Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

334 13. INTRODUCTORY CHEMISTRY REVIEW
Hydrogen bonding
4.
In which of the following compounds is hy-
drogen bonding possible?
a) RNHjb) RCHO
d) H^S e) CO2
g) EtOHh) RCHjCl
j) RCH2OH
c) RCOOH
f) HF
i) HjSOj
5.
6.
Draw the partial charges involved with hy-
drogen bonding of HCl in water.
Which of the following three compounds is
expected to have the highest boiling point?
Why?
a) CH3CH2OCH2CH3
b) CH3CH2CH2OCH3
c) CH3CH2GH2CH2OH
The mole and mass percentage
7.
Calculate the number of moles in each of the
following:
a) 98.3 g C5H12O6
b) 17.3gNa2SO4-10H2O
c) 210gNa2S
8. Calculate the mass percentage of sulfur in the
following chemicals:
a) NajS
d) SO2
b) Na2S04
e) NaHS03
c) H2SO3
9. 50 g of a mixture of NaCl and sucrose
(C12H22O11) was burned leaving 30.5 grams
of NaCl ash. How much
CO2
was produced?
Equivalency, molarity, and normality
10.
150 g of water contains 20 g of glucose,
C6HJ2O6, with MW = 180 g/mol. What is
the molality of the solution.
11.
How many grams of NaOH are required to
make 700 ml of 1.25 M NaOH solution?
What volume of 0.50 M H2SO4 is required to
neutralize this solution?
Acids, bases, and
the
pH scale
12.
What is the pH of the following solutions?
a) 0.047 M HCl
b) 0.0013
^^
NaOH
c) O.OIMH2SO4
13.
What is the pH of a solution containing 0.5
M acetic acid and 0.2 M sodium acetate?
14.
WhatisthepHof0.1MNaHCO3?
Oxidation-reduction
reactions
15.
What is the oxidation number of chromium in
16.
In the reaction 2Na + 2 H2O ^2NaOH +
Hjt, what species is the reducing agent?
Electrochemistry and corrosion
17.
The voltage required to produce electrolysis
of NaCl is about 4 volts. How much electri-
cal energy is required to produce one pound
of NaOH?
18.
Describe how pitting leads to high localized
corrosion.
19.
Why is it important to periodically replace
the magnesium strip in hot water heaters
equipped with these?
20.
What is galvanized metal?
Properties of gases
21.
A pure liquid is analyzed as 85.6% C and
14.4%
H. Under STP conditions the density
of its vapor is 3.75 g/L. What is the molec-
ular formula of
the
substance? Show a possi-
ble structure.
14
ANALYTICAL AND COORDINATE CHEMISTRY
This chapter presents concepts of analytical
chemistry. Acid-base chemistry is important for
pulping and bleaching operations. Redox reactions
are important to understand bleaching, corrosion,
and ion-specific electrodes. Coordinate chemistry
is central to alum and wet end chemistry and
involves many principles of analytical chemistry.
For more detail on these concepts one should
consult an introductory text on quantitative analy-
sis or analytical chemistry.
14.1 STRONG ACID-STRONG BASE
TITRATIONS
Neutralization is the formation of water from
a strong acid and a strong base to form a neutral
compound, i.e., a compound that is neitiier an acid
nor a base, such as NaCl. Consider the reaction
of HCl + NaOH -^ H2O + NaCl. Since HCl,
NaOH, and NaCl are all completely ionized in
solution, this reaction can be written as: H"^ +
CI"
+ Na+ + OH- -* Na+ + CI" + HOH. Na+
and CI" are spectator ions, that is, they are not
involved in the reaction, so the overall reaction is
written simply as H"^ + OH- -* HOH; this is the
essence of neutralization reactions. The enthalpy
of neutralization of a strong acid and strong base
is about -57.2 kJ/mol. The term "neutralization"
is sometimes used for the reaction of any acid and
base,
such as the reaction of acetic acid and
sodium hydroxide to form sodium acetate and
water, but this is not strictly correct use of the
word, since sodium acetate is a weak base.
