Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

324 13. INTRODUCTORY CHEMISTRY REVIEW
13.8 SOLUBILITY PRODUCTS
Many salts have limited solubility in water.
This leads to scale formation on processing equip-
ment. Conditions are chosen to minimize scale
formation by understanding the solubility proper-
ties of these compounds. For a salt of the form
A^B^,
the following relationship can be written:
AJ3^(s) ^ aA + bB. The effective concentration
of a solid in dilute aqueous solutions is a constant,
provided there is excess solid. The solubility
constant can be derived from the law of mass
action where the concentration (strictly speaking
activities) of the reactant (the solid, which is of
constant activity) is incorporated into the equilibri-
um constant and defined as follows:
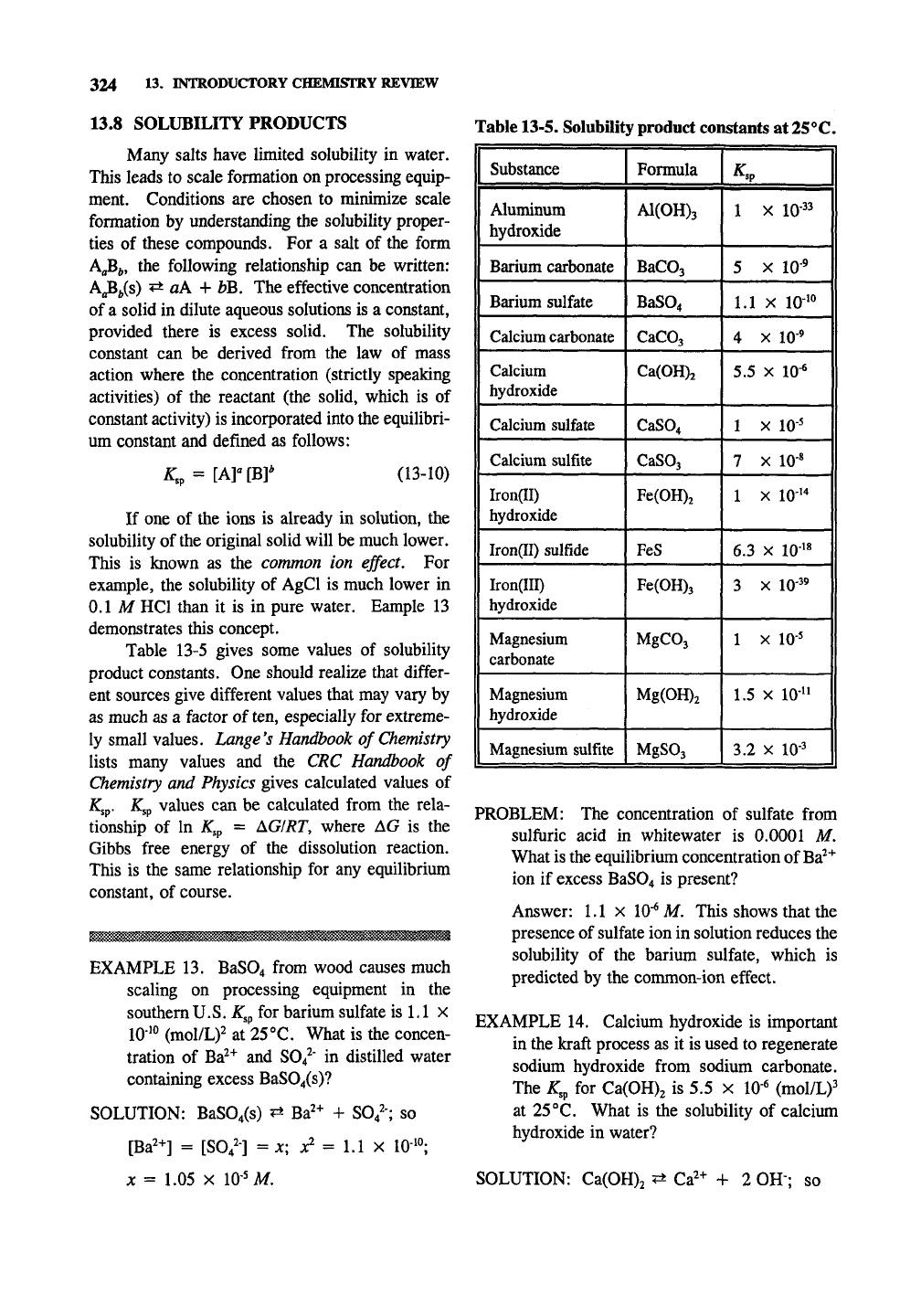
Table 13-5. Solubility product constants at 25°C.
i^sp = [Ar[Br
(13-10)
If one of the ions is already in solution, the
solubility of
the
original solid will be much lower.
This is known as the common ion effect. For
example, the solubility of AgCl is much lower in
0.1 M HCl than it is in pure water. Eample 13
demonstrates this concept.
Table 13-5 gives some values of solubility
product constants. One should realize that differ-
ent sources give different values that may vary by
as much as a factor of
ten,
especially for extreme-
ly small values. Lange's Handbook of
Chemistry
lists many values and the CRC Handbook of
Chemistry and Physics gives calculated values of
^sp-
^sp values can be calculated from the rela-
tionship of In X,p = AG//?r, where AG is the
Gibbs free energy of the dissolution reaction.
This is the same relationship for any equilibrium
constant, of course.
EXAMPLE 13. BaS04 from wood causes much
scaling on processing equipment in the
southern
U.S.
^^p for barium sulfate is 1.1 x
10'^^
(mol/L)2 at 25
°C.
What is the concen-
tration of Ba^"^ and 804^" in distilled water
containing excess BaS04(s)?
SOLUTION: BaS04(s) ^ Ba^^ + SO,^'
[Ba^+l = [SO42-] =;c; jc' = 1.1 X
X = 1.05 X
10-5
M.
so
10-^
Substance
Aluminum
1 hydroxide
Barium carbonate
Barium sulfate
1 Calcium carbonate
Calcium
hydroxide
Calcium sulfate
Calcium sulfite
Iron(II)
hydroxide
Iron(II) sulfide
Iron(III)
hydroxide
Magnesium
carbonate
Magnesium
hydroxide
Magnesium sulfite
Formula
A1(0H)3
BaCOj
BaS04
CaCOj
Ca(0H)2
CaS04
CaSOj
Fe(0H)2
FeS
Fe(0H)3
MgC03
Mg(0H)2
M^S03
^sp 1
1 X 10-33
5
X
10-^
1
1.1
X
10-^*^
II
4
X
10-^
1
5.5 X 10-^
1
X
10-5
1
7 X 10-«
II
1 X 10-^*
6.3
X 10-^«
II
3 X 10-3^
1 X 10-5
1.5 X 10-^*
3.2
X
10-3
1
PROBLEM: The concentration of sulfate from
sulfuric acid in Whitewater is
0.0001
M.
