Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

304 12. METRIC AND ENGLISH UNITS AND UNIT ANALYSIS
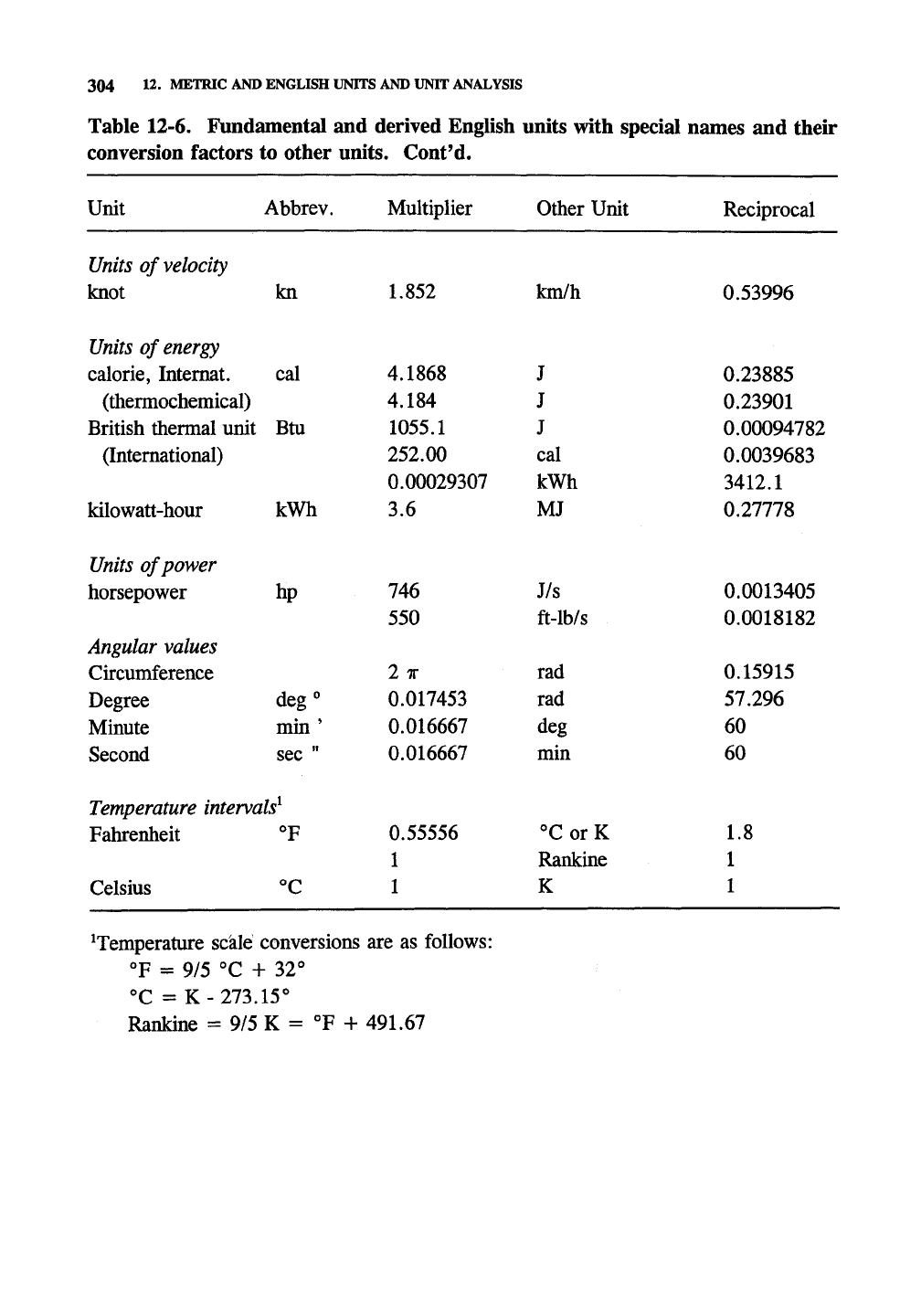
Table 12-6. Fundamental and derived English units with special names and their
conversion factors to other units. Cont'd.
Unit
Units
of
velocity
knot
Units
of
energy
calorie, Internal.
(thermochemical)
British thermal unit
(International)
kilowatt-hour
Units
of power
horsepower
Angular values
Circumference
Degree
Minute
Second
Abbrev.