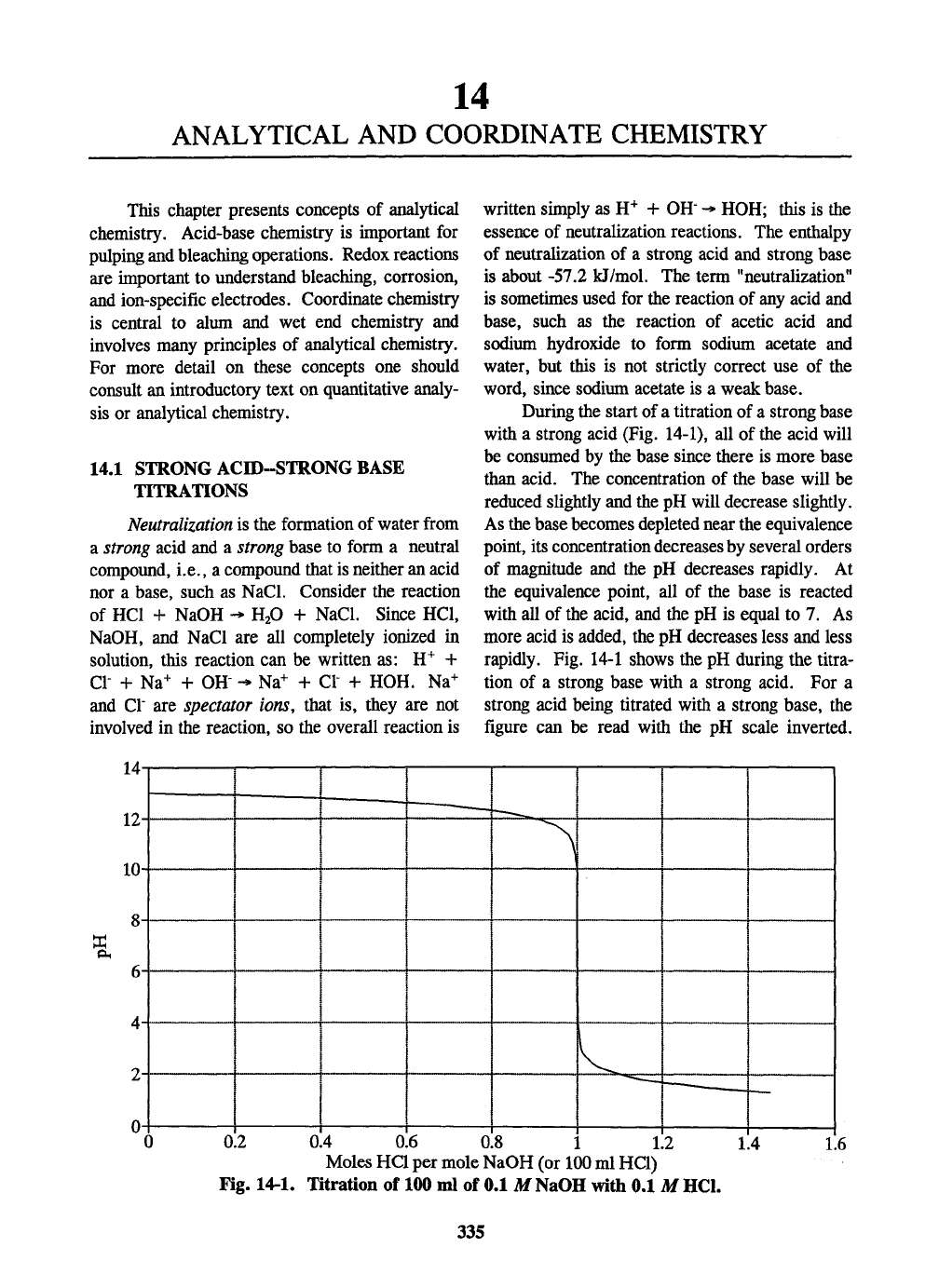
During the start of a titration of a strong base
with a strong acid (Fig. 14-1), all of the acid will
be consumed by the base since there is more base
than acid. The concentration of the base will be
reduced slightly and the pH will decrease slightly.
As the base becomes depleted near the equivalence
point, its concentration decreases by several orders
of magnitude and the pH decreases rapidly. At
the equivalence point, all of the base is reacted
with all of the acid, and the pH is equal to 7. As
more acid is added, the pH decreases less and less
rapidly. Fig. 14-1 shows the pH during the titra-
tion of a strong base with a strong acid. For a
strong acid being titrated with a strong base, the
figure can be read with the pH scale inverted.
14-
10-
IZ
iU
0
0
A-
4
Z
n-
""^^
L_
0.2 0.4 0.6 0.8 1 1.2
Moles HQ per mole NaOH (or 100 ml HCl)
Fig. 14-1. Titration of 100 ml of 0.1 M NaOH with 0.1 M HCl.
1.4 1.6
335
336 14. ANALYTICAL AND COORDINATE CHEMISTRY
The endpoint is detected with a pH meter or
suitable indicator (usually phenolphthalein) as
discussed in the next section.
Since normality is equivalents per liter, if the
normality of a solution is multiplied by the volume
of solution, the number of equivalents is deter-
mined for that solution: normality x volume =
equivalents. Also, at the endpoint of a titration,
by definition, the number of equivalents in the
sample being analyzed (the analyte) is equal to the
number of equivalents in the titrant (the standard
solution used in the buret). This leads to the very
useful titration equation of
N,V,=N,V,
(14-1)
The normality of titrant multiplied by the volume
of titrant equals the normality of
analyte
multiplied
by the volume of analyte. During titrations the
volume is measured in ml; therefore it is useful to
remember that I N = I eq/L = 1 meq/ml.
EXAMPLE 1. 5.00 ml of HCl solution of un-
known strength required 15.35 ml of 0.500 N
NaOH to achieve the endpoint (neutraliza-
tion).
Calculate the following:
1.
2.
3.
4.
5.
6.
7.
9.
10.
Milliequivalents of NaOH consumed.
Milliequivalents of HCl consumed, or
neutralized by the NaOH.
Equivalents of HCl consumed.
The equivalent weight of HCl.
The milliequivalent weight of HCl.
The weight of HCl neutralized.
The normality of the original HCl solu-
tion.
The strength of the original HCl solu-
tion in g/L.
The strength of the original HCl solu-
tion in lb/gal.
The weight of the NaOH consumed.
SOLUTION:
1.
15.35 ml NaOH x 0.5 meq/ml = 7.67
meq NaOH.
2.
By definition of equivalency,
eq NaOH = eq HCl = 7.67 meq.
3.
7.67 meq/(1000 meq/eq) = 0.00767 eq
HCl.
6.
The equivalent weight of HCl is equal
to the formula weight; therefore, the
equivalent weight = 1 + 35.5 = 36.5.