What is the equilibrium concentration of
Ba^^
ion if excess BaS04 is present?
Answer: 1.1 x
10-^
M. This shows that the
presence of sulfate ion in solution reduces the
solubility of the barium sulfate, which is
predicted by the common-ion effect.
EXAMPLE 14. Calcium hydroxide is important
in the kraft process as it is used to regenerate
sodium hydroxide from sodium carbonate.
The K,^ for Ca(0H)2 is 5.5 x 10'^ (mol/L)^
at 25°C. What is the solubility of calcium
hydroxide in water?
SOLUTION: Ca(0H)2 ^ Ca^^ + 2 OH'; so
SOLUBILITY PRODUCTS 325
[Ca'-'ILOH-]^
= 5.5 X
10"^
M^
[Ca2+] = x\ [OH] = 2x
X'{2xf
= 5.5 X
10-^
M^
jc =
0.0111
M; [OH] =0.0222
the pH is 12.34 and the original concentra-
tion of OH' (10'^) does not interfere with its
solubilization by the common ion effect.
Salts containing anions that are basic (for
example, OH",
COj^",
or S^) have more complicat-
ed chemistry. For example ^sp for calcium car-
bonate is 4 X 10-^ (mol/L)^ at 25°C. However,
the carbonate anion is a weak base and at pH
10.32 half of the COa^" is actually in the form of
HCOj'.
Since calcium bicarbonate is fairly soluble
in water, below pH 7 calcium carbonate is fairly
soluble and exists as calciimi bicarbonate.
Salts may contain cations that are acidic
because they complex with OH" groups (leaving
H"^ behind). The solubilities of such salts may be
strongly dependent on pH. Aluminum will be
considered in detail because of its importance in
wet end chemistry. Aluminimi hydroxide has very
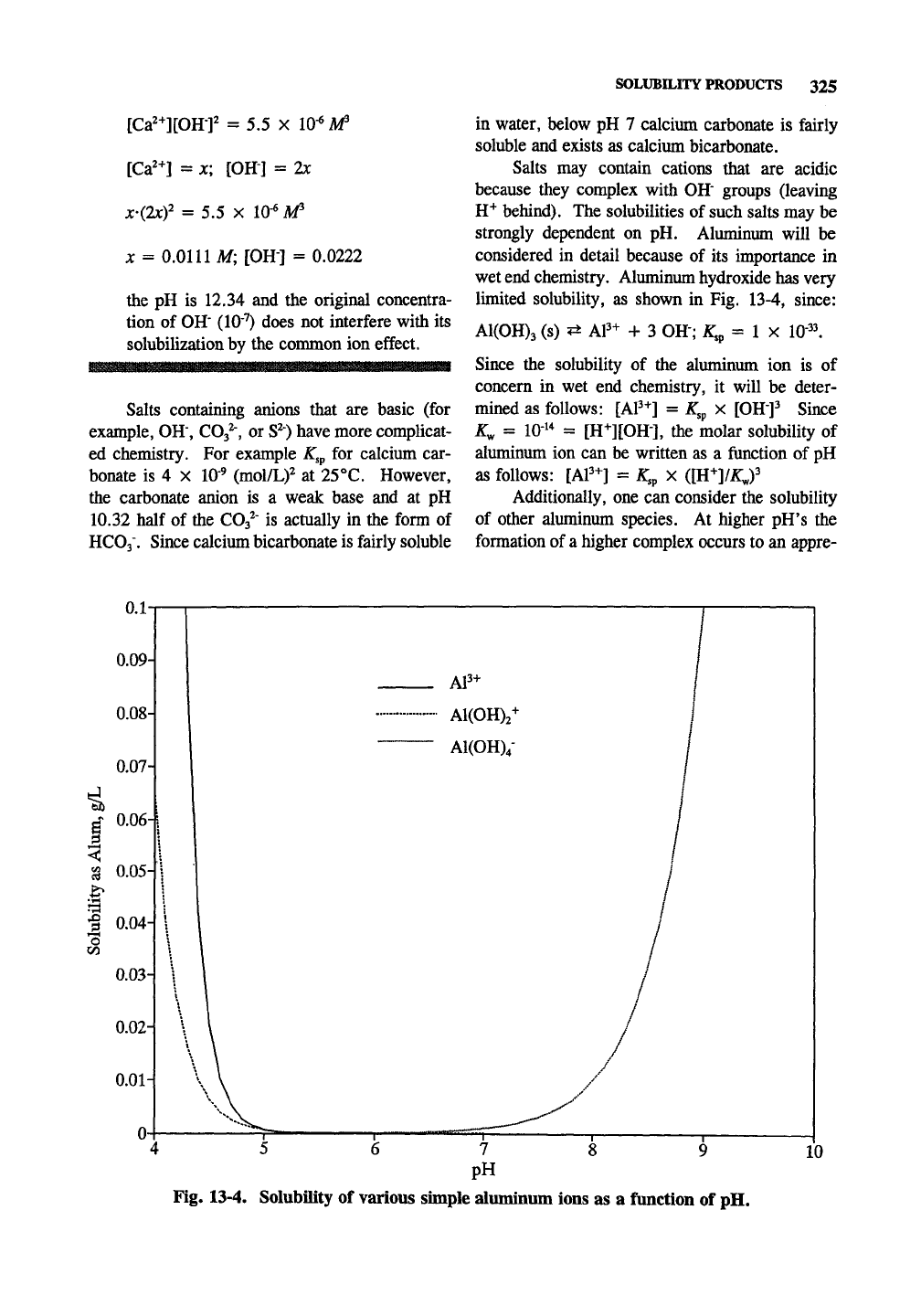
lunited solubility, as shown in Fig. 13-4, since:
A1(0H)3 (s) ^ AP-^ -h 3 OH"; ^sp = 1 x 10"^^
Since the solubility of the aluminum ion is of
concern in wet end chemistry, it will be deter-
mined as follows: [AP+] = ^,p x
[OH"]^
Since
K^ = 10"^* = [H-^][OH"], the molar solubility of
aluminum ion can be written as a function of pH
as follows: [AP+] = K,^ X ([H+]/X;,)^
Additionally, one can consider the solubility
of other aluminum species. At higher pH's the
formation of a higher complex occurs to an appre-
Fig. 13-4. Solubility of various simple aluminum ions as a function of pH.