kn
cal
Btu
kWh
hp
deg"
min '
sec "
Temperature
intervals^
Fahrenheit
Celsius
op
"C
Multiplier
1.852
4.1868
4.184
1055.1
252.00
0.00029307
3.6
746
550
2
IT
0.017453
0.016667
0.016667
0.55556
1
1
Other Unit
km/h
J
J
J
cal
kWh
MJ
J/s
ft-lb/s
rad
rad
deg
min
°CorK
Rankine
K
Reciprocal
0.53996
0.23885
0.23901
0.00094782
0.0039683
3412.1
0.27778
0.0013405
0.0018182
0.15915
57.296
60
60
1.8
1
1
'Temperature sckle conversions are as follows:
°F = 9/5 °C + 32°
°C =
K-273.15°
Rankine = 9/5 K = °F + 491.67
ENGLISH AND METRIC UNITS 305
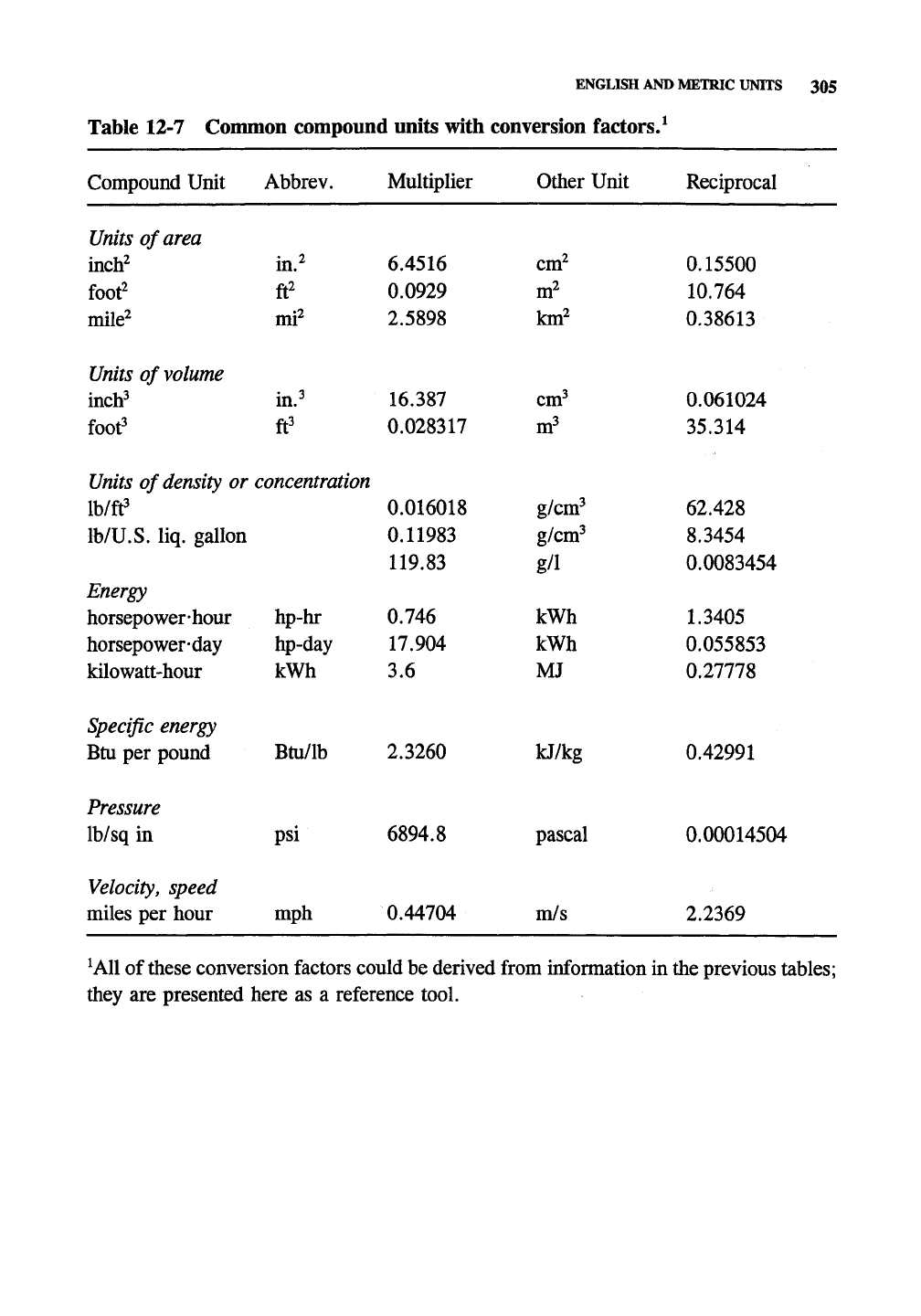
Table 12-7 Common compound units with conversion factors/
Compound Unit
Units
of area
inctf
foot^
mile^
Units
of
volume
inctf
foot'
Abbrev.
in.2
ft^
mi^
in.3
ft'
Units
of density or
concentration
lb/ft'
Ib/U.S.
liq. gallon
Energy
horsepower-hour
horsepower day
kilowatt-hour
Specific energy
Btu per pound
Pressure
Ib/sq in
Velocity,
speed
miles per hour
hp-hr
hp-day
kWh
Btu/lb
psi
mph
Multiplier
6.4516
0.0929
2.5898
16.387
0.028317
0.016018
0.11983
119.83
0.746
17.904
3.6
2.3260
6894.8
0.44704
Other Unit
cm^
m^
km^
cm'
m'
g/cm'
g/cm'
g/1
kWh
kWh
MJ
kJ/kg
pascal
m/s
Reciprocal
0.15500
10.764
0.38613
0.061024
35.314
62.428
8.3454
0.0083454
1.3405
0.055853
0.27778
0.42991
0.00014504
2.2369
^AU
of
these
conversion factors could be derived from information in the previous tables;
they are presented here as a reference tool.