(36.5 g/eq HC1)/(1000 meq/eq) =
0.0365 g/meq HCl.
0.00767 eq HCl x 36.5 g/eq = 0.280
g HCl.
Since
NiVi=N2V2,
with rearrangement
one obtains the following:
^1 =
N^xV^
^i^l5^
= L534NHCl
5.0
m{
(0.280 g HCl)/(0.005 L) = 56 g/L HCl
Dimensional analysis of the concentra-
tion in g/L gives:
U lib
454 g 0.264 gal
=0.467-5!^
HCl
gal
10.
7.67 meq NaOH x 40 mg/meq X 1
g/(1000 mg) = 0.306 g NaOH
EXAMPLE 2. How many ml of concentrated
sulfuric acid (density = 1.86 g/ml) should
one add to make 1.00 L of 0.05 N H2SO4
solution? Note the notation of normality!
SOLUTION:
ImolH^SO,
0.05 eq/5 x
1^
x —- x 98g/mol H2SO4
2
eq H2SO4
1
md
H2SO4
L86gH2S04
=
1.32mC
cone. H^SO^
14.2 pH PROPERTIES OF WEAK ACID-
CONJUGATE BASE PAIRS
The pH of a weak acid can be expressed in
terms of the ionization constant and the concen-
trations of the weak acid, HA, and its conjugate
base.
A". The conjugate base is sometimes re-
ferred to as the salt of the weak acid. Rearrange-
ment of Eq. (13-2) gives the following equation
that is applicable when pKJ[C] > 0.1; i.e.,
greater than 10% ionization of A':
pH PROPERTIES OF WEAK ACID-CONJUGATE BASE PAIRS 337
[H^] = ^ [A]/[A-]
Taking the logarithm of each side gives:
pH = pi^ - log([A]/[A-])
Using the properties of logarithms, this equation
becomes:
pH = pi^ + log([A-]/[A]) (14-2)
Eq. 14-2 is called the
Henderson-Hasselbalch
equation. This equation should be used with some
care if the ratio of the salt to acid is very high or
very low. A
10"^
M solution of acetic acid without
sodium acetate might be expected to have a very
low pH as the ratio approaches 0 (and the log
approaches -00). However, as shown in Example
13-7,
the acid does ionize and appreciable amounts
of the conjugate base are present. Thus, Eq. 14-2
cannot be used near equivalence points (endpoints)
of titrations. Eq. 14-2 can be written in the form
of a weak base as follows:
pOH = vK, + log([BH^]/[B])
= pjR; + log([salt]/[base]) (14-3)
The Henderson-Hasselbalch equation is very
useful for describing the pH behaviors of mixtures
of weak acids and their conjugate bases in solu-
tion. If equal molar concentrations of an acid and
its conjugate base are in solution, the pH will be
equal to the pX^ of that acid, since -log(/z//i) = 0
and pH = pX^. This is a useful fact to remember
because it gives a rough estimate of pH of a
solution containing an acid and its conjugate base.
Furthermore, even if this solution is diluted, the
pH will not change. Also this solution can absorb
small amounts of acid or base without appreciably
changing the ratio of acid and salt form. There-
fore,
such a solution would be a good buffer, that
is,
a solution which resists changes in pH by
dilution or by addition of acid or base. Therefore,
any solution of a weak acid and its conjugate base
is a buffer. The amount of buffering capacity is
proportional to the concentration of these species.
The maximum buffering action is achieved for a
given concentration of weak acid when the ratio of
the concentration of salt to acid is unity (i.e., both
the acid and its conjugate base are of equal con-
centration). Later, it will be observed that this is
the flattest part of a titration curve for a weak acid
or base.
EXAMPLE 3. What is the pH of a solution of
0.1 M
H2SO3
and 0.2 M NaHSOa?
SOLUTION: Table 11-3 gives a p^, of 1.89 for
sulftirous acid at 25
^'C.
Therefore:
pH = 1.89 + log (0.2/0.1) = 2.19^
EXAMPLE 4. A solution that is 1 M acetic acid
and 1 M sodium acetate has a pH of 4.76,
which is the p^a fo^* acetic acid. What will
the pH be after 0.5 mole of HCl is added to
1 liter of this solution?
SOLUTION: The strong acid reacts completely
with the weak base to give 0.5 mol/L HAc
and leaving 0.5 mol/L Ac'. The total [HAc]
= 1.5 M. Therefore, pH = 4.76 +
log(0.5/1.5) = 4.28, a small change.
PROBLEM: What would be the pH of 1 L of
water with 0.5 moles HCl? Answer: The
pH would be 0.30, which shows the effect
of a buffer solution when compared to this
example.
14.3 pH INDICATORS
The pH of solutions can be monitored using
indicators. Indicators are themselves weak acids
or bases whose ionized forms differ in color from
the un-ionized forms. The color of these species
should be intense so only small amounts of indica-
tor are used; after all, the indicators are also
reacting with
the
titrant. Usually these compounds
involve conjugated aromatic systems. A common-
ly used indicator is phenolphthalein. Fig. 14-2
shows the un-ionized, colorless form and the
ionized, deep red-purple form. The p^a of pti^-
nolphthalein is about 9. Using Eq. 14-2, this
338 14. ANALYTICAL AND COORDINATE CHEMISTRY
"°-^0^?-o
^'-'
COO"
Na"^
Acid form - colorless Salt form - deep red-purple
Fig. 14-2. Phenolphthalein indicator forms.
means that at pH 9 about half the indicator will be
in the ionized form, while at pH 8 only 1/10 the
indicator will be ionized. This is the range in
which one observes the most color change. Most
pH indicators change color over a pH range of
about 2, going from 90% of one color to 90% of
the other color. If one of the forms is colorless,
the color change is over one pH unit.