326 13- INTRODUCTORY CHEMISTRY REVffiW
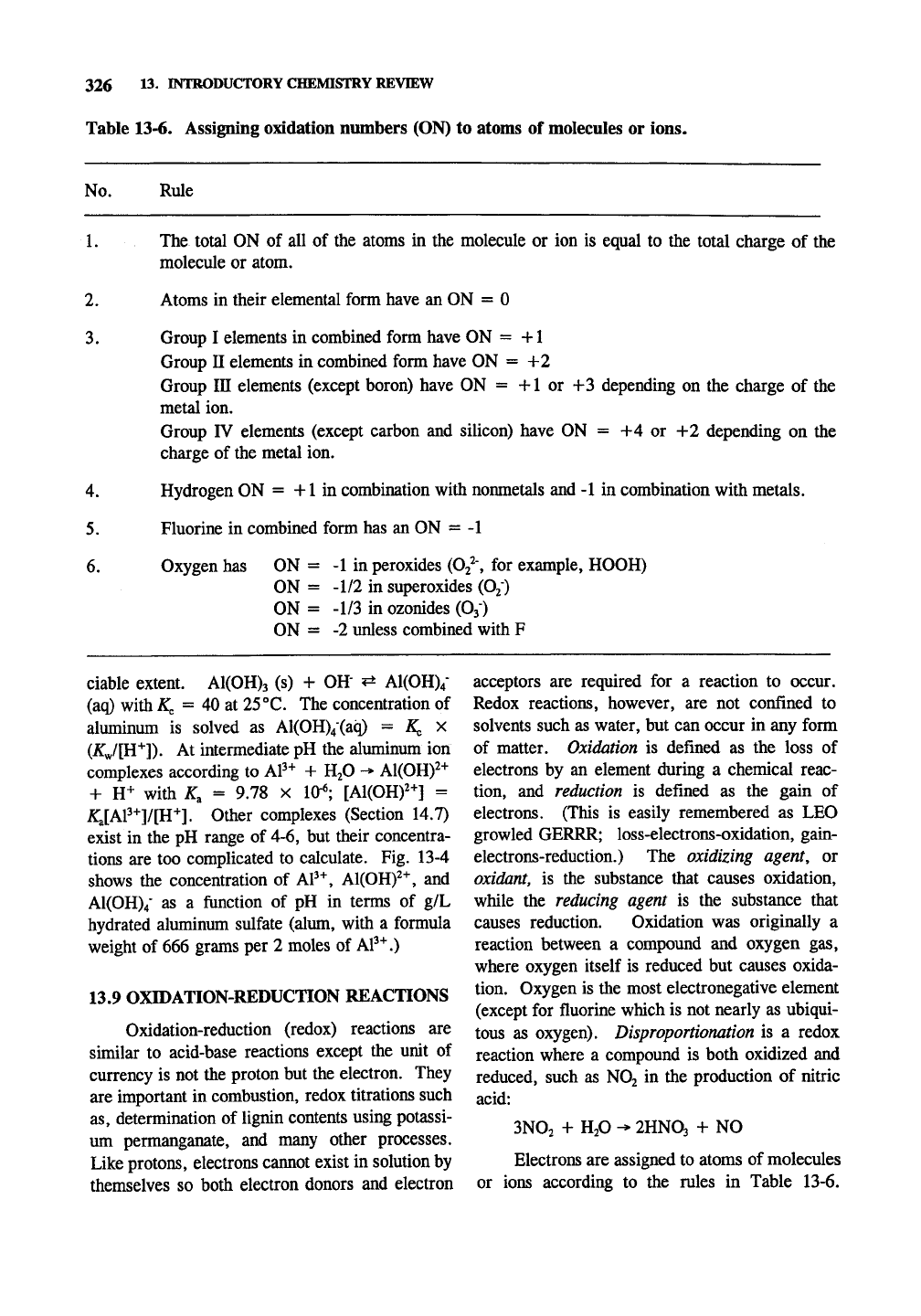
Table 13-6. Assigning oxidation numbers (ON) to atoms of molecules or ions.
No.
1.
2.
3.
Rule
The total ON of all of the atoms in the molecule or ion is equal to the total charge of the
molecule or atom.
Atoms in their elemental form have an ON = 0
Group I elements in combined form have ON = +1
Group n elements in combined form have ON = +2
Group in elements (except boron) have ON = +1 or +3 depending on the charge of the
metal ion.
Group IV elements (except carbon and silicon) have ON = +4 or +2 depending on the
charge of the metal ion.
4.
Hydrogen ON = +1 in combination with nonmetals and -1 in combination with metals.
5.
Fluorine in combined form has an ON = -1
6. Oxygen has ON = -1 in peroxides
(Oj^',
for example, HOOH)
ON = -1/2 in superoxides (Oj)
ON = -1/3 in ozonides (O3)
ON = -2 unless combined with F
ciable extent. A1(0H)3 (s) + OH" ^ AKOH)/
(aq) with
K^
= 40 at 25
°C.
The concentration of
aluminum is solved as Al(0H)4"(aq) = ^ x
{KJ\R^]),
At intermediate pH the aluminum ion
complexes according to AP+ + HjO -^ A1(0H)2+
+ H+ with K, = 9.78 x 10^; [A1(0H)2+] =
^a[Al^"']/[H''].
Other complexes (Section 14.7)
exist in the pH range of 4-6, but their concentra-
tions are too complicated to calculate. Fig. 13-4
shows the concentration of AP+, Al(OH)^"^, and
A1(0H)4" as a function of pH in terms of g/L
hydrated aluminum sulfate (alum, with a formula
weight of 666 grams per 2 moles of AP^.)
13.9 OXIDATION-REDUCTION REACTIONS
Oxidation-reduction (redox) reactions are
similar to acid-base reactions except the unit of
currency is not the proton but the electron. They
are important in combustion, redox titrations such
as,
determination of lignin contents using potassi-
um permanganate, and many other processes.