306 12. METRIC AND ENGLISH UNITS AND UNIT ANALYSIS
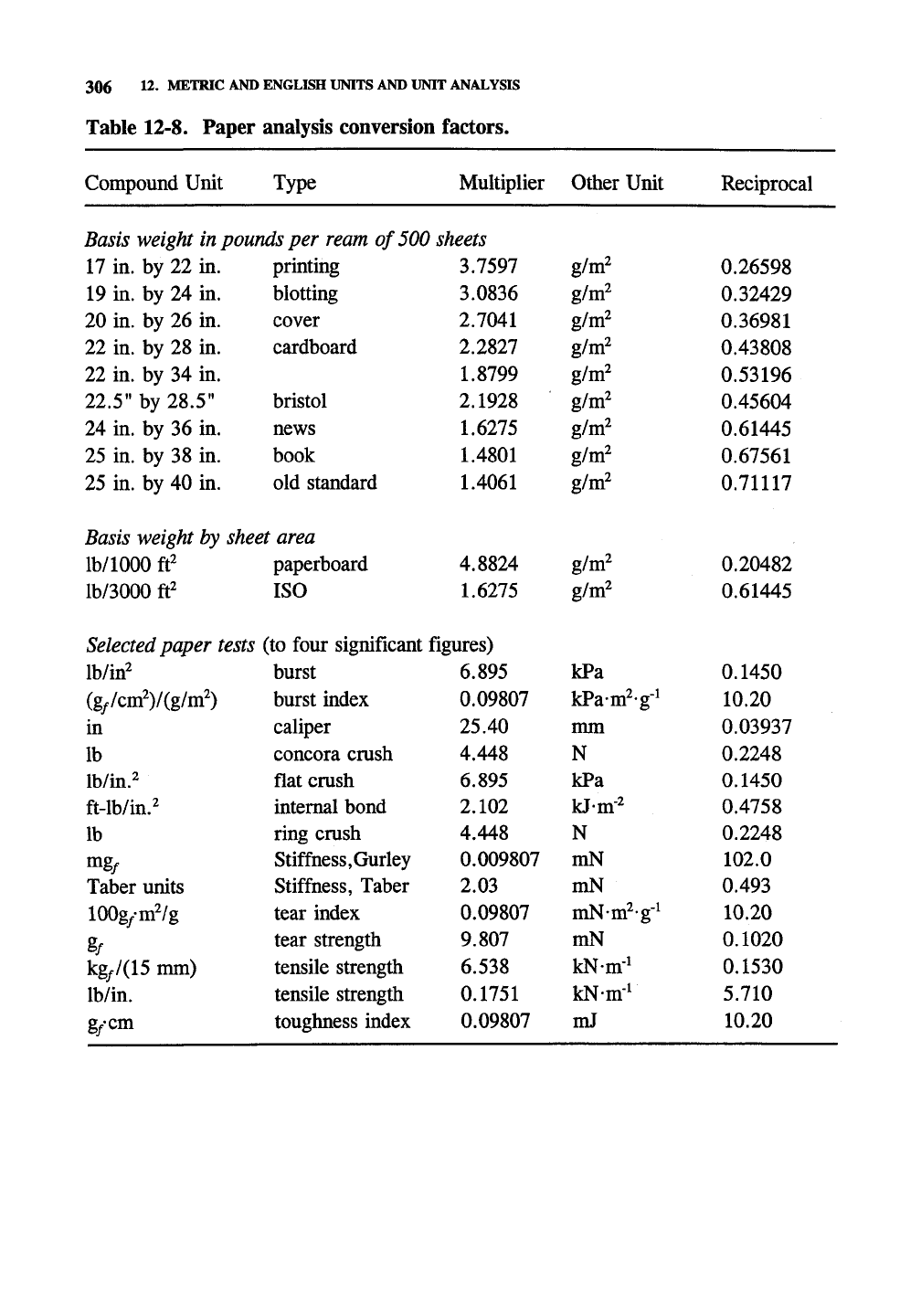
Table 12-8. Paper analysis conversion factors.
Compound Unit
Type Multiplier
Basis weight in pounds per ream of 500 sheets
17 in. by 22 in.
19 in. by 24 in.
20 in. by 26 in.
22 in. by 28 in.
22 in. by 34 in.
22.5"
by 28.5"
24 in. by 36 in.
25 in. by 38 in.
25 in. by 40 in.
Basis weight by,
lb/1000 ft^
lb/3000 ft2
printing
blotting
cover
cardboard
bristol
news
book
old standard
sheet area
paperboard
ISO
3.7597
3.0836
2.7041
2.2827
1.8799
2.1928
1.6275
1.4801
1.4061
4.8824
1.6275
Selected paper tests (to four significant figures)
Ib/in^
(g^/cmyig/w?)
in
lb
lb/in.2
ft-lb/in.2
lb
mg/
Taber units
lOOg/m^/g
8/
kg^/(15 mm)
lb/in.
g/cm
burst
burst index
caliper
concora crush
flat crush
internal bond
ring crush
Stiffness.Gurley
Stiffness, Taber
tear index
tear strength
tensile strength
tensile strength
toughness index
6.895
0.09807
25.40
4.448
6.895
2.102
4.448
0.009807
2.03
0.09807
9.807
6.538
0.1751
0.09807
Other Unit
g/m^
g/w?
g/tn?
g/m^
g/m?
g/m^
g/m^
g/m^
g/m^
g/m^
g/m^
kPa
kPam^g"'
mm
N
kPa
kJm-2
N
mN
mN
mN-m^-g"^
mN
kN-m-^
kN-m-^
mJ
Reciprocal
0.26598
0.32429
0.36981
0.43808
0.53196
0.45604
0.61445
0.67561
0.71117
0.20482
0.61445
0.1450
10.20
0.03937
0.2248
0.1450
0.4758
0.2248
102.0
0.493
10.20
0.1020
0.1530
5.710
10.20
ENGLISH AND METRIC UNITS
307
Japan, China,
and
many European countries
use
the DIN
standards
for
paper size.