Phenolphthalein is a suitable indicator for
strong acid-strong base reactions. It reacts with
CO2,
so the CO2 must be boiled off near the end-
point to give a sharp endpoint for titrations involv-
ing carbonate. This will be discussed in greater
detail in Chapter 16 on pulping liquor analysis.
Phenolphthalein is suitable for titration of borax if
glycerol is present to avoid color fading. The
color change goes from colorless up to pH 8.5 to
deep red-purple above pH 9. Phenolphthalein is
usually used as a few drops of a
1 %
ethanol-water
solution, since it is only slightly soluble in water
in its un-ionized form.
Methyl orange is another common pH indica-
tor and goes from red below pH 3.1 to yellow
above pH 4.4; it is used as a 0.1% aqueous solu-
tion. There are about 60 additional pH indicators
available.
14.4 TITRATION OF A WEAK ACID WITH
A STRONG BASE OR WEAK BASE
WITH A STRONG ACID
The tools are now available to describe the
titration curve of a weak acid by a strong base or,
more likely in kraft pulping liquor analysis, the
titration of a weak base by a strong acid. Consid-
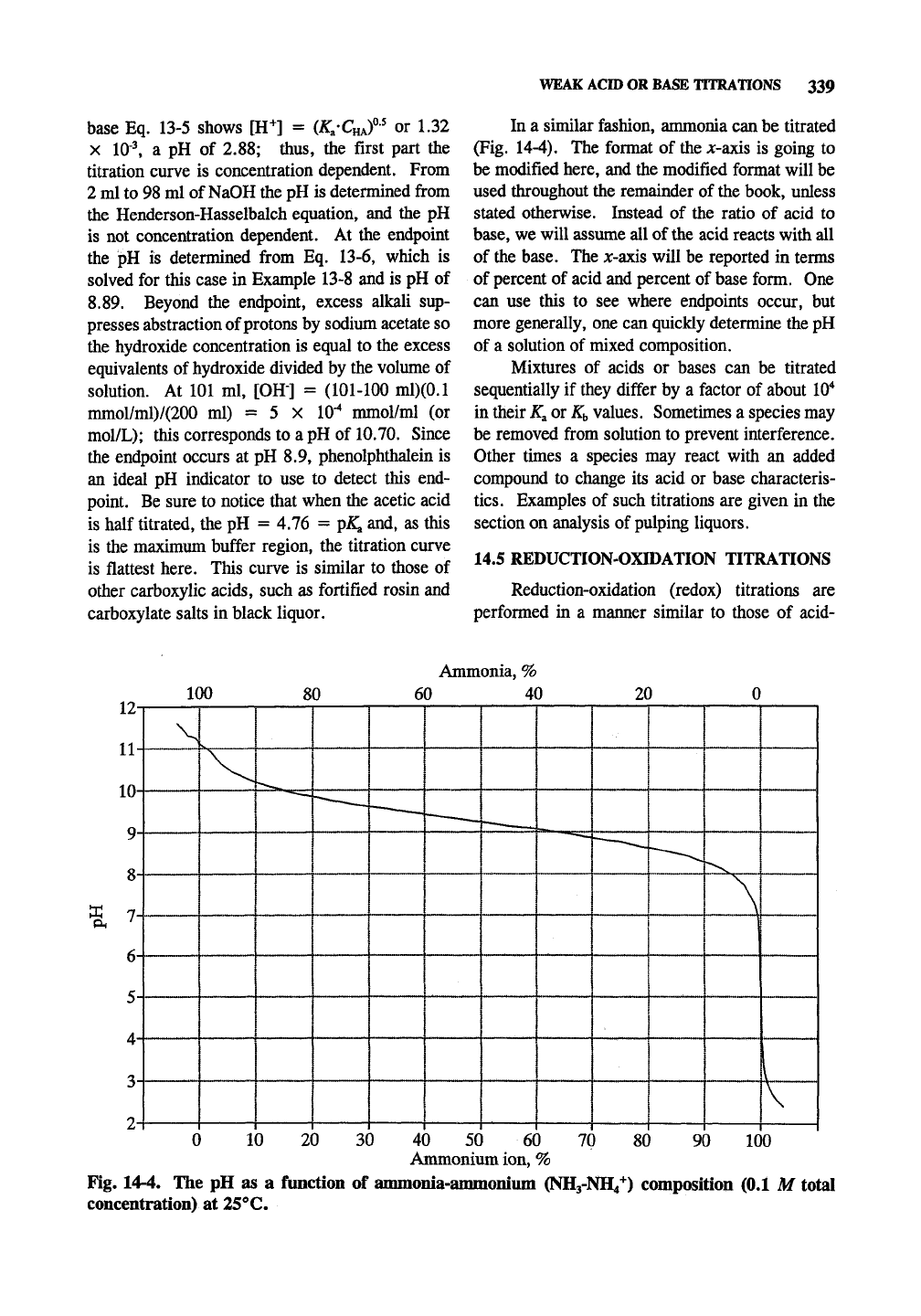
er the titration of 100 ml of 0.1 iV acetic acid by
0.1 N NaOH (Fig. 14-3). With no addition of
1
1
X
4.76
L3n
10
LZ
1
Ll"
in
LU"
0
y
Q
0
n
1
0-
\
4
2-
,^
J)
f
0.2 0.4 0.6 0.8 1 1.2
Moles NaOH per mole HAc (xlOOs ml NaOH)
1.4 1.6
Fig. 14-3. Titration of 100 ml of 0.1 M acetic acid with 0.1 M NaOH.