Like protons, electrons cannot exist in solution by
themselves so both electron donors and electron
acceptors are required for a reaction to occur.
Redox reactions, however, are not confined to
solvents such as water, but can occur in any form
of matter. Oxidation is defined as the loss of
electrons by an element during a chemical reac-
tion, and reduction is defined as the gain of
electrons. (This is easily remembered as LEO
growled GERRR; loss-electrons-oxidation, gain-
electrons-reduction.) The oxidizing agent, or
oxidant, is the substance that causes oxidation,
while the reducing agent is the substance that
causes reduction. Oxidation was originally a
reaction between a compound and oxygen gas,
where oxygen itself is reduced but causes oxida-
tion. Oxygen is the most electronegative element
(except for fluorine which is not nearly as ubiqui-
tous as oxygen). Disproportionation is a redox
reaction where a compound is both oxidized and
reduced, such as NO2 in the production of nitric
acid:
3NO2 + H2O -* 2HNO3 + NO
Electrons are assigned to atoms of molecules
or ions according to the rules in Table 13-6.

OXTOATION-REDUCTION REACTIONS 327
Oxidation numbers (ON) are assigned to elements
with these rules. The lower number rule always
takes precedence over a later numbered
rule.
Rule
6 is sunmiarized as follows: except for hydrogen
peroxide where oxygen has a charge of-1, oxygen
ahnost always has a charge of -2. While these
rules are somewhat arbitrary, it allows reactions
involving transfer of electrons to be balanced. Let
us consider the reaction of sodium metal with
water used to make sodium hydroxide. 2Na +
2H2O -* 2Na"^ + 20H- + Hj. Clearly sodium has
gone from neutral to a
1
+ charge by losing an
electron; in this reaction sodium was the reducing
agent (for hydrogen) and was oxidized. Some of
the hydrogen of water went from
1
+ to neutral by
accepting an electron; hydrogen was the oxidizing
agent, and it was reduced in the reaction.
EXAMPLE 15. Calculate the charge on sulfur in
each of the following compounds: Sg, HjS,
SO2,
H2SO3, and SO3.
SOLUTION: In each case hydrogen has an ON
= 4-1 and oxygen = -2; therefore the charg-
es on sulfur are 0, -2, +4, +4, +6. The
chemistry of sulfur is relatively complex due
to all of the possible oxidation numbers in a
variety of compounds.
PROBLEM: What are the oxidation numbers of
carbon in each of the following compounds:
C, CO, CO2, and H2CO3?
Answer: 0, +2, +4, +4.
One can determine whether or not a redox
reaction has occurred by determining whether the
oxidation number of any element has changed in
the course of a reaction. For example, the reac-
tion of
H"*"
-f OH" -* H2O is not a redox reaction,
but C + O2
-»•
CO2 is a redox reaction.
13.10 ELECTROCHEMISTRY
Many materials are capable of both oxidation
and reduction reactions. The type of reaction de-
pends on what the other reactant
is.
For example,
sulfur may be reduced with hydrogen to form HjS
or oxidized with oxygen to form SOj. Oxygen,
being more electronegative than sulfur, causes
oxidation, while hydrogen is more electropositive
than sulfur and causes reduction. Therefore,
redox reactions are conveniently split into two
half
reactions with one half reaction corresponding to
oxidation and the other half reaction corresponding
to reduction. Zinc metal dissolves in hydrochloric
acid with the overall reaction as follows:
Zn(s) + 2 HCl ^ ZnCl2 + H2(g) (overall)
Zn(s) -> Zrf+ + 2e- (oxidation)
2H+ + 2e- -> H,
(reduction)
It is useful to prepare a table of half reactions
with the energy released or absorbed in the reac-
tion to predict whether or not overall reactions will
occur based on thermodynamics. This technique
also simplifies the balancing of complex chemical
equations. Lange
*s
Handbook of
Chemistry
is one
good source for half reaction potentials. Some
half reactions are listed in Table 13-7 with com-
pounds on the left in decreasing order of oxidation
power. This means the higher a compound is on
the left hand portion of the table, the more likely
it is to induce oxidation of another compound or
ion. Similarly, compounds or ions on the lower
right are powerful reducing agents, the compounds
higher up on the right hand side of the table are
lower in reduction potential. Remember these are
thermodynamic values; they say nothing about the
reaction kinetics.
EXAMPLE 16. Balance the following equation,
which represents the Palmrose method of
titrating sulfite liquors:
H2SO3 + KIO3 ^ H2SO4 + KI
SOLUTION: First write the two half reactions.
Each mole of sulfur in sulfurous acid looses
two electrons as it goes from +4 to +6 va-
lence. Each mole of iodine gains six elec-
trons as it goes from +5 to
-1.