DIN is an
acronym
for
Deutsche Industrie Normen, which
might
be
thought
of as the
German Industry Stan-
dard.
The
standard sized sheet
for a
particular
DIN series
is
indicated
by a
capital letter such
as
A,
B, C, or D. The A
series
is
usually used
for
printing
and
writing papers. Envelopes, file
folders,
and so on use the B and C
series.
The
standard sheet size
of a
series
is
indicated
by 0
such
as AO.
Subsequent designations within
a
series
are
indicated
by the
number
of
folds needed
to make
the
sheet.
For
example,
Al is
defined
as
a sheet
of
AO
folded
in half.
The base sheet
of
paper
has a
length
to
width
ratio
of
(2)^^:
1,
or
approximately 1.414:1. Since
smaller sheets
are
defined
by
folding
the
larger
sheet
in
half successively,
the
length
to
width
of
any sheet
of
paper will maintain the
1.414:1
ratio.
The most common series
is the A
series where
the
base sheet
is
exactly 1
m^.
Since
the
area
and the
ratio
of
the length
to
width
are
both stipulated,
the
base sheet
is
"forced"
to
have
the
dimensions
of
0.841
m by 1.189 m. The A
series
in
millimeters
and inches
is
given
in
Table
12-8. If one
knows
the weight
of a
single sheet
of A4, the
basis
weight
in g/m^ can be
determined
by
multiplying
by 42
(or 16).
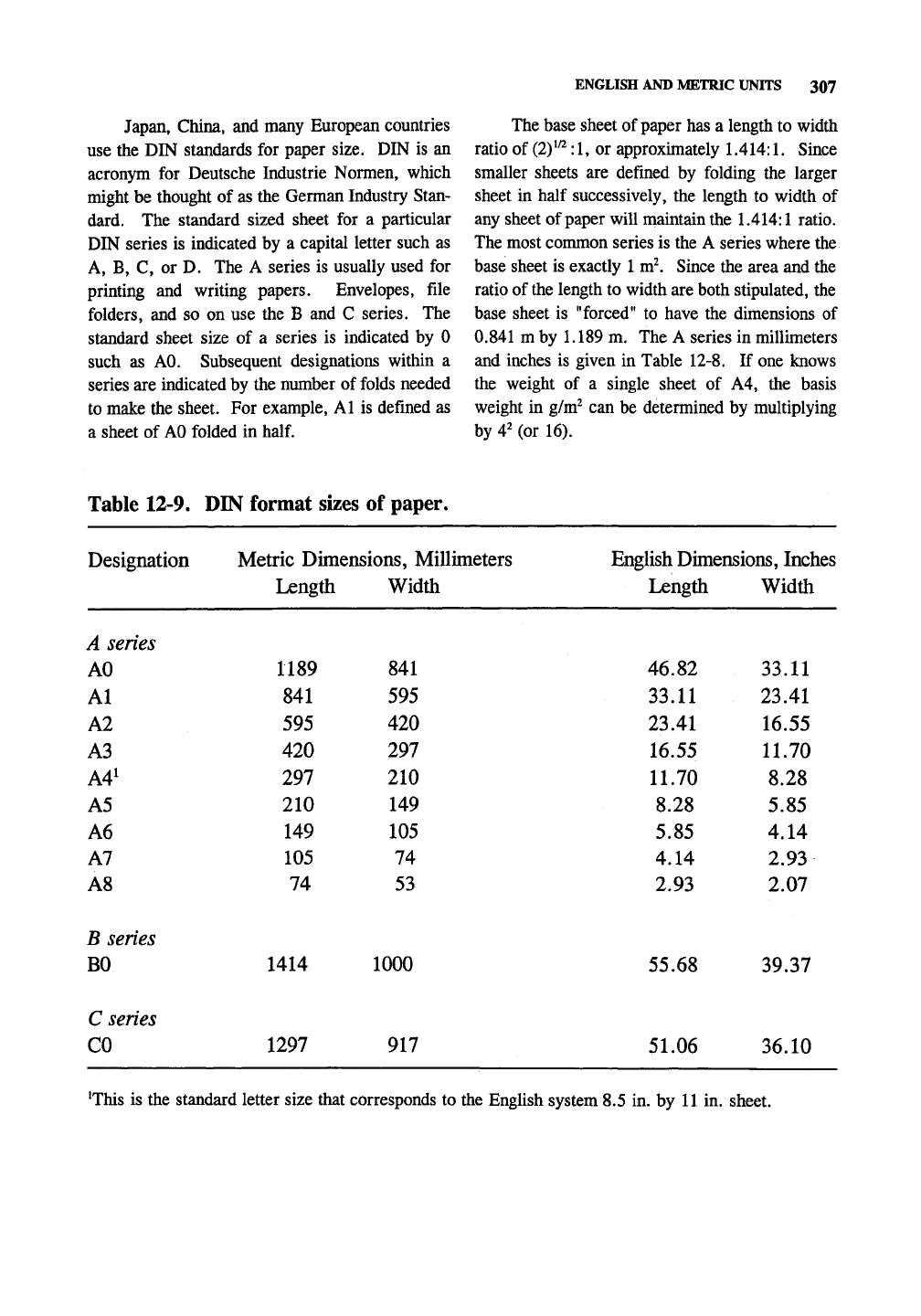
Table
12-9. DIN
format sizes
of
paper.