WEAK ACID OR BASE TITRATIONS 339
base Eq. 13-5 shows [H"*^] =
(K-C^^f'
or 1.32
X 10•^ a pH of 2.88; thus, the first part the
titration curve is concentration dependent. From
2 ml to 98 ml of NaOH the pH is determined firom
the Henderson-Hasselbalch equation, and the pH
is not concentration dependent. At the endpoint
the pH is determined from Eq. 13-6, which is
solved for this case in Example 13-8 and is pH of
8.89. Beyond the endpoint, excess alkali sup-
presses abstraction of protons by sodium acetate so
the hydroxide concentration is equal to the excess
equivalents of hydroxide divided by the volume of
solution. At 101 ml, [OH] = (101-100 ml)(0.1
mmol/ml)/(200 ml) = 5 X 10*^ mmol/ml (or
mol/L);
this corresponds to a pH of 10.70. Since
the endpoint occurs at pH 8.9, phenolphthalein is
an ideal pH indicator to use to detect this end-
point. Be sure to notice that when the acetic acid
is half titrated, the pH = 4.76 =
pK^
and, as this
is the maximum buffer region, the titration curve
is flattest here. This curve is similar to those of
other carboxylic acids, such as fortified rosin and
carboxylate salts in black liquor.
In a similar fashion, ammonia can be titrated
(Fig. 14-4). The format of the A:-axis is going to
be modified here, and the modified format will be
used throughout the remainder of the book, unless
stated otherwise. Instead of the ratio of acid to
base,
we will assume all of the acid reacts with all
of the base. The jc-axis will be reported in terms
of percent of acid and percent of base form. One
can use this to see where endpoints occur, but
more generally, one can quickly determine the pH
of a solution of mixed composition.
Mixtures of acids or bases can be titrated
sequentially if they differ by a factor of about 10"^
in their
K^
or
K^,
values. Sometimes a species may
be removed from solution to prevent interference.
Other times a species may react with an added
compound to change its acid or base characteris-
tics.
Examples of such titrations are given in the
section on analysis of pulping liquors.
14.5 REDUCTION-OXIDATION TITRATIONS
Reduction-oxidation (redox) titrations are
performed in a manner similar to those of acid-
11
Iz-
11-
10-
9-
X
1.
6-
4-
2-
100
\
SS-
'
Ammonia, %
80 60 40 20 0
"\
1
' ]vn
0
10
20 30
80
90 100
40 50 60 70
Ammonium ion, %
Fig. 14-4. The pH as a function of ammonia-ammoniiun (NH3-NH4*) composition (0.1 M total
concentration) at 25°C.

340 14. ANALYTICAL AND COORDINATE CHEMISTRY
base titrations. The titration is monitored by the
potential (voltage) with respect to a standard elec-
trode or by the use of indicators that are oxidized
or reduced after the analyte. In redox titrations,
equivalents are based on moles of electrons trans-
ferred in the reaction. A compound with a certain
equivalent weight for an acid-base reaction may
have a different equivalent weight in a redox
reaction; it may even have an equivalent weight
that varies depending on the particular redox
reaction.
In order for an analyte to be titrated with a
titrant there should be at least 0.2 to 0.3 V differ-
ence between the standard two electrode poten-
tials.
This does not say the reaction will occur
quickly, only that thermodynamically the reaction
is predicted to occur.
When the two materials have reacted, i.e.,
they are in equilibrium, both of their actual
half-
reaction potentials are equal. The general form of
the titration reaction with species (1) and (2) with
stoichiometric reaction coefficients of a, b, c, and
^is:
aOxi +
bRed2
^ cRed^ +
dOx2
Activity coefficients are ignored here since
the concentrations are low and the change in
potential at the endpoint will be sharp.