So,
KIO3 + 6e- + 6H+ -^ 3H2O + KI
3H2SO3 + 3H20-> 3H2SO4 + 6H+ +6e-
3H2SO3 +
KI03-^
3H2SO4 + KI
328 13. INTRODUCTORY CHEMISTRY REVIEW
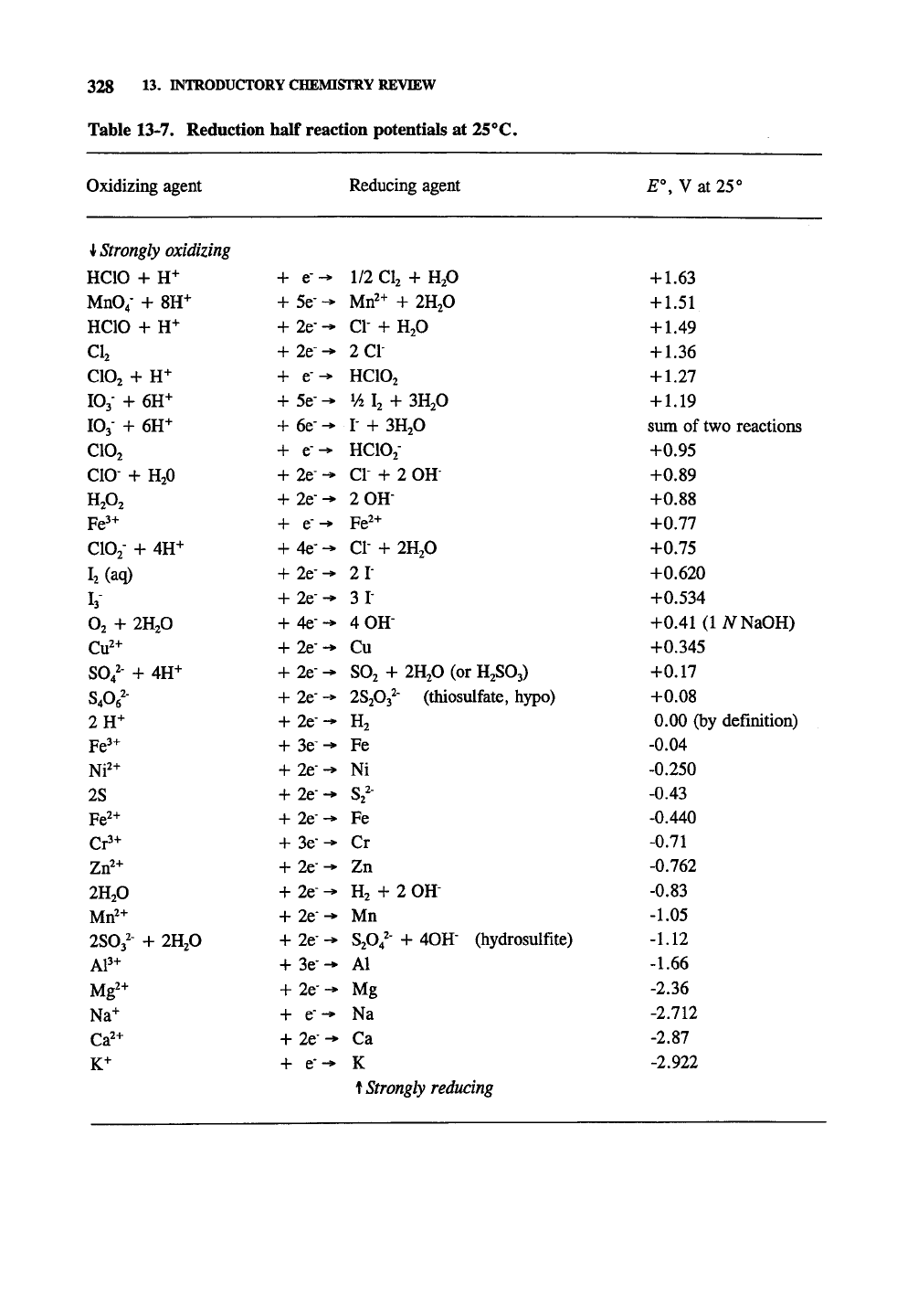
Table 13-7. Reduction half reaction potentials at 25^C.
Oxidizing agent
1 Strongly oxidizing
HCIO
+ H+
Mn04-
+ 8H+
HCIO
+ H+
CIj
CIO2
+ H+
lOj-
+ 6H+
IO3-
+ 6H-^
CIO2
CIO
+ H2O
HA
Fe'^
ClOj-
+ 4H+
IjCaq)
V
O2
+
2H2O
Cu^+
S04^-
+ 4H+
SA^-
an-'
Fe'^
Ni^^
2S
Fe^+
Cr3+
Zrf+
2H2O
Mrf+
2SO3'-
+ 2H2O
AP+
Mg^+
Na-*-
Ca^+
K+
+ e-»
+ 5e-^
+ 2e--»
+ 2e-^
+ e--»
+ 5e-^
+ 6e-^
+ e-^
+ 2e--*
+ 2e--*
+ e-^
+ 4e--*
+ 2e--»
+ 2e--»
+ 4e--»
+ 2e--*
+ 2e-^
+ 2e--»
+ 2e"-
+ 3e-^
+ 2e-^
+ 2e--»
+ 2e-^
+ 3e-^
+
2er-*
+ 2e-^
+ 2e--»
+ 2e-^
+ 3e--*
+ 2e--»
+ e-^
+ 2e-^
+ e-^
Reducing agent
1/2
CI2
+ H2O
Mrf+ + 2H2O
CI-
+ H2O
2C1-
HCIO2
V4
I2 +
3H2O
I-
+
3H2O
HCA"
CI-
+ 2 OH-
2 OH
Fe^+
CI + 2H2O
21-
3 1-
4 0H-
Cu
SO2 + 2H2O (or H2SO3)
28203^-
(thiosulfate, hypo)
H2
Fe
Ni
S2^-
Fe
Cr
Zn
H2
+ 2
OH-
Mn
S2O4'-
+ 40H-
(hydrosulfite)
Al
Mg
Na
Ca
K
^Strongly reducing
E",
V at 25°
+ 1.63
+ 1.51
+ 1.49
+ 1.36
+ 1.27
+ 1.19
sum of two reactions
+0.95
+0.89
+0.88
+0.77
+0.75
+0.620
+0.534
+0.41(liVNaOH)
+0.345
+0.17
+0.08
0.00 (by definition)
-0.04
-0.250
-0.43
-0.440
-0.71
-0.762
-0.83
-1.05
-1.12
-1.66
-2.36
-2.712
-2.87
-2.922
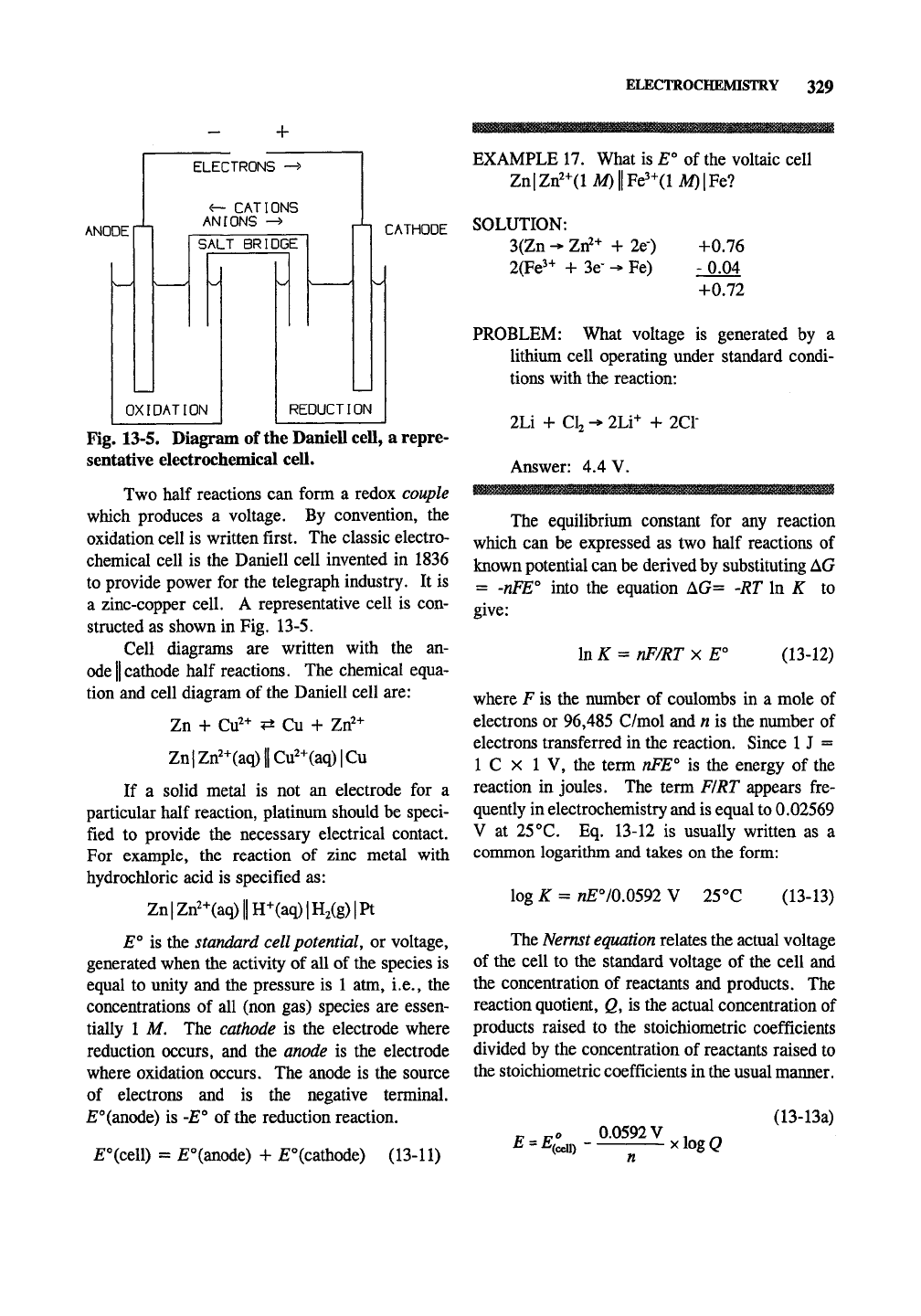
ELECTROCHEMISTRY 329
ANODE
ELECTRONS
<
CATIONS
ANIONS -^
SALT BRIDGE
OXIDATION
CATHODE
REDUCTION
Fig. 13-5. Diagram of the Daniell cell, a repre-
sentative electrochemical cell.
Two half reactions can form a redox couple
which produces a voltage. By convention, the
oxidation cell is written first. The classic electro-
chemical cell is the Daniell cell invented in 1836
to provide power for the telegraph industry. It is
a zinc-copper cell. A representative cell is con-
structed as shown in Fig. 13-5.
Cell diagrams are written with the an-
ode
II
cathode half reactions. The chemical equa-
tion and cell diagram of the Daniell cell are:
Zn + Ctf-^ <:i Cu + Zrf+
Zn|Zrf-^(aq)||Cu2+(aq)|Cu
If a solid metal is not an electrode for a
particular half reaction, platinum should be speci-
fied to provide the necessary electrical contact.
For example, the reaction of zinc metal with