Designation Metric Dimensions, Millimeters
Length Width
English
Dimensions,
Inches
Length Width
A series
AO
Al
A2
A3
A4'
A5
A6
A7
A8
B series
BO
C series
CO
1189
841
595
420
297
210
149
105
74
1414
1297
841
595
420
297
210
149
105
74
53
1000
917
46.82
33.11
23.41
16.55
11.70
8.28
5.85
4.14
2.93
33.11
23.41
16.55
11.70
8.28
5.85
4.14
2.93
2.07
55.68
51.06
39.37
36.10
'This
is the
standard letter size that corresponds
to the
English system
8.5 in. by 11 in.
sheet.

308
12.
METRIC
AND
ENGLISH
UNITS AND UNIT
ANALYSIS
12.3 UNIT ANALYSIS
Unit analysis (sometimes called dimensional
analysis) is a powerful way of solving problems by
keeping track of the units (mass, length, area or
length^, density or mass/length^ time, etc.) of
numbers in order to solve problems. By using this
method, one can solve a wide variety of problems
by memorizing very few things. All solutions to
problems should show units throughout the solution
in order to prevent "silly" oversights and to be
considered correct; a number without the proper
units is meaningless. In many of the examples
throughout this book, conversion factors are listed
for convenience. In other examples it will be
necessaiy to find conversion factors from the tables
presented in this chapter. One important tip for
solving word problems is that "of" usually implies
multiplication while "per" usually implies division.
One straightforward application of unit analy-
sis lies in multiplying a starting figure by conver-
sion factors which are equal to unity (one) to
convert the original number to new units. These
conversion factors merely substitute one set of units
for another set of
units
without making any changes
in the quantity considered.
EXAMPLE 1. You are a Canadian buying a car in the
U.S.
and the salesman tells you it gets 30 miles per
gallon, but you are not really sure this is good gas milage since your point of reference is
kilometers per liter. Convert 30 mi/gal (mpg) to km/L.
SOLUTION. One realizes that both miles and km are units of distance and comparable, and that gallons
and liters are units of volume and comparable. We can look
up
conversion factors and fmd
out that 1 mile = 1.609 km and 1 gallon = 3.637 liters. Thus, we can take 30 mpg and
multiply by appropriate conversion factors that cancel out the units we have and leave us
with the units desired:
^^ mile 1.609 km
1
gallon .^_-km
30 X
X
—5 = 12.75
gallon 1 mile 3.785
H
H
One can use any conversion factor (with the correct units) that is equal to unity. For
example, we could just as easily use 1 km = 0.6215 mile and 1 L = 0.2642 gal.
30
mile
1 km 0.2642 gallon ^
-
^ __ km
gallon 0.6215 mile
1 it
Of course, there are an infinite number of equally valid ways to achieve the same result.
In the above case one might remember the following:
1 mile = 5280 feet
1 cm = 0.00001 km
1 foot.= 12 in.
1 gal = 3.785 L
1 inch = 2.54 cm
Thus,
within rounding error, the same result is achieved as follows:
^^ mile
5280
ft
12
in.
2.54cm
10"^
km 1 gal
30 X X X X X
—
gallon
1
mile
1
ft 1 in.
1
cm 3.785
H
km
H

UNIT ANALYSIS 309
To find a general conversion factor to convert mpg to km/L start with 1 mpg as follows:
- mile 1.609 km 1 gallon ^ .^-- km
1 X
X
—^
=
0.4251
—
gallon 1 mile 3.785 d d
This is often abbreviated by saying "multiply mpg by
0.4251
to give km/liter"; it is
preferable, but alas not common, to indicate "multiply mpg by
0.4251
gal
km
mile"^
liter"^"
to give km/L. The difference is that
0.4251
is not equal to 1.000 (unity); however, the
term
0.4251
gal km
mile"^
liter'' is equal to unity.
EXAMPLE 2. The basis weight of one type of linerboard is 79 lb/1000 ft^. Convert this to g/ml
SOLUTION. This involves two conversion factors to get appropriate units. Notice how the conversion
factor for area is derived by squaring the linear conversion factor. Instead of squaring the
numerator and denominator separately, the entire fraction could be squared; the result, of
course, is the same since (a/bf =
AW.
79Axi^^x-iLiel_=38.6^
ft2 1 lb (0.3048 m)^
m"
EXERCISE. In Table 12-8 the conversion factor for basis weight in terms of pounds per 17 in. by 22
in.
printing ream (500 sheets) to
g/m^
of individual
paper
sheets is given as 3.7597; show
how this factor is derived.
Unit analysis is very important when using
equations. The units of the answer must be appro-
priate for those used in the equation; in short, they
must match.