Consider the titration of I2, which usually
occurs as I3', with Na2S203 (sodium thiosulfate)
that is used in lignin determinations with
permanganate or determination of bleaching
chemicals with iodine. The reaction becomes:
I3-
+ 2S2O32 ^ 31 + SA^-
At any time during the titration the E of the
iodine/iodide cell is equal to the E of the thiosul-
fate/tetrathionate cell. The Nernst
equation
for the
half reaction of the compound that is reduced
(iodine) is :
n [OxV
at25°C
The iodine is generated from excess iodide
that is oxidized by permanganate or bleaching
chemicals in many pulp and paper laboratory
determinations. Therefore, let us consider the
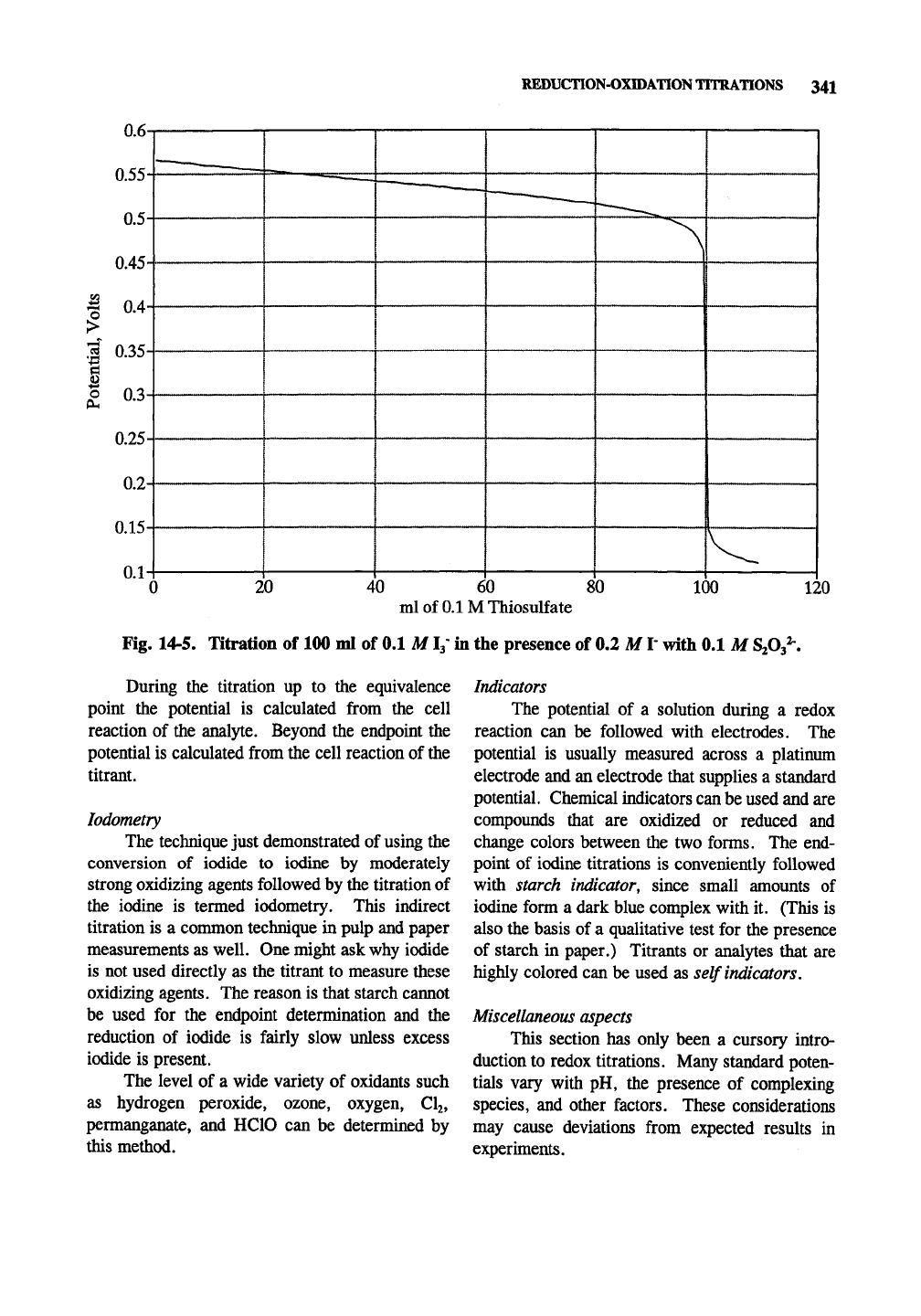
titration of 100 ml of 0.1
M13"
in the presence of
0.2 M iodide with 0.1 M thiosulfate (Fig. 14-5).
£° for the iodine/iodide cell is 0.534 V. The
E""
for the thiosulfate cell is 0.08 so the £° (cell) is
0.454 V, enough of a difference to give a sharp
endpoint. As soon as the first 0.5 ml or so is
added, the [I] will be known (if none had been
present initially) and the cell potential can be
calculated.
EXAMPLE 5. What is E of the iodine/iodide
cell just described after 0.5 ml of thiosulfate
is added in the reaction above?
SOLUTION: For each I3- reacted 3 l form.
Ignoring the small dilution:
E = £^-0.05915/2 X log(0.2015)V0.0995
= 0.534 + 0.032 = 0.566 V.
EXAMPLE 6. Knowing that the £eeii of the thio-
sulfate cell is 0.566 V, what is the concentra-
tion of thiosulfate?
SOLUTION: Assume all of the thiosulfate
reacted except for a minuscule amount in
equilibrium.
0.566 = 0.08 -0.05915/2 X log(x/0.0005)
3.69 X 10-^^ = (x/0.0005)
X = 1.83 X
10'^^
M (correct assumption)
PROBLEM: Redo this example with 2 ml of
titrant added. The answers are 0.565 V and
2 X 10-2« M
EXAMPLE 7. Shortly before the endpoint. Fig.
14-5 shows the potential as 0.40 V. What is
the concentration of thiosulfate if
tetrathionate is 0.1 Af?
0.40 = 0.08 -0.05915/2 X log(x/0.1000)
1.51 X 10" = (jc/0.1000)
x= 1.51 X
10-^0
M
REDUCTION-OXIDATION TITRATIONS 341
u.o-
U.4D-
1
"•'
.2 U.^D-
i
n 9-
u.z
n
1^-
U.ID"
0.1-
~~^^
v^
0 20 40 60 80 100 120
ml of
0.1
M Thiosulfate
Fig. 14-5. Titration of 100 ml of 0.1 M !{in the presence of 0.2 M
T
with 0.1 M
SiOj^.