hydrochloric acid is specified as:
Zn|Zrf^(aq)||H^(aq)|H2(g)|Pt
E° is the standard
cell
potential, or voltage,
generated when the activity of all of the species is
equal to unity and the pressure is 1 atm, i.e., the
concentrations of all (non gas) species are essen-
tially 1 M. The cathode is the electrode where
reduction occurs, and the anode is the electrode
where oxidation occurs. The anode is the source
of electrons and is the negative terminal.
£°(anode) is -£^° of the reduction reaction.
£:°(cell) = £°(anode) + £:°(cathode) (13-11)
EXAMPLE 17. What is
E""
of the voltaic cell
Zn|Zrf+(l A^fFe^-'d A^|Fe?
SOLUTION:
3(Zn ^ Zrf+ + If)
2{Ve^ + 3e- -* Fe)
+0.76
-0.04
+0.72
PROBLEM: What voltage is generated by a
lithium cell operating under standard condi-
tions with the reaction:
2Li + CI2 ^ 2Li+ + 2C1-
Answer: 4.4 V.
The equilibrium constant for any reaction
which can be expressed as two half reactions of
known potential can be derived by substituting AG
=
-nFE""
into the equation AG= -RT In K to
give:
InK^nF/RTxE''
(13-12)
where F is the number of coulombs in a mole of
electrons or 96,485
C/mol
and n is the number of
electrons transferred in the reaction. Since 1 J =
1 C X 1 V, the term nFE° is the energy of the
reaction in joules. The term F/RT appears fre-
quently in electrochemistry and is equal to 0.02569
V at 25°C. Eq. 13-12 is usually written as a
common logarithm and takes on the form:
log K = nEVO.0592 V 25°C (13-13)
The Nernst
equation
relates the actual voltage
of the cell to the standard voltage of the cell and
the concentration of reactants and products. The
reaction quotient, Q, is the actual concentration of
products raised to the stoichiometric coefficients
divided by the concentration of reactants raised to
the stoichiometric coefficients in the usual manner.
^ = ^(ceU)
0.0592 V
n
(13-13a)
xlog<?
330
13.
INTRODUCTORY CHEMISTRY REVIEW
BB
EXAMPLE 18. Give the equilibrium constant
for the reaction: Ikg" + Zn -* Zrf+ + 2Ag
SOLUTION: E'' = 1.56 V;
log K = 2(1.56)/0.0592 = 52.7;
EXAMPLE 19. What is the potential of a Zn-
Cu^^ cell with [Cu^+J = 2 M and [Zrf+] =
0.2 M?
SOLUTION: £ = 1.10 - 0.0592/2 x log 0.2/2
= 1.13 V.