There are often difficulties in unit analysis in
pulp and paper because units may be mixed be-
tween English and metric, or even unmatched
within either system.
EXAMPLE 3. Velocity (v) is equal to distance (s) traveled divided by the time (f) it took to travel that
distance (v = s/t = s x r'). Suppose we know that 60 miles per hour is equal to 26.82
meters per second. Does
a paper
machine that travels 140 feet in two seconds travel faster
or slower than this?
SOLUTION. s = v/t= 140 ft/(2 s) = 70 ft/s
The units of the answer are not directly comparable, but we can compare the results after
the use of conversion factors.
70—
X
m
21.3
s 3.281 ft s
or
-^ft 3600s 1 mi
70 —
X
X
1 hr 5280 ft
47.7 5E
hr
In either case, whether we convert ft/s to m/s or mi/hr, one obtains values which are
comparable to (and less than) the original speeds given.
310 12. METRIC AND ENGLISH UNITS AND UNIT ANALYSIS
EXAMPLE 4. Use Table 2-2 (page 32) to determine an approximate conversion factor of cords to units.
SOLUTION. Since a direct relationship is not available, two relationships are used in series.
- J
3.62
m^
stacked
wood
1 cord X X •
1 cord
1 unit
3.28
m^
stacked wood
= 1.10 units
EXAMPLE 5. The relationship for converting mass into energy is the famous equation of Albert Einstein,
E=mc^, While this is applicable to any change of energy, it is usually applied to nuclear
reactions where very large amounts of energy are released. In normal chemical reactions
the changes in mass are minuscule. For the sake of argument, assume one could
completely convert coal into energy via a nuclear reaction. How many horsepower-days
of energy would be produced from 1 gram of coal?
c = speed of light in a vacuum = 3x10^ m/s.
hp = 746 J/s; thus 1.00 hp x s = 746 J
SOLUTION. (Note that if one were to try to solve this problem in English units one might be tempted
to use the units of
pounds;
however, pounds are
a
unit of
weight,
not mass, and this would
lead to incorrect results. This
is
just one of
many
advantages to the metric system.) Since
energy is in units of joules, which in
turn
is units of
m,
kg, and s, it is convenient to solve
as follows:
E = 0.001kgx(3-10«—)2 = 9
10
,2
9-10^3
J
9
•
10^^
J
1 hp -s
X X
746 J
JL^
X
iJS =
1,396,000
hp-day
3600 s 24 hr ^ ^
The amount of energy generated is enough to keep a 4000 hp motor operating for almost
one year. This calculation indicates the tremendous amounts of energy released in nuclear
reactions. Although coal is not converted to pure energy on the face of the earth, nuclear
reactions can occur. For example, one mole (235.0439 grams) of uranium-235
decomposes (fission) to form 234.8286 grams of products while 0.2153 grams of mass is
converted to energy. Analogously, 140.4634 grams of hydrogen can be fused (fusion) to
form 139.4635 grams of helium while 1.000 gram of mass is converted to energy. The
larger amount of energy given off and the high availability of hydrogen and deuterium
makes fusion a very good potential source of energy, although the conditions required for
fusion reactions are extremely difficult to obtain under controllable conditions. For this
reason fusion has not been used as an energy source to generate electricity. Energy of
nuclear reactions is given off as heat. About 30% of the heat energy is converted to
electricity.

UNIT ANALYSIS 311
EXAMPLE 6. How much work in horsepower is accomplished by pumping 3000 gallons (22,500 lb) of
water per minute up 70 vertical feet (equal to a pressure of 30 psi)?
SOLUTION. The horsepower is a rate of energy. One horsepower is the work accomplished by lifting
550 pounds one foot high (perpendicular to gravity) per second. This is equal to 33,000
foot-poimds per minute. The work required to lift 3000 gallons of water every minute a
height of 70 feet is easily converted to foot pounds of work. This is about the amoimt of
work the primary fan pump must accomplish for a paper machine that produces 80 tons/day
with a speed of 4000 ft/min.
1 hp
224001b x70ftx
33000ft-lb
47.4 hp
EXERCISES
1.
Calculate the number of gallons of paint
required to paint the walls of a room 6 m by
8 m by 3.25 m high with a dry paint thickness
of 0.1 mm. Use these conversion factors:
1 gal paint = 0.30 gallons solids when dry
1 gal = 3.785 liters
1 L = 0.001 m'
1 m =100 cm
One approach is to calculate the wall area to
be painted. Convert this to the volume of dry
paint (surface area times thickness) in a sepa-
rate calculation, then convert to wet paint
gallons. The details remain.
8.
2.
Refining energy is often reported in units of
horsepower-days/ton; however one buys
electricity by kilowatt hours. Convert
hp-days/ton to kWh/ton. 9.
3.
Derive a conversion factor to convert Btu/hr
to watts.
Derive a conversion factor to convert
pounds/(24 in. by 36 in. ream) to g/m^ of a
single sheet of paper. Remember one ream
has 500 sheets, 1 lb is 453.59 g, and 1 cm is
2.54 in.
Derive a conversion factor for kg-s-m^
A/mol.
to
A certain type of paper is 78 lb/3000 ft^ with
a thickness of 0.009 in. What is its density?
How many seconds would a one ampere flow
of current take to move one mole (6.0220 x
10^^) of electrons?