During the titration up to the equivalence
point the potential is calculated from the cell
reaction of the analyte. Beyond the endpoint the
potential is calculated from the cell reaction of the
titrant.
lodometry
The technique just demonstrated of using the
conversion of iodide to iodine by moderately
strong oxidizing agents followed by the titration of
the iodine is termed iodometry. This indirect
titration is a common technique in pulp and paper
measurements as well. One might ask why iodide
is not used directly as the titrant to measure these
oxidizing agents. The reason is that starch cannot
be used for the endpoint determination and the
reduction of iodide is fairly slow unless excess
iodide is present.
The level of a wide variety of oxidants such
as hydrogen peroxide, ozone, oxygen, Clj,
permanganate, and HCIO can be determined by
this method.
Indicators
The potential of a solution during a redox
reaction can be followed with electrodes. The
potential is usually measured across a platinum
electrode and an electrode that supplies a standard
potential. Chemical indicators can be used and are
compounds that are oxidized or reduced and
change colors between the two forms. The end-
point of iodine titrations is conveniently followed
with starch indicator, since small amounts of
iodine form a dark blue complex with it. (This is
also the basis of a qualitative test for the presence
of starch in paper.) Titrants or analytes that are
highly colored can be used as self indicators.
Miscellaneous aspects
This section has only been a cursory intro-
duction to redox titrations. Many standard poten-
tials vary with pH, the presence of complexing
species, and other factors. These considerations
may cause deviations from expected results in
experiments.
342 14. ANALYTICAL AND COORDINATE CHEMISTRY
14.6 COLORIMETRIC ANALYSIS
Colorimetric analysis is important in the area
of effluent color of mill discharges and some
determinations of concentrations of chemical spe-
cies.
Glucose liberated by enzymatic hydrolysis of
starch is measured colorimetrically in the assay of
starch in corrugated board (TAPPI Standard T
532).
In liquid solutions, the amount of mono-
chromatic light absorbed by a solution is described
by
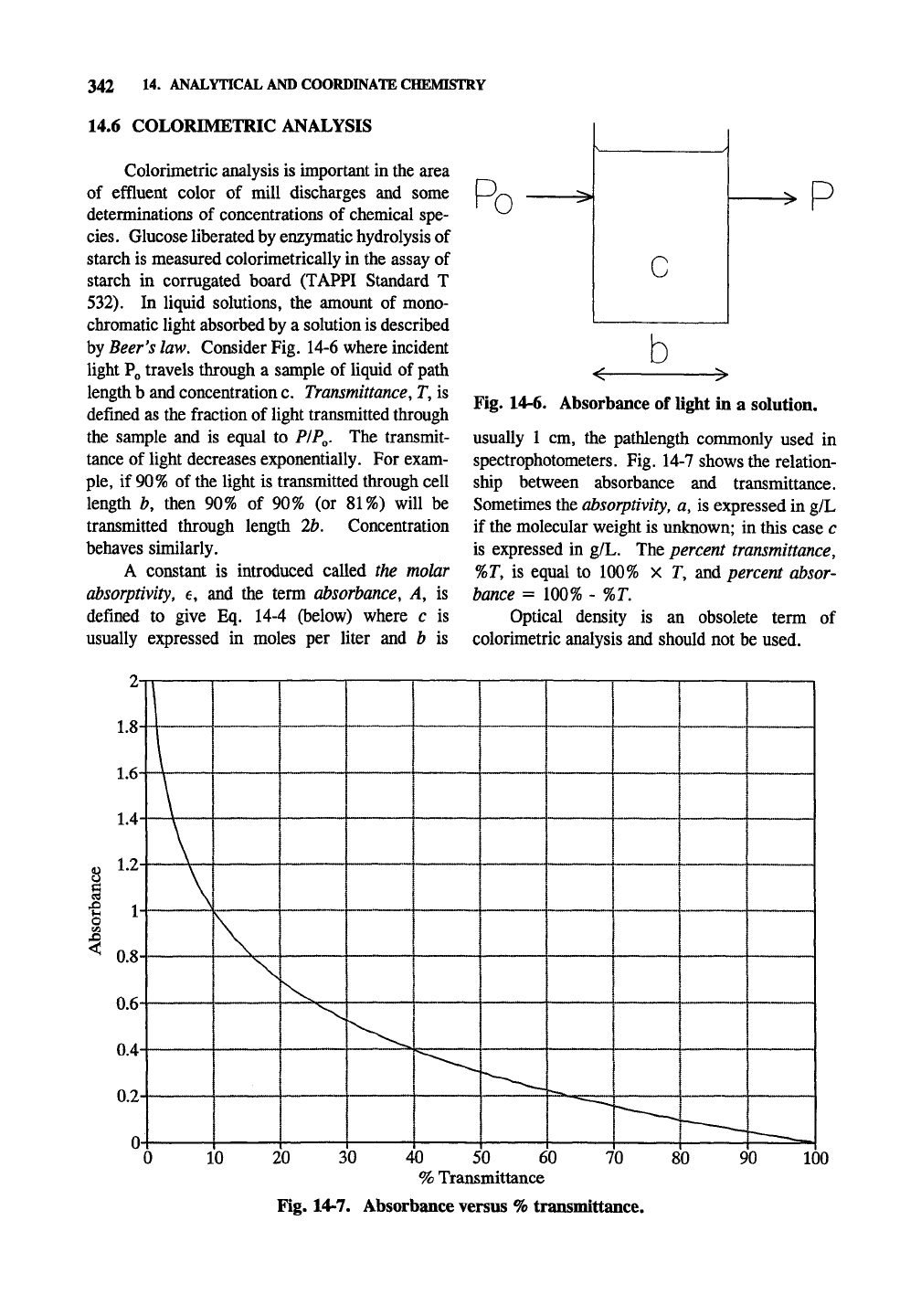
Beer^s
law. Consider Fig. 14-6 where incident
light Po travels through a sample of liquid of path
length b and concentration c. Transmittance, T, is
defined as the fraction of light transmitted through
the sample and is equal to PIPQ. The transmit-
tance of light decreases exponentially. For exam-
ple,
if
90%
of the light is transmitted through cell
length b, then 90% of 90% (or 81%) will be
transmitted through length lb. Concentration
behaves similarly.