13.11 PRACTICAL ASPECTS OF
ELECTROCHEMISTRY
Corrosion
Corrosion of metals is the result of electro-
chemical reactions. For example, in the zinc-
copper cell, the zinc metal is lost or corroded.
Since metal becomes soluble (corroded) as it goes
from a neutral to cationic state, corrosion occurs
at the anode where electrons are given up by
metal. These electrons must be used by a cathodic
reaction to complete the cell. An obvious corro-
sion reaction occurs when zinc or iron is put into
hydrochloric acid. Numerous bubbles form as the
metal displaces hydrogen from water. Parts of the
metal act as the anode where electrons are given
up and the metal goes into solution as ions, while
other parts of the metal act as the cathode where
electrons are accepted to convert hydrogen ions
into hydrogen gas. As the anode and cathode
areas move around the surface, the entire piece of
metal is ultimately dissolved.
In water near neutral pH, hydroxide ion is
produced at the cathode (by loss of hydrogen ions)
which precipitates ferrous ion in solution to give
ferrous hydroxide. The ferrous hydroxide is
further oxidized to ferric hydroxide, which is the
rust observed on the surface of iron. The behav-
ior of die rust (whether it forms on the surface or
away from the surface) depends on the pH and
oxygen content of the water and gives rise to
several types of corrosion mentioned below.
When two metals of different composition
(dissunilar metals) are electrically connected and
placed in a solution with an electrolyte, a voltage
is generated. The resultant corrosion is called
galvanic action. This can occur as potentials exist
across various parts of
the
same piece of metal due
to differences in surface compositions (like varia-
tions in oxygen concentrations caused by dirt that
excludes oxygen, leading to pitting), solution
composition at the surface, stress in the metal, etc.
When two dissimilar metals are in contact, the
more strongly reducing metal (Table 13-7) will
corrode. A large difference in potential may lead
to severe corrosion. For example, zinc will
corrode if attached to iron. When the surface area
of the metal that corrodes is small relative to the
other metal, corrosion is particularly quick. For
example, iron rivets holding copper sheets together
will corrode very quickly. Also, pitting represents
a rapid corrosion of a small area made anionic by
oxygen starvation. (These are thermodynamic
considerations, they do not say anything about the
rate of corrosion.)
Often an easily corroded metal is deliberately
attached to a large piece of metal to protect the
large piece of metal from corroding. The metal
that is corroded is called a sacrifice anode and
keeps the metal it protects cathodic, thereby not
allowing it to corrode. This technique is known as
cathodic protection. Galvanized (zinc coated)
iron, magnesium blocks inside hot water tanks,
and zinc anodes attached to the submerged metal
parts of marine vessels are three examples of
sacrifice anodes. It is used in water heaters,
marine vessels and
engines,
pipelines, and bridges.
The sacrificial anode (usually magnesium or zinc)
corrodes in preference to the material that it
protects. The sacrificial anode is periodically
replaced to maintain protection.
A related method of protection is to apply a
small, negative direct current voltage to the device
being protected if it is electrically insulated from
its environment. It must be insulated from its
environment or else it would take too much power
(amperage) to maintain the voltage. The applied
voltage makes the metal surface cathodic. This
technique has been used to protect underground
metal pipes (if they are electrically insulated from
ground).
PRACTICAL ASPECTS OF ELECTROCHEMISTRY 331
A more strongly reducing metal can displace
another metal from solution. A copper film forms
over most of a piece of iron that is dipped in
copper sulfate. If a surface of a less strongly
reducing metal is desired, the reaction can be
forced by the application of an external voltage in
the process of electroplating. Electroplating
occurs when reduction of a metal from solution is
forced by an external voltage, much like the
reaction at the cathode in a Daniell cell.
Some materials, like aluminum, would seem
to be unstable based on their position in the elec-
trochemical series. However, aluminum and chro-
mium both form oxides on their surfaces that are
very hard, insoluble, nonporous, self-healing, and
inert. The formation of such films renders metals
passive. These surfaces protect the underlying
metal from corrosion. For example, 12-18%
chromium in stainless steels protects the accompa-
nying iron from corrosion. Actually, stainless
steel (active) is vulnerable to corrosion; however,
the coating (passive) that forms on stainless steel
is inert. Crevice corrosion results when oxygen is
kept from the surface of an active material. Oxy-
gen gains electrons during its reaction so high
areas of oxygen react at the cathode and low areas
of oxygen provide an area of decreased potential
for the anode reactions. This occurs under depos-
its,
barnacles, or plastic washers used to insulate
fastenings on stainless steel plates used in sea
water. Table 13-8 shows metals presented in
increasing resistance to galvanic corrosion in sea
water. In other solutions the order may change.
Corrosion can be decreased in a number of
ways.
Painting prevents air, water, and salts from
reaching the surface of the metal. Galvanization
is the process of coating the entire surface with an
unbroken coat of
zinc
by immersion in molten zinc
or by electroplating. The zinc is protected by
passivation with a zinc oxide coating. The iron is
protected since zinc is oxidized in preference to it.
Electrolysis
Some other practical aspects of electrochem-
istry include production of NaOH and Clj and pro-
duction of
ClOj.
Ion-selective electrodes
Ion-selective electrodes are cells that obey the
Table 13-8. Galvanic series of metals and
alloys in sea water with increasing resistance.
Metal or alloy
Magnesium
Zinc
Aluminum alloys
Aluminum
Cadmium
Mild Steel
Alloy Steel
Cast Iron
Type 410 SS, Active
Type 430 SS, Active
Type 304 SS, Active
Type 316 SS, Active
Nickel Cast Iron
Yellow Brass
Copper
Nickel
Type 304 SS, passive
Type 316 SS, passive
Monel
Silver
Hastelloy, Alloy C
Titaniimi
Platinum
Nominal composition
Mg
Zn
Al
Cd
Low carbon
Various below 5%
3-4% C, 0.5-3.5% Si
11.5-13.5%
Cr
12-16%
Cr
18%
Cr, 8% Ni
18%Cr,8%Ni,3%Mo
0.5-10% Ni, 3-4% C,Cr
65%
Cu, 35% Zn
Cu
Ni
18%
Cr, 8% Ni
18%Cr,8%Ni,3%Mo
67%
Ni, 28% Cu
Ag
Ni-Cr-Mo
Ti
Pt
rules of electrochemistry. The Nemst equation
shows that the electrochemical potential of a cell
depends on the chemical concentration of the
reactive species. This principle is used in pH
electrodes and other ion-specific electrodes for
sodium, sulfide, carbonate, fluoride, etc. Ion-
selective electrodes, other than for pH, have not
been used extensively by the pulp and paper indus-
try, but they offer the potential for rapid laborato-
ry analysis or on-line sensors for process control.