Keeping in mind that one watt is one J/s, if
electricity is $0.10 per kilowatt hour, how
much is the energy generated by one gram of
mass (Example 5) worth?
If a mill makes 1000 tons/day of paper and a
certain additive worth $1.15/lb is used at
0.03%
on paper, how much will this mill
spend on this additive in one year?

13
INTRODUCTORY CHEMISTRY REVIEW
This book presumes a knowledge of intro-
ductory chemistry. Basic concepts especially
related to the pulp and paper industry are briefly
reviewed here. One should consult a good intro-
ductory chemistry book if more detail is required.
13.1 THE ELEMENTS
Unless one works in the field of subatomic
particle physics, all matter may be considered to
be made up of the 103 naturally occurring ele-
ments. About one dozen of these are important to
pulp and paper products. Another dozen are
important to the metallurgy of equipment, an area
covered in this volume in regards to corrosion.
Each element is made up of protons, elec-
trons, and neutrons arranged to form atoms.
Protons and neutrons together are called nucleons
because they are the constituents of the nucleus,
the center of the atom. Protons and neutrons have
about the same mass and make up most of the
mass of an atom. Electrons have
1/1836
the mass
of protons and a negative charge assigned at -1
unit, since the electron is the basis of all charges.
Electrons orbit the nucleus in shells. The outer-
most shell of electrons are the valence electrons,
which are the electrons primarily involved in
forming chemical bonds. Protons are positively
charged (opposite in charge of the electron) with
exactly the same magnitude of charge as the
electron, that is +1. Like charges repel each
other, and opposite charges attract each other;
therefore, electrons repel each other and electrons
are attracted to protons.
Each atom of a pure element has equal num-
bers of electrons and protons and, therefore, is
electrically
neutral.
The particular type of element
an atom forms (and its chemical properties) de-
pends on the number of protons in the nucleus;
this is called the atomic number. The periodic
table of the elements (on the inside cover) shows
the elements in increasing atomic numbers. There
are many reasons why the table has this arrange-
ment. For example, vertical columns of elements
have similar properties.
Atoms tend to have approximately equal
numbers of neutrons and protons. The relative
masses of individual atoms have approximately
whole number relationships to each other because
protons and neutrons constitute most of the mass
and have almost identical masses. For this reason
it is useful to define an atomic mass unit (amu).
An amu is precisely 1/12 the mass of the carbon-
12 isotope. (Carbon-12 is made up of 6 neutrons,
6 protons, and 6 electrons.) If one looks up the
atomic weight of carbon, it is actually 12.01 amu
as shown in the periodic table of the elements.
The reason is that carbon in nature exists as
98.9%
of carbon-12 and about 1.1% ofcarbon-13.
Carbon-13 atoms have an extra neutron, which has
a slight effect on the physical properties but little
effect on the chemical properties of carbon.
Carbon-12 and carbon-13 are said to be isotopes
of each other; an alternate way of writing isotopes
is by adding a superscript to the left of the chemi-
cal symbol as in ^^C or ^^C. Isotopes have equal
atomic numbers but differ in atomic weights and
the number of neutrons in the nucleus.
While much more information is available in
chemistry textbooks on the arrangement of the
orbiting electrons in atoms and molecules, the
important features of orbitals will be briefly sum-
marized
here.
The arrangement of electrons deter-
mines the reactivity of the elements with each
other. Electrons can occupy orbitals in pairs.
Numbers before orbitals designate the shell num-
ber; shells of electrons may be thought of as layers
of electrons around the nucleus. In any shell the
single s orbital can accommodate 2 electrons; the
three/? orbitals, 6 electrons; the five dorbitals, 10
electrons; and the seven/orbitals, 14 electrons.
(The first shell only has a single s orbital, the
second shell has one s and three p orbitals, the
third shell has s, p, and d
orbitals,
and the remain-
der of the shells have s, p, d, and/orbitals.) The
orbitals are filled (with some exceptions involving
shifting of electrons within the outermost d, f, and
s orbitals) in the order that describes the arrange-
ment of the elements in the periodic table of the
312
THE ELEMENTS 313
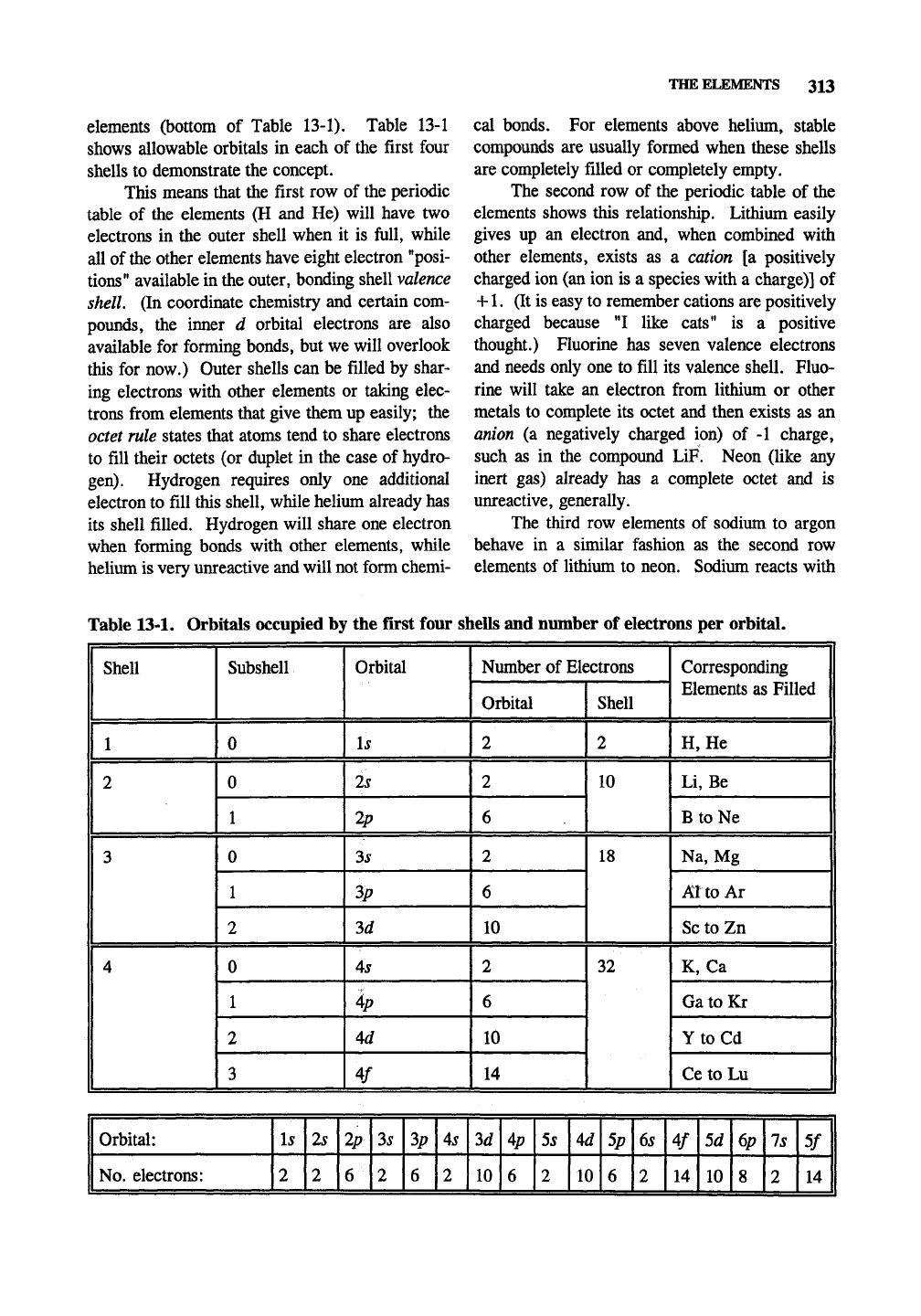
elements (bottom of Table 13-1). Table 13-1
shows allowable orbitals in each of the first four
shells to demonstrate the concept.
This means that the first row of the periodic
table of the elements (H and He) will have two
electrons in the outer shell when it is fiiU, while
all of the other elements have eight electron "posi-
tions"
available in the outer, bonding shell valence
shell (In coordinate chemistry and certain com-
pounds, the inner d orbital electrons are also
available for forming bonds, but we will overlook
this for now.) Outer shells can be filled by shar-
ing electrons with other elements or taking elec-
trons from elements that give them up easily; the
octet rw/^ states that atoms tend to share electrons
to fill their octets (or duplet in the case of hydro-
gen).
Hydrogen requires only one additional
electron to fill this shell, while helium already has
its shell filled. Hydrogen will share one electron
when forming bonds with other elements, while
helium is very unreactive and will not form chemi-
cal bonds. For elements above helium, stable
compounds are usually formed when these shells
are completely filled or completely empty.
The second row of the periodic table of the
elements shows this relationship. Lithium easily
gives up an electron and, when combined with
other elements, exists as a cation [a positively
charged ion (an ion is a species with a charge)] of
+1.
(It is easy to remember cations are positively
charged because "I like cats" is a positive
thought.) Fluorine has seven valence electrons
and needs only one to fill its valence shell. Fluo-
rine will take an electron from lithium or other
metals to complete its octet and then exists as an
anion (a negatively charged ion) of -1 charge,
such as in the compound LiF. Neon (like any
inert gas) already has a complete octet and is
unreactive, generally.
The third row elements of sodium to argon
behave in a similar fashion as the second row
elements of lithium to neon. Sodium reacts with
Table 13-1. Orbitals occupied by the first four shells and number of electrons per orbital.
Shell
1 1
2
3
4
Subshell
0
0
1
0
1
2
0
1
2
3
Orbital
Is
2s
2p
3s
ip
3d
As
Ap
Ad
¥
Niunber of Electrons
Orbital
2
2
6
2
6
10
2
6
10
14
Shell
2
10
18
32
Corresponding
Elements as Filled
H,
He
J
Li,
Be
II
BtoNe
Na, Mg 1
Al to Ar 1
Sc to Zn
K,
Ca
1
GatoKr |
Y to Cd 1
Ce to Lu
1
Orbital:
No.
electrons:
Is
2
2s
2
2p
6
3s
2
3p
6
As
2
3d
10
Ap
6
5s
2
Ad
10
5p
6
6s
2
4/
14
5d
10
6p
8
7s
2
IT]
14
1
