax^
+ &c + c = 0 where a, b and c are
coefficients
and
x is the variable. The gener-
al solution to the quadratic equation is:
JC=
-'b±yjb^-4ac
(pK, + pK2)/2 = (1.89 +
5.3)/2
= 3.59.
EXAMPLE 11. Determine the pH of 0.10 M
HK2PO3?
SOLUTION: Use of Eq. 13-7, but with
K2
and
^3,
gives a pH of 9.72.
2a
THE LAW OF MASS ACTION 323
13.7 THE LAW OF MASS ACTION
All chemical reactions are reversible (al-
though many to an infmitesimally small degree),
and an equilibrium condition is eventually estab-
lished where the rate of the forward reaction is
equal to the rate of the reverse reaction. A gener-
alized form of a chemical reaction is given as ah
+ fcB 4± cC + dD, where the lowercase letters in
italics are stoichiometric coefficients and the
capital letters are reacting species or products.
Strictly speaking, the effective concentration
of ionic species is lower than the actual concentra-
tion. The ratio of effective concentration to the
actual concentration is known as the activity
coefficient of the ion. Generally, as the total ionic
strength of a solution increases, the activity coeffi-
cient of a given species decreases from unity. The
activity is the effective concentration of ionic spe-
cies.
All equilibrium constants are correctly stated
only in terms of activity. Fortunately, in dilute
solutions the activity coefficients are approximately
equal to unity, and one can use concentration
directly instead of activities to simplify the calcula-
tions.
Even concentrated solutions are analyzed
(such as by titration) under relatively dilute condi-
tions.
Assuming activity coefficients of unity, the
molar concentrations, [A], [B], etc. are related at
equilibrium as follows:
[AfEBf
(13-8)
K^ is called the equilibrium constant and
varies with temperature. Solubility products and
acid ionization constants are specific examples of
the law of mass action. This law applies to gas-
eous or solution reactions. A key concept is that
the law of mass action does not predict the reac-
tion rate, only the final equilibrium concentration.
For example, a gaseous mixture of hydrogen and
oxygen at 25 °C is predicted to be almost com-
pletely reacted to form water; however, at 25 °C
the reaction will take thousands of years to reach
equilibrium. Catalysts are used to increase reac-
tion rates, but do not affect equilibrium constants.
Sometimes it is difficult to know whether there is
no change observed in the concentration of reac-
tants because equilibrium is achieved or because
the reactions are very slow.
With gases, it is often easier to express con-
centrations as partial pressures (the pressure
exerted by an individual species). When this is
done, the term
K^
is used and often the units are
atmospheres. K^ is usually highly dependent on
the temperature. For example, the equilibrium
constant for the oxidation of sulftir dioxide to
sulfiir trioxide is 4.0-10'" atm'^ at 298 K, 2.5-10'°
atm-' at 500 K, and 3.4 atm'^ at 1000 K. (Of
course, K^ can be determined from K^ using the
ideal gas law.) When the reaction species are
expressed as their pressures, P, Eq. 13-8 becomes:
(13-9)
EXAMPLE 12. Nitrogen gas reacts with hy-
drogen gas at elevated temperature in the
presence of a catalyst to produce ammonia in
the Haber synthesis. At 500°C and equilibri-
um the concentration of ammonia is 0.43 M,
the concentration of hydrogen gas is 1.09 M,
and the concentration of nitrogen gas is 1.3
M. Calculate the equilibrium constant for the
formation of ammonia.
SOLUTION: The reaction is Nj + 3H2 ^ 2NH3.
Using Eq. 13-8 with a=l,Z? = 3andc =
2,
and substituting in the concentrations in
molarity, it is found that K^ = 0.11 M.
PROBLEM: During the production of SO2 for
use in sulfite pulping, if excess oxygen is
present, SO3 may be formed and interferes
with the pulping process. At 1000 K, K^ =
3.4 atm-^ for the reaction 2SO2 + 02^
2SO3.
If the partial pressures of
SO2
and O2
are 0.25 atm and 0.001 atm, respectively,
what is the partial pressure of
SO3
at equilib-
rium? Answer: 0.0146 atm.