13.12 PROPERTIES OF GASES
Ideal gas law
The kinetic theory of gases is a set of three
assumptions about the behavior of ideal gases.
While the assumptions are not completely true.

332 13- INTRODUCTORY CHEMISTRY REVIEW
they are approximately true at low pressures,
generally below one atmosphere pressure. (Much
of
the
non-ideal behavior of
gases
is a result of
the
nonzero volume of
molecules,
which increases the
pressure compared with ideal behavior, and the
formation of dimers, trimers, and clusters, which
decrease the actual pressure compared with ideal
behavior.) From these assumptions, much about
gas behavior can be predicted. The assumptions
are:
1.
A gas consists of a collection of mole-
cules or atoms in continuous random motion.
2.
The molecules or atoms are infinitely
small particles which move in straight lines
until they collide.
3.
Gas molecules do not affect each other
except during collisions.
The ideal gas law relates the temperature,
pressure, volume,
and
nimiber of moles of
an
ideal
gas.
T
is
always expressed in the absolute temper-
ature scale of Kelvin, while volume is usually
expressed in liters. When pressure is in atmo-
spheres, R = 0.08206 L-atm-K-^-mor^; when
pressure is in torr, R = 62.37 L-torr-K'^-mol'^;
when pressure is in Pascals, R = 8.314
J-K"^-
mol'^ R is called the gas
constant.
The ideal gas
law is usually written as Eq. 13-14. Standard
temperature and pressure, STP, conditions are
defined as 273.15 K and 760 torr (or 1 atm);
under these conditions one mole of an ideal gas
occupies 22.41 L.
EXAMPLE
20.
1.5 g of argon occupies 4.03 L at
298 K. What is the pressure exerted by this
gas inside the vessel, in atm, if argon be-
haves as an ideal gas?
SOLUTION: First the number of moles of argon
is calculated to be 0.0375 based on its atomic
weight of 40. Using Eq. 13-14 with R =
0.08206 L-atm'K"^-mol
^
the pressure is de-
termined to be
0.228
atm.
PROBLEM: What is the volume of 0.154 mol of
an ideal gas at 745 torr and 30.0°C? An-
swer: 3.91 L.
Often one desires to compare a single system
under two different states of pressure, volume, and
temperature. In this case the two states will be
designated as
1
and 2 with subscripts. Rearrange-
ment of Eq. 13-14, with nR a constant, leads to
Eq.
13-16.
PiVi/r, =
P2V2IT2
(13-16)
EXAMPLE 21. 5.0 liters of a gas are collected
at a temperature of 300 K and a pressure of
720 torr. What would the volume be at STP
conditions?
SOLUTION:
PV= nRT
(13-14)
.^^^720tMr^273K
^760 torr'^300K
=4.315
The compressibility factor, Z, is defined in Eq.
13-15,
and is equal to 1 for an ideal gas. Z is a
measure of the deviation from ideal behavior and
is usually less than one except for highly volatile
gases at low pressures.
Z = PV/nRT
(13-15)
The root-mean-square velocity of the mole-
cules V is related to the molar mass M by the
relationship v =
(3RTIM)^^.
Since diffusion is
proportional to velocity, it is also inversely pro-
portional to the square root of molecular weight
(Graham's law). Since kinetic energy is Vimv^,
PROPERTIES OF GASES 333
it follows that the internal energy (not to be con-
fused with work done by an expanding gas which
is an external energy) of a gas is
3/2'RT.
The
latter equation can be used to calculate the heat
capacity of an ideal gas.
Real gas equations
There are many empirical equations that
approximate the behavior of real gases at elevated
pressures where deviations from ideal become
appreciable. The most famous is the van der
Waals equation developed by the nineteenth
century Dutch chemist. The constant a represents
the attractive forces which decrease the actual
pressure from ideal, and the constant b represents
the effect of repulsions or the finite volume of the
molecules which increase the actual pressure from
ideal. The equation is not useful for the liquid
phase.
The equation is not useful at very high pres-
sures;
for example, while the van der Waals equa-
tion predicts a compressibility factor, Z, of 0.375
at the critical point of a gas, in fact, gases usually
have a compressibility factor on the order of 0.25
to 0.30 at their critical point. The
CRC
Handbook
of
Chemistry
and Physics gives values of
A
and 6
for many gases (Table 13-9). The constants may
also be obtained from the critical data of gases,
usually using P^ and
T^.
as follows:
a «
21R^T^I6AP,\
b « RTJ^P,
One of
the
most accurate equations of state is
the
Redlich'Kwong
equation,
which is useful over
wide ranges of temperature and pressure. The
constants a and b are different than those of the
van der Waals equation.
Table 13-9. Van der
Waals'
constants of sever-
al gases.
(P +
an
V(V+bln)T^^
)
X
(Y-nb)
= nRT
13.13 ANNOTATED BIBLIOGRAPHY
Corrosion
1.
Perry's
Chemical Engineers * Handbook
has
1
Gas
1 COj
1
CI2
Ua
loj
1 (^113)20^2
SO2
1
H2O
1
CH4
a L^-atm-mol"^
3.59
6.49
1.39
1.36
8.66
6.71
5.46
2.25
b,
L-mol' 1
0.0427
II
0.0562
1
0.0391
II
0.0318
II
0.0845
1
0.0564
1
0.0305
1
0.0428
1
a very useful, detailed chapter on the subject
(section 23 of the 6th ed.).
2.
Corrosion in Action, International Nickel
Co.,
1961. This was used as a source of
information about corrosion for this chapter.
EXERCISES
The
elements
1.
Draw the Lewis structures for the following
compounds showing all valence electrons.
a) NajS
c) CCI4
b) CO2
d) CH4
Ionic and
covalent bonds
2.
Which of the following statements is true of
a solution containing an electrolyte?
a) An electrolyte solution contains ions.
b) Water is a good solvent to use to pro-
duce an electrolyte solution.
c) An electrolyte solution will conduct
electricity.
d) All of the above.
3.
Which of the following is the most ionic
compound?
a) BF3
c) Na2S
b) CCI4
d) Si02