A constant is introduced called the molar
absorptivity, e, and the term absorbance, A, is
defined to give Eq. 14-4 (below) where c is
usually expressed in moles per liter and b is
R
0
P
Fig. 14-6. Absorbance of light in a solution.
usually 1 cm, the pathlength commonly used in
spectrophotometers. Fig. 14-7 shows the relation-
ship between absorbance and transmittance.
Sometimes the
absorptivity,
a, is expressed in g/L
if the molecular weight is unknown; in this case c
is expressed in g/L. The percent transmittance,
%T, is equal to 100% X T, and percent absor-
bance = 100% - %T.
Optical density is an obsolete term of
colorimetric analysis and should not be used.
1 S-
l.o
1 #^-
l.O
1 A-
1.4
1 9-
8 ^-^
•S 1-
S
1
CO
U.o
U.o
U.4
n
9-
U.Z"'
0-
\
10 20 30 40 50 60
% Transmittance
70
80
90 100
Fig. 14-7. Absorbance versus
%
transmittance.
COLORIMETRIC ANALYSIS 343
Absorbance, and not transmittance nor
percent absorbance, is proportional to the concen-
tration and path length. Absorbance much above
2.5 cannot be measured accurately since this
corresponds to only 0.31% transmittance.
.4 = - log r = log 1/r
= log PJP = ebc
log 1/r = log PJP = abc
(14-4)
EXAMPLE 8. A sample in a 1.0 cm cell absorbs
10%
of the light at a certain wavelength. If
the absorptivity of the material is 3.0
L(g-cm) at this wavelength, what is the
concentration of the material?
SOLUTION: The transmittance is 0.90,
-log 0.90 = 3.0 L(gxm) x 1.0 cm x c;
c = 0.0153 g/L.
EXAMPLE 9. 10% of the light of a certain
wavelength of a pulp mill effluent is trans-
mitted through a distance of 1 feet. What
will the percent transmittance be at the same
wavelength through the same distance be if
the effluent is diluted
1:100?
SOLUTION: A^ = abc, = 1; ^2 = cibc^ =
abcJ\OQ\ A2 = 0.01; foT^ = 97.7%
PROBLEM: For this same problem what would
the apparent transmittance be for a river 2.5
feet deep? Remember the effective distance
will be 5 feet since the light will reflect off
the bottom and back through the water before
reaching the eye of the observer. Answer:
89.1%.
Beers law ahnost always applies. However,
apparent deviations may occur. For example, the
color of pulp mill effluents are pH dependent. If,
in the problem above, the pH were different
before and after dilution, then Beer's law may not
appear to hold. This is why the pH*s of mill
effluents are adjusted to that at which they will
ultimately be discharged before the absorbance
(using color units. Section 11.3) is measured.
14.7 COORDINATE CHEMISTRY
Introduction
Coordinate chemistry involves the for-
mation of complexes when two atoms share an
electron
pair.
One species makes the donation and
the second species accepts the electron pair.
Coordinate covalent bonds are formed when one
atom donates both electrons of the electron pair;
with other covalent bonds each atom donates one
electron to the pair. The distinction is arbitrary,
but has historical precedence. More information
and detail on this important subject may be found
in advanced inorganic chemistry textbooks.
Coordinate chemistry
explains
the behavior of
alum (which is central to many aspects of wet end
chemistry) in aqueous solutions. Aluminum ions
will be used to demonstrate some aspects of
coordinate chemistry. Also, many size press
formulations contain zirconium compounds or
synthetic polymers containing carboxylated poly-
mers that are "set" with metal ions. Applications
of coordinate chemistry will undoubtedly receive
much wider recognition in the future by the pulp
and paper industry.
The first explanation of the actual nature of
complexes is credited to the classic 1906 work of
Werner, for which Werner received the Nobel
Prize in 1913. Werner showed that neutral com-
pounds were bound directly to metal atoms in
many complexes. Thus, CuCl2*6NH3 is correctly
written as Cu(NH3)6Cl2. In general, coordination
complexes are formed upon the reaction of Lewis
acids, compounds which accept electron pairs,
with Lewis bases, compounds which donate elec-
tron pairs, to form the complex. Acid-base reac-
tions involving proton transfers are examples of
coordinate chemistry reactions. The reaction of
H^ and OH" to form the product H2O proceeds as
H^ + :0H' -• H:OH, where the electron pair is
shared in the product. Lewis greatly expanded the
class of acid-base reactions over that of the
Bronsted-Lowry theory by making electron pairs
of central importance rather than the proton.
Many other reactions besides acid-base
reactions are included as well. One classic exam-
ple is the reaction of the two gases BF3 and :NH3
to form the complex H3N:BF3, which is a white
solid:
