Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


immunocomplex formation. Whereas the general
principle of EIA is similar to RIA, there is an amplifi-
cation system present in this assay, and thus it is
more sensitive. Since no radioactive substances are
used, the assays avoid the problems encountered
in handling radioactivity. Enzyme labeling can be
achieved by conjugation of the enzyme to an antigen
or antibody via periodate oxidation with subsequ-
ent reductive alkylation method, cross-linking
using glutaraldehyde or others. Some of the methods
used in the conjugation of hapten to proteins can
also be used. However, to avoid nonspecific inter-
action, methods for coupling of protein/hapten to
enzyme should be different from the one that had
been used for conjugating the hapten to protein
for immunization purpose. Although horseradish
peroxidase and alkaline phosphatase are the two
enzymes most commonly used, others, such as
glucose-6-phosphate dehydrogenase, coupled with
oxidoreductase and luciferase, glucose oxidase,
beta-galactosidase, urease, and others have also
been employed.
0011 Depending on whether or not the immunocomplex
is separated from the free antigen, EIAs are further
divided into two types. One type is a homogeneous
system, which is based on the modification of enzyme
activity occurring when antibody binds with the
enzyme-labeled antigen/hapten in solution. No separ-
ation is necessary in this assay. One such type of this
system, which is called enzyme mulitiplied immuno-
assay (EMIT), has been used for the analysis of some
antibiotics and hormones in the clinical diagnosis
area. Because modification of enzymatic activity is
generally not significant, this system is not very sensi-
tive (mgml
1
to mg ml
1
range) and has not been
widely used in food analysis.
0012 The other is a heterogeneous system involving sep-
aration of free and bound antigen–antibody. In this
system, either antigen or antibody is bound to the
solid matrix noncovalently, or conjugated to it cova-
lently. Unreacted antibody or antigen/hapten is
simply removed by washing or centrifugation. The
term ‘enzyme-linked immunosorbent assay’ (ELISA)
is used for this type of assay. Solid phases such as
microtiter plates, cellulose, nylon beads/tubes, nitro-
cellulose membrane, polystyrene tubes/balls, and
modified magnetic beads have been used. In some
cases, staphylococcal protein A or protein G is coated
on the solid surface, entrapping the antibody for sub-
sequent analysis. This method is further divided into
two major types. One type is competitive ELISA,
which can be used for the analysis of both hapten
and macromolecule; the other is noncompetitive
sandwich-type ELISA, which is only used for divalent
and multivalent antigens.
Competitive ELISA
0013Depending on whether enzyme-labeled antigen or
antibody is used or whether antigen or antigen is
coated to the solid phase, several types of competitive
ELISAs have been developed. Two major types, i.e.,
direct competitive ELISA (dC-ELISA) and indirect
competitive ELISA (inC-ELISA), are used most com-
monly in food analysis.
0014Direct competitive ELISA In this assay, specific anti-
bodies are first coated to a solid phase. The sample or
standard solution of analyte is generally incubated
simultaneously with enzyme–conjugate or incubated
separately in two steps. The amount of enzyme bound
to the plate is then determined by incubation with a
chromogenic substrate solution. The resulting color/
fluorescence, which is inversely proportional to the
analyte concentration present in the sample, is then
measured instrumentally or by visual comparison
with the standards. A typical standard curve for
dC-ELISA of aflatoxin is shown in Figure 1 of
Immunoassay: Principles and general considerations.
Excluding the time for sample preparation, the assay
itself can generally be completed in 1–2 h. In general,
dC-ELISA is approximately 10–100 times more
sensitive than RIA when purified standard is used.
0015Indirect competitive ELISA (or double-antibody
ELISA) In the inC-ELISA, an antigen or a hapten-
protein (or polypeptide) conjugate (preferably with a
carrier protein/polypeptide that is different from the
original carrier protein) is coated to the solid phase,
e.g., microtiter plate, to facilitate antibody capture.
Antibody and analyte are then incubated together in
the wells of the plate. The amount of antibody bound
to the wells is then determined by reaction with a
second antibody–enzyme complex such as goat anti-
rabbit IgG–enzyme complex or goat antimouse-IgG
(IgM)–enzyme complex and by subsequent reaction
with substrate. Thus, analyte in the sample and anti-
gen in the solid phase compete for the same antibody-
binding site in the solution. The sensitivity of the in
C-ELISA is comparable to or slightly better than the
dC-ELISA, with the advantage that less antibody is
required. In addition, it is not necessary to prepare an
antigen/hapten–enzyme conjugate. The disadvantage
of the method is that an additional incubation step is
necessary for the assay and, thus, it requires longer
analytical time (2–3 h).
0016Two modifications of the inC-ELISA have been
made. One involves the conjugation of antibody,
especially monoclonal antibody, to an enzyme; the
conjugate is then used directly in the assay. Thus,
this modification converts the inC-ELISA format
to a direct assay. The second involves premixing
3250 IMMUNOASSAYS/Radioimmunoassay and Enzyme Immunoassay

the antibody with a second antibody–enzyme
conjugate. Both modifications have shortened assay
time without sacrificing sensitivity. Because only
small amounts of antibody are needed, inC-ELISA
has been used extensively for screening hybridoma
cultures for monoclonal antibody production.
Noncompetitive Sandwich Immunoassay
(or Two-Site ELISA)
0017 In this assay, the antibody is coated on to the solid
phase, most commonly to the wells of a microtiter
plate. The analyte is added and incubated for an
appropriate time. After washing, enzyme-labeled
antibody is added and incubated, followed by reac-
tion with substrate to develop the color. Under opti-
mal antibody and enzyme antibody concentrations,
the color intensity is directly proportional to the con-
centration of analyte. Thus, it is not necessary to
prepare an enzyme-labeled antigen in this assay. A
number of modifications of this method have been
made.
0018 Monoclonal antibody-based sandwich assay In this
assay, a monoclonal antibody (type ‘a’) is coated on
the plate. The plate is sequentially incubated with
analyte and another monoclonal antibody (type ‘b’)
labeled with enzyme. The antibody–enzyme conju-
gate must recognize a different epitope on the analyte
than does the antibody (type ‘a’) used to coat the
plate. Determination of analyte–antibody–enzyme–
complex is then made by incubation with substrate.
With this format, one can select appropriate pairs
of monoclonal antibodies for special analytical
objectives.
0019 Double-antibody sandwich assay In this assay, anti-
body originating from animal species ‘a’ (e.g., rabbit)
is coated on to the plate. The plate is sequentially
incubated with analyte and antibody against the
same analyte from animal species ‘b’ (e.g., mice),
followed by antibody against animal species ‘b’
(goat antimouse) labeled with enzyme, and then
with substrate.
Quick Immunoscreening Tests
0020 Based on the ELISA principle described above, a
number of quick screening tests have been developed.
For example, by adjustment of reagent concentra-
tions, microtiter plate ELISA assay can be completed
in less than 20 mins. Other approaches involve immo-
bilizing the antibody on a paper disk or other mem-
brane which is mounted either in a plastic card or in a
plastic cup, or on the top of a plastic tube. In the
‘dipstick’ assay, antibody or antigen is coated on to
a stick, which is then dipped in various reagents for
subsequent reactions. Substrates leading to the for-
mation of a water-insoluble chromogenic product are
used in these assays. Most of these screening tests are
very simple and easy to perform and take 10–15 min
to complete. They are designed to provide semiquan-
titative information at certain cut-off concentrations
for the substance in which one is interested. The
immunoscreening tests have gained wide application
for monitoring microbial toxins in foods and food
contaminants such as mycotoxins in the field and
versatile assay kits are commercially available.
New Developments and Biosensors:
Nonisotopic Labeling Other than with Enzymes
0021Among many new nonisotopic labeling systems de-
veloped in recently years, FIAs in which the immu-
noreactants are tagged with fluorescent probes are
used most often. For example, in the so-called
‘hit-and-run’ assay for T-2 toxin, a T-2 toxin column
was equilibrated with a fluorescein isothiocyanate
(FITC)-labeled Fab fragment of IgG (anti-T-2 toxin).
Samples containing T-2 toxin were injected into the
column. The FITC-Fab that eluted together with the
samples containing T-2 toxin was then determined in
a standard flowthrough fluorometer. While fluores-
cein isothiocyanate (FITC) has been used most often
for this purpose, a number of other high-quantum-
efficiency reagents, such as the phycobiliproteins,
which include phycoerythrins, phycocyanins, and
allophycocyaninins, have gained wide applications
in recent years. These reagents replace the enzyme as
the markers in both the heterogeneous and homoge-
neous immunoassays.
0022Fluorescence-based substrate has also been used to
improve the sensitivity. New instrumentation further
led to the development of several unique FIAs. For
example, the fluorescence polarization method can
detect the antigen/antibody reaction in solution with
good sensitivity in the homogeneous system (ng to mg
ml
1
range). A time-resolved fluoroimmunoassay,
which involves the use of europium ion (Eu)-labeled
antibodies, has a sensitivity similar to most ELISAs
for aflatoxin analysis (with an IC
50
of 0.2 ng ml
1
). In
the particle concentration FIA (PCFIA), the antigen/
antibody is bound to the solid phase such as submic-
rometer polystyrene particles and the fluorescence-
labeled reagent and analyte bind to the particle
surface competitively. The sensitivity of this for both
high-molecular-weight proteins and haptens reaches
the ng and sub-ng range in each assay. Similar to FIA,
both bioluminescent and chemiluminescent reagents
have been used as the markers as well as enzyme
substrates in different types of immunoassays.
IMMUNOASSAYS/Radioimmunoassay and Enzyme Immunoassay 3251

Enhanced Amplifications Leading to more Sensitive
and Ultrasensitive Immunoassays
0023 The sensitivity of different immunoassays has also
improved considerably by incorporation of various
amplification systems in conjunction with EIA and
RIA. Whereas biotin (BT) has been used commonly
as a secondary label in systems in conjunction with
labeled avidin (AV) or streptavidin, amplification
with multiple labeling further improved the sensitiv-
ity. The power of amplification is well demonstrated
in the ultrasensitive immunoassay where several
steps of amplifications and solid-phase concentra-
tions are involved. For example, in a noncompetitive
IA of hapten, the hapten is first biotinylated and then
reacted with antihapten IgG-coated solid phase. After
washing, the bound hapten–BT is removed from the
solid phase at pH 1.0. The eluted solution is adjusted
to neutral pH, then reacted with antihapten-Fab’-
enzyme conjugate, followed by incubation with AV
(or streptavidin)-coated solid phase. After washing,
the hapten-BT-antihapten-Fab’-enzyme-solid phase is
reacted with the substrate. Although this involves
many steps, the sensitivity of this system for the hap-
ten reached to 10 attomoles (10
18
mol) per assay.
Automated Immunoassay and Development of
Antigen/Antibody-Based Biosensors
0024 Development of new sensitive immunoassays that
require repeated precise handling of reagents as well
as the demand for large numbers of samples for an-
alysis have led to the establishment of automated
immunoassay systems. Rapid progress on the anti-
gen/antibody-based biosensors for the detection of
various food components, toxins, and contaminants
has also emerged. Only a few examples are cited here
to show the dimension of this type of technology. A
biosensor for a homogeneous immunoassay for T-2
toxin, which involves the use of liposomes and com-
plement, was developed. A fiberoptic immunosensor
has been demonstrated to be effective for several
microbial toxins, including staphyloccocal entero-
toxin B (SEB), T-2 toxins, and fumonisins, in foods.
In the assay of hapten-related toxins, monoclonal
antibodies are covalently bound to an optical fiber
and an evanescent wave effect was utilized to excite
the fluorescent-tagged toxin near the surface of the
monoclonal antibody fiber as the tagged toxin bound
to the fiber. In the presence of unlabeled toxin, it
competes with the labeled toxin for binding with
monoclonal antibody and results in a decrease in
signal. Thus, it is also a competitive assay. For SEB,
the sandwich system rather than the competitive
format was used and an automated immunosensor
detected 5 ng of SEB g
1
of cream in 10 min. In the
automated particle-based immunosensor (API), the
antigen or hapten–protein conjugate is coated on to
the polymethylmethacrylate beads (98 mm), and this
is then pumped into the 1.5-mm diameter glass capil-
lary to serve as the flow cell within the final lens and
trapped on a filter. The sample or calibrated standard
solutions that had been incubated with antibodies
were then allowed to pass through for a short period
(120 s), followed immediately by FITC-labeled goat
antirabbit antibody (120 s), and then finally washed
with buffer to remove excess label. The fluorescence
during each step of the reaction was recorded. Thus,
this is a kinetic exclusion assay and can easily detect
down to a level of 4 ng g
1
of sample (4 p.p.b.) for
aflatoxin in 8 min. In a miniaturized ELISA, urease
acts as an enzyme marker to produce a pH-shift that
can be detected by the ion-sensitive field effect tran-
sistor. This system consisted of a flowthrough set-up
with a pretreated activated fused silica capillary as a
reaction cartridge to serve as a solid phase for the
reversible inC-ELISA with a detection limit of 10 pg
ml
1
for T-2 toxin. In the surface plasmon resonance
(SPR) immunosensor, antibody is adsorbed on to a
thin gold film substrate coupled to a glass prism.
The output beam of a planar light-emitting diode is
focused through the prism to excite SPR at the surface
of the gold film. When a sample containing antigen/
hapten is added to a cell on the outside of the gold
film, the angular profile of reflected light intensity
shifts. This changes the resonance angle and the re-
flected beam intensity at a selected angle, both of
which are proportional to the antigen/hapten concen-
tration. A detection limit of 50 ng ml
1
is obtained
for the mycotoxin fumonisin with an analysis time
under 10 min. A commercial electrochemilumines-
cence (ECL) sensor can detect 100 and 1000 of
Escherichia coli O157 and Salmonella typhimurium,
respectively, in 1 ml of pristine buffer (or 1000–2000
bacteria ml
1
of food samples) in less than 1 h.
Application of Immunoassays in Foods
Immunoassays for Normal Food Components and
Adulteration in Foods
0025Whereas immunochemical methods have been estab-
lished for most normal food components, one of the
major applications of immunoassay in foods is to
detect adulteration. This includes the detection of
nonmeat proteins in meats and meats from different
species. Combinations of electrophoresis with various
classic methods, which provided only some qualitative
information, were used in early investigations. Both
RIA and ELISA are more commonly used at present.
For example, RIA has been used to differentiate cattle
3252 IMMUNOASSAYS/Radioimmunoassay and Enzyme Immunoassay

meat from sheep, donkey, pig, horse, and kangaroo at
5% contamination level. Various ELISA methods
were used to detect albumin and immunoglobulins as
a means of detecting blood residues in the muscles of
various species. Sensitive RIAs and ELISAs for non-
meat proteins, including soybean proteins, cows’
milk, avian egg white protein, wheat gluten, and
other nonmeat proteins (legume, cotton seed, peanut,
sunflower), have also been developed. These assays
are specific and could be used to detect the presence
of these proteins in meat products. Since heat treat-
ment or acid hydrolysis can result in a change of
protein structure, immunoassays for some of these
proteins have also been developed by using antibodies
against the denatured proteins and soluble proteins.
Immunoassays have also been established for some
antinutrients such as trypsin inhibitors, lectins, and
allergens. The so-called radioallergosorbent inhib-
ition test (RAST) involving the use of
125
I-labeled
antihuman IgE, has been used for the detection of
allergens in foods. Allergens in foods are also detected
with double-antibody sandwich ELISA as well as
dC-ELISA and inC-ELISA.
0026 The application of immunoassay for quality con-
trol in food processing has also emerged. For
example, immunoassays of certain forms of casein,
lactoserum, a- and b-lactoalbumins, IgG, lactoferrin,
and certain enzymes produced by psychrotropic bac-
teria in milk were used to determine quality of milk
and dairy products. A sensitive ELISA method is also
available to differentiate animal rennet and other
rennet substitutes. Immunoassay of barley proteins
and other soluble proteins and enzymes has been
used by the beverage industry. Immunoassays of
cortisol and corticosterone were used to monitor
meat quality. An ELISA was established for insect
myosin, which serves as an indicator for the presence
of insects in foods.
Immunoassays for Naturally Occurring
Contaminants and Toxins in Foods
0027 Foodborne pathogens and toxins Immunochemical
methods have been used for more than 30 years for
the detection of foodborne pathogens and high-
molecular-weight microbial toxins in foods. Different
immunochemical methods for foodborne toxins, in-
cluding staphylococcal enterotoxins, Clostridium
perfringens type A enterotoxins, C. botulinum toxins,
Bacillus enterotoxins, and E. coli toxins, have been
developed. Immunoassays of these toxins in foods
have moved from classical immunodiffusion and
hemagglutination methods to modern RIA, and
now, more commonly, to ELISA. Increasingly, mono-
clonal antibodies are used. The sensitivity of various
types of RIA for the detection of staphylococcal
enterotoxins is in the range of 1–5ng ml
1
as com-
pared to that of ELISA of 0.1–5ngml
1
. An EMIT
assay using b-amylase with a sensitivity of 5 ng ml
1
has been developed for SEB. The ELISA sensitivities
for the detection of botulinum toxin and C. perfrin-
gens type A toxin are in the range of 0.125–5ngml
1
and 0.5–500 ng ml
1
, respectively. Recent develop-
ments have led to more sensitive ELISA methods for
monitoring important foodborne bacterial pathogens
such as Campylobacter jejuni, E. coli, Listeria, Sal-
monella, and Yersinina enterocolitica.
0028During the last few years, many studies focused on
the development of immunoassays for E. coli
O157:H7. For example, a bead-ELISA with a sensi-
tivity of about 200 pg ml
1
was developed for specific
detection of the VT2 variant VT2e and a monoclonal
antibody-based sandwich ELISA for VT1 and VT2.
Using an antigen competition format, a solid-phase
fluorescence-based immunoassay was developed. In
this assay, a soft glass capillary tube served as the
solid support, to which heat-killed E. coli O157:H7
were adsorbed. Polyclonal anti-E. coli O157:H7 anti-
body, conjugated with biotin, was used; the bound
antigen–antibody complex was detected using avidin
molecules labeled with a fluorescent cyanine dye Cy5.
Anti-E. coli O157 antibody coated to the inexpensive
macroporous polyester fabric Polymacron was used
to capture E. coli O157 antigens for subsequent
immunoenzymatic detection of the bacteria. In a
rapid and inexpensive sandwich enzyme-linked re-
ceptor-based immunodot assay method, the purified
glycosphingolipid digalactosylceramide (diGalCer)
was used as in the solid-phase matrix to entrap for
the assay.
0029Naturally occurring food toxicants Rapid progress
on the development and application of immunoassay
for low-molecular-weight naturally occurring toxins,
contaminants, and agricultural residues in foods
has also occurred during the last two decades.
Both monoclonal and polyclonal antibodies against
low-molecular-weight food toxins, including marine
toxins (brevetoxin, palytoxin, okadaic acid, paralytic
shellfish toxins, and ciguatoxins) and mycotoxins
(aflatoxins, ochratoxin A, fumonisin, sterigmatocys-
tin, trichothecenes, and zearalenone), are available.
Various immunoassay formats have been developed.
These methods are generally very simple and
sensitive. For example, the lower limits of RIA and
ELISA for purified mycotoxin standards are in the
range of 0.05–0.5 ng and 2.5–5 pg in each assay, re-
spectively. Because of the interference of sample
matrix, the detection limits of most of the mycotoxins
in foods/feed are generally about 1–5 p.p.b. for RIA
and 0.05–0.5 p.p.b. for ELISA with no sample
IMMUNOASSAYS/Radioimmunoassay and Enzyme Immunoassay 3253

clean-up treatment. The detection limits for other
food contaminants by RIA and ELISA varied consid-
erably, but generally are less sensitive than for myco-
toxins.
0030 Since immunoassays for these haptens in foods
share some common problems, strategies to over-
come these problems are discussed here. In general,
the use of affinity purified antibody in combination
with the use of good radioactive-labeled or enzyme-
conjugate marker, or with other systems such as
chemiluminescence, or amplification systems such as
biotin-avidin, can improve the assay sensitivity. How-
ever, one of the major concerns is still interference by
sample matrix. This problem is generally overcome
by dilution of the sample to a range which does not
affect the assay or by using sample extract in the
preparation of the standard curve. Sample clean-up
may sometimes be necessary. For example, the sensi-
tivity of the RIA, dC-ELISA and inC-ELISA for myco-
toxins in foods has improved after such treatment.
The combination of using high-specific-activity radio-
labeled compounds and clean-up samples has allowed
the sensitivity for the detection of aflatoxin M
1
in
tissues and milk to reach the 0.01 ng g
1
level in
both RIA and ELISA.
0031 Other naturally occurring contaminants In addition
to mycotoxins and phycotoxins, sensitive immuno-
assays have also been developed for many naturally
occurring toxicants and contaminants, including
DNA-adducts of psoralens, caffeine, naringin,
limonin, and selected isoflavones. Sensitive ELISA
(ng g
1
) for ergot alkaloids can detect about one
sclerotium 20 kg
1
of grain. Immunoassay for potato
glycoalkaloids such as a-solanine, a-chaconine,
and solasonine, and tomato glycoalkaloids such as
a-tomatine, tomatidine (aglycon), and digitonin are
also available. The sensitivity of these assays is in the
1–200 ng g
1
range. Contaminants such as mercury
(100 ng g
1
in scallops) and histamine (10 mgg
1
salmon) are now detectable by immunoassay.
0032 Agricultural chemicals and residues and pestici-
des This is another area for which immunochemical
methods have been established in the last two decades
and have gained wide application. Immunoassays are
available for almost all the important antibiotic resi-
dues that might be present in our foods. For example,
b-lactone antibiotics such as ampicillin, cloxacillin,
and penicillin G, in the range of 1–10 ng g
1
, could
easily be measured in milk. The sensitivity for the
detection of other antibiotics by immunoassay falls
in a similar range. Likewise, immunoassays for many
pesticides are available. The sensitivity for measuring
ethyl parathion, diflubenzuron, and dieldrin in milk
are around 5 ng g
1
. Less than 1 ng g
1
of benomyl/
carbendazim in fruits and vegetables could be deter-
mined by immunoassay. ELISA kits with a sensitivity
in the range of 0.1–5ngml
1
are available for ala-
chlor, aldicarb, atrazine, carbaryl, carbendazim, car-
bofuran, cyanazine, 2,4-D, and metolachlor.
Concluding Remarks
0033The development of immunoassays for the detection
of food components and contaminants has progressed
rapidly in the last few years. Antibodies against
almost all the important agricultural and food-related
compounds are currently available. Sensitive, simple,
and specific immunoassays have been established.
Modern immunoassays such as RIA and ELISA are
gradually replacing the classic immunodiffusion and
agglutination methods for food analyses. These
methods are very simple, sensitive, and specific. To
avoid the use of radioactive compounds, ELISAs are
more commonly used than RIA. The development of
new labeling reagents and enhanced amplification
systems in conjunction with solid-phase technology
further improves the sensitivity and precision of dif-
ferent types of immunoassays, including those of pre-
cipitation and agglutination. With the availability of
immunochemical reagents and sensitive instrumenta-
tion, automation of immunoassays has become a real-
ity. Versatile immunoassay kits that can be used in the
field are commercially available and are widely used
for the detection of toxins and contaminants in foods.
A number of immunoassay protocols have been
adopted as first action by the Association of Official
Analytical Chemists.
0034Whereas immunoassays have been used extensively
for the analysis of contaminants/toxicants/residues in
foods, applications of these methods for the analysis
of food components and food adulteration are also
merging. However, immunoassays for the control of
food processing are not common. Future develop-
ments in the area of rapid test, automation, and
immunosensors will certainly widen the scope of ap-
plications to some of these areas. As such methods are
developed, more collaborative studies are needed to
validate the efficacy of the protocol and assay kits.
Since all immunoassays are based on the specific
interaction of antibody with antigen, it is imperative
for those who run immunoassays to have a clear
understanding of the specificity of the antibody used
in the assay as well as the purity and authenticity of
the standards. Immunochemical methods, including
affinity chromatography as a clean-up tool and
immunoassay as a monitoring system for high-
performance liquid chromatography, thin-layer chro-
matography, and electrophoresis have been used in
3254 IMMUNOASSAYS/Radioimmunoassay and Enzyme Immunoassay

conjunction with other analytical methodologies. The
innovative approaches have led to a new dimension in
the immunochemical methods for food analysis. Un-
questionably, immunoassays will be advanced to an-
other new era in the new millennium and they will be
used extensively in food and agriculture in the coming
years. It will not be surprising to see immunoassay
screening kits will some day reach the consumer
level. Such progress will certainly not only provide
safer foods to eat and a better environment in which
to live, but will also enhance agricultural productivity
by using these new technologies to improve crop qual-
ity and yield and enhance animal and plant health.
See also: Biosensors; Chromatography: Thin-layer
Chromatography; High-performance Liquid
Chromatography
Further Reading
Beier RC and Stanker LH (1996) Immunoassays for Resi-
due Analysis: Food Safety. ACS Symposium Series book
no. 621. Washington, DC: American Chemical Society.
Chu FS (1991) Development and use of immunoassays in
detection of the ecologically important mycotoxins. In:
Bhatnagar D, Lillehoj EB and Arora DK (eds) Handbook
of Applied Mycology, Mycotoxins, vol. V, pp. 87–136.
New York: Dekker.
Fukal L and Kas J (1989) The advantages of immunoassay
in food analysis. Trends in Analytical Chemistry 8:
112–116.
Maggio ET (1980) Enzyme-immunoassay. Boca Raton,
Florida: CRC Press.
Morgan MRA, Smith CJ and Williams PA (1992) Food
Safety and Quality Assurance Application of Immuno-
assay Systems. London: Elsevier Applied Science.
Pestka JJ (1994) Application of immunology to the analysis
and toxicity assessment of mycotoxins. Food and Agri-
culture Immunology 6: 219–234.
Rittenburg JH (1990) Development and Application of
Immunoassay for Food Analysis. London: Elsevier Ap-
plied Science.
Schuurs AHWM and van Weemen BK (1977) Enzyme-
immunoassay. Clinica Chimica Acta 81: 1–40.
Tijssen P (1985) Practice and Theory of Enzyme Immuno-
assays. Amsterdam: Elsevier Scientific.
Vanderlaan M, Stanker LH, Watkins BE and Roberts DW
(1991) Immunoassays for Trace Chemical Analysis:
Monitoring Toxic Chemicals in Humans, Foods and
the Environment. ACS symposium series 451. Washing-
ton, DC: American Chemical Society.
Yallow RS (1978) Radioimmunoassay: a probe for the fine
structure of biologic systems. Science 200: 1236–1245.
IMMUNOLOGY OF FOOD
K M Burghardt and F W Scott, Ottawa Health
Research Institute, Ottawa, Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The process of eating brings us into intimate contact
with a wide variety of foods derived from plants and
animals. These foods contain carbohydrates, pro-
teins, lipids, micronutrient vitamins and minerals,
dietary fiber, other nonnutrient chemicals, bacteria,
fungi, viruses, environmental contaminants, food
additives, and byproducts of processing and cooking.
Food is the most important modifiable environmental
exposure to which we are subjected. The aim of this
chapter is to explore, in a selective manner, food
components that have the potential to alter the activ-
ity of the immune system. The gut immune system is
continously challenged by a wide spectrum of dietary
macromolecules, their metabolic products, resident
bacteria, and invading pathogens. Luminal antigens
and other molecules are thus a source of information
about our immediate environment. The main inter-
action between food and the immune system occurs in
the gastrointestinal tract, the largest lymphoid organ
in the body. We shall examine the arsenal of immune
cells in the gut-associated lymphoid tissue (GALT),
the defense mechanisms, and selected examples of
immunomodulatory foods and food components
that contribute to health or disease.
Dietary Modulation of the Immune System
0002Human milk provides optimal nutritional, immuno-
logical, physiological, and psychological benefit to a
newborn child. At this vulnerable time when its
immune system is ill equipped to protect itself, the
baby relies on antiidiotypic antibodies and lympho-
cytes it received in utero and which it continues to
acquire through mother’s milk. Breast-fed infants
have been reported to experience significantly fewer
gastrointestinal, respiratory, urinary, and ear infec-
tions. Some studies have shown long-term enhanced
IMMUNOLOGY OF FOOD 3255

protection against Haemophilus influenzae type b
infections, otitis media, diarrhea, respiratory tract
infections, and wheezing bronchitis. Mother’s milk
provides an immediate boost for the young immune
system because it delivers antibodies, cytokines, hor-
mones, growth factors, and cells that promote the
maturation of the intestinal mucosa as well as modu-
late immune responses. It contains factors that estab-
lish a normal, symbiotic microbial gut flora that
protects the newborn from pathogenic bacteria.
There is a debate whether infants at risk for develop-
ment of atopy should be breast-fed because small
amounts of food allergens have been detected in
human breast milk. A prospective study of neonates
identified prolonged (> 9 months) exclusive breast-
feeding as the most likely risk factor for atopy by
2 years of age. In contrast, another study showed
that supplementation with cows’ milk prior to
breast-feeding resulted in fewer cases of atopy by 18
months. Numerous other studies have examined this
question, and it is yet to be established whether ex-
posure to antigens acquired via the mother’s milk
induces oral tolerance or priming. Several confound-
ing factors such as antigen dose, timing, and fre-
quency of exposure contribute to the variable results
obtained.
0003 The type and quantity of essential fatty acids, such
as linoleic acid (n-6), dictate the structure and func-
tion of the cell membrane. Long-chain and saturated
fatty acids reduce fluidity, while short-chain and cis-
unsaturated fatty acids increase membrane fluidity.
Changes to membrane fluidity influence cellular per-
meability as well as reorganize the distribution and
activity of membrane-associated enzymes and recep-
tors. Consequently, this alters the presentation of
membrane surface antigens, which affects cellular
communication, migration, and immune stimulation.
0004 Linoleic acid is a precursor of arachidonic acid
(AA). AA serves as the starting material for eicosa-
noid synthesis. The membranes of lymphocytes and
macrophages contain high concentrations of AA,
> 25% of all esterified membrane fatty acids. Mito-
gens stimulate the release of AA from lymphocytes,
which will be converted to eicosanoids by macro-
phages. Other stimuli that release AA from the cell
membrane are reactive oxygen intermediates (ROI)
and cytokines such as interleukin-1 (IL-1) and tumor
necrosis factor (TNF). Eicosanoids are composed of
two families of molecules, the prostanoids (prosta-
glandins and thromboxanes) and the leukotrienes
(lipoxins and hydroxy acids). Release of these oxida-
tion products has physiological and immunological
consequences. Prostaglandins regulate immune respon-
ses such as IL-2 production, T-cell mitogenesis, and
antibody production. Leukotrienes cause leukocyte
infiltration, stimulate vascular permeability, and
induce smooth-muscle contraction.
0005Linolenic acid, of the n-3 series, was reported to
inhibit leukotriene B
4
synthesis and decrease inflam-
mation. Linolenic acid is a precursor for eicosapen-
taenoic acid (EPA), which is structurally similar to
AA, but has antiinflammatory effects. In fact, dietary
supplementation with n-3 fatty acids increases the
incorporation of EPA into the membrane with a pro-
portional decrease in the amount of AA. EPA is me-
tabolized to prostacyclins and thromboxanes which
have a net antiinflammatory and antiaggregatory
effect. Thus, the amount and type of essential fatty
acids in the diet can regulate the type of eicosanoid
synthesis. Furthermore, polyunsaturated fatty acids
(PUFA) modulate immune responses by enhancing
antibody production and decreasing cell-mediated re-
sponses, as demonstrated by a shift from T helper
1 (Th1) to Th2 responses that is consistent with IL-2
receptor inhibition. Incorporation of EPA, derived
from dietary n-3, at the expense of AA reduces the
level of PKC activation and thus decreases IL-2 recep-
tor production. There is now considerable evidence
that atherosclerosis is an inflammatory disease of the
vascular system, the prevention of which involves not
only the modification of serum lipids but also a de-
crease in inflammatory reactions with the endothe-
lium. Dietary n-3 PUFA and vitamin E have received
particular attention as protective agents.
0006Dietary fat is also important as a delivery vehicle
for the lipid-soluble vitamins, D, E, A, and K. These
lipophilic vitamins have immunomodulatory func-
tions. Vitamin A, D, and b-carotene regulate leuko-
cyte activity. Vitamin A controls the recruitment
of cells to lymphocyte and macrophage lineages.
Vitamin E has antioxidant properties that prevent
excessive tissue damage by ROI during stress or in-
fection. Since ROI are also involved in activation of
proinflammatory gene expression (e.g., nuclear factor
kappaB (NFkB)), antioxidant vitamins can inhibit the
inflammatory cascade. These vitamins have been
shown to scavenge free radicals that initiate lipid
peroxidation. Lipid peroxidation in polymorphonuc-
lear leukocytes, macrophages, and mast cells also
releases AA.
0007Garlic has been used since the times of the early
Egyptians as a medicinal herb. There are several bio-
logically active ingredients, including protein fraction
4, diallyl disulfide, and S-allyl cysteine in garlic. S-allyl
cysteine can inhibit activation of NFkB in neuroblas-
toma and melanoma cells. Thus, garlic is reported to
inhibit directly tumor cell growth and may potentiate
the immune system by stimulating macrophages, NK
(nuclear killer cells), and killer cells, lymphokine-
activated killer cells, and increasing production of
3256 IMMUNOLOGY OF FOOD

IL-2, TNF (tumor necrosis factor), and interferon-g
(IFN-g). This could be considered an example of indu-
cing a Th1 response that benefits the immune system
fight against certain tumors. It is important to be
aware that individual foods are unlikely by themselves
to be ‘magic bullets’ that boost the immune response
against cancer or regulate other complex chronic dis-
eases. It is the aggregate effect of all dietary exposures
that is important. There are several other examples of
foods and food components that influence the
immune response, including glutamine, arginine, nu-
cleotides, probiotics, vitamins (e.g., vitamin D
3
), min-
erals (e.g., zinc, iron, copper), and oligosaccharides
(e.g., 1,3 b-glucans), to name a few.
Micronutrient Modulators of the Immune
System
0008 Nutrient status may determine the defense capacity of
the immune system. Many vital relationships between
trace elements such as zinc, iron, selenium, copper,
and cellular function have been described. Most often
these elements are an integral part of enzymatic reac-
tions as cofactors and molecular scaffolds. Recently,
micronutrients have been shown to regulate directly
the immune system.
0009 Zinc has catalytic, structural, and regulatory func-
tions that are well described in cellular metabolism.
Zinc deficiency also has immunological conse-
quences, such as lymphopenia, poor cell- and anti-
body-mediated responses, adrenal hypertrophy, and
thymic atrophy. When zinc becomes limiting, it is
redistributed to vital organs such as the liver and
diverted to the innate arm of the immune system.
Zinc deficiency induces a chronic increase in gluco-
corticoid synthesis, a steroid which promotes apop-
tosis in precursor lymphoid cells. Thus myeloid cells
such as macrophages and neutrophils are spared
while lymphoid compartments are depleted. Although
macrophages are spared from apoptosis, they are still
sensitive to zinc availability. Macrophages isolated
from zinc-deficient mice have a reduced capacity to
phagocytose and generate an oxidative burst, likely
due to the dependence of superoxide dismutase and
certain phospholipases on zinc as a cofactor. Similar
findings were shown in human monocytes. Clearly,
zinc status can alter the choice of defense mechanisms
of the immune system.
0010 Iron is an essential trace element because it is part
of heme compounds, hemoglobin and myoglobin,
which are responsible for oxygen transport and stor-
age, as well as being part of nonheme compounds,
and acting as a cofactor for certain critical enzymes
like mitochondrial aconitase and ribonucleotide
reductase. Moreover, iron availability influences
immune effector functions. In the event of chronic
infection, iron uptake and storage are increased by
proinflammatory cytokines to limit the availability of
this essential cofactor to invading pathogens. In-
creased iron uptake and storage by activated macro-
phages and monocytes also serve to increase IFN-g
and TNF-a activity and thus enhance their cytotoxic
effect. These cytokines also induce the production of
nitric oxide, and can form iron–nitrosyl complexes
and further decrease iron availability. The counter-
effect of the diversion of iron traffic is a reduction in
heme synthesis, which contributes to a reduced
oxygen transport capacity and possibly chronic
anemia. A case in point is anemia of chronic disease,
whose pathogenesis has been linked to activated
macrophages and monocytes.
0011Dietary selenium is covalently bound to methionine
and cysteine and converted to selenide. Selenide pro-
vides an essential cofactor for the synthesis of seleno-
proteins such as glutathione peroxidases and type
1 iodothyronine deiodinase. Selenium has been ex-
ploited in rheumatoid arthritis therapy to reduce
oxygen-derived free radicals that damage inflamed
joints. Furthermore, selenium also has immunomo-
dulatory properties. Selenium-deficient mice show
decreased lymphocyte proliferation in response to
mitogen. Selenium supplementation has been shown
to enhance cell-mediated immune responses in
clinical trials with elderly individuals or subjects
receiving parenteral nutrition.
0012A copper deficit is associated with a small thymus,
anemia, decreased antibody production, and neutro-
penia. In more extreme cases, severe copper defi-
ciency compromises the number and function of
neutrophils and macrophages. Individuals fed a
copper-deficient diet exhibit reduced T-cell prolifer-
ation following mitogen stimulation, an effect that is
attributed to decreased IL-2 production. Acquired
immunity is also impaired in an animal model of
marginal copper deficiency. Pregnant rats were
placed on a marginally copper-deficient diet halfway
through the gestation period. The rat pups showed
reduced cell-mediated responses, yet they had normal
copper status as determined by common indices:
tissue copper, serum copper, and ceruloplasmin activ-
ity. This highlights the exquisite sensitivity of the
immune system to nutrient deficiencies and the inher-
ent difficulties in defining ‘normal’ nutrient require-
ment for optimum immune function.
How Luminal Antigens are Handled by the
Gut Immune System
0013Nonspecific barriers, such as gastric acid, mucus,
digestive enzymes, and peristalsis, as well as specific
IMMUNOLOGY OF FOOD 3257
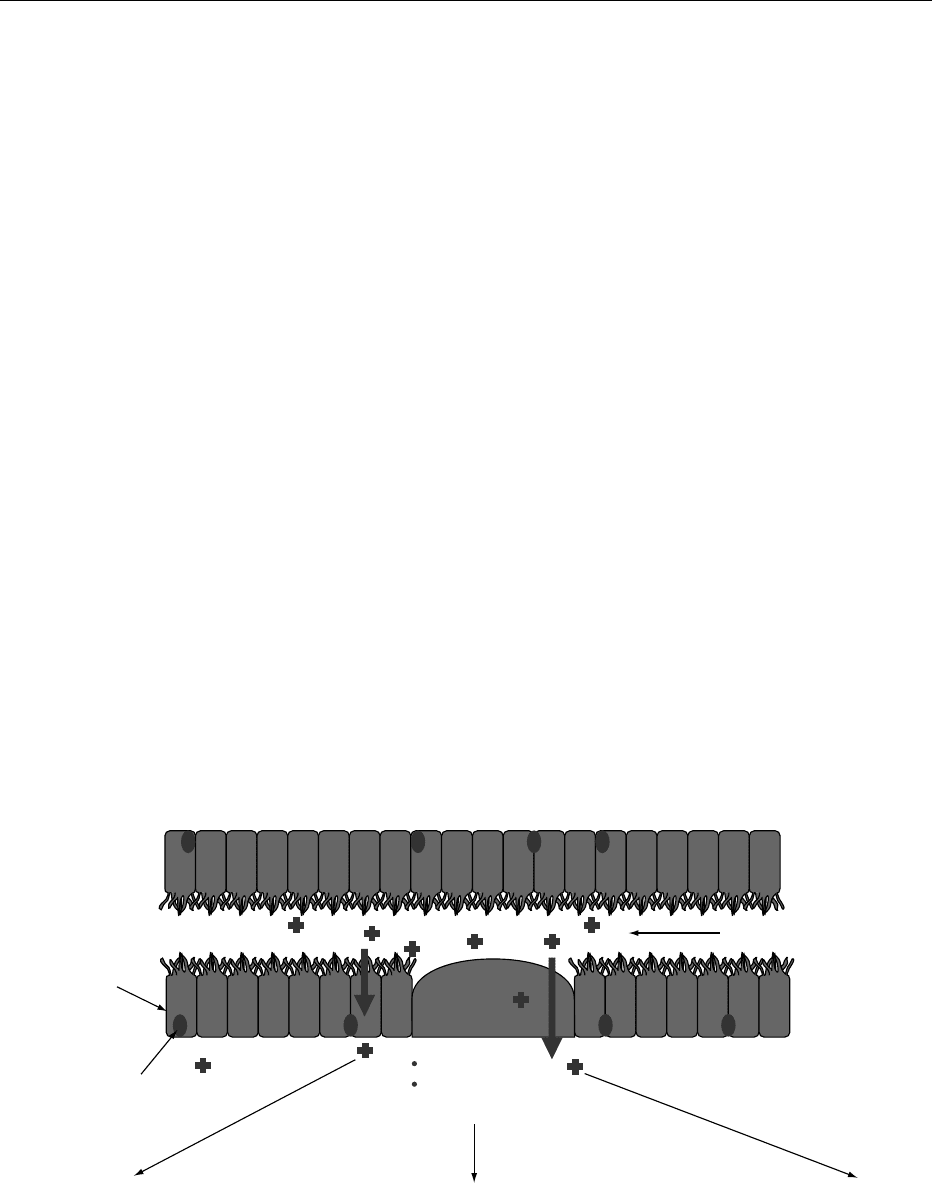
immunological barriers, including secretory immuno-
globulin A (sIgA) and sIgM, limit antigen access to
the GALT. It is estimated that 1 out of 10
5
ingested
antigens is absorbed intact by the gastrointestinal
tract, which is in the order of microgram per liter
quantities. Antigens that cross the gut epithelium
are surveyed by immune cells and classified as harm-
less or harmful. The controlled manner by which
luminal antigens gain entry to the GALT is depicted
in Figure 1. Lymphoid follicles, called Peyer’s patches,
are overlaid by membranous epithelial cells (M cells)
whose morphology is typified by a smooth apical
surface, lack of glycocalyx, and deep invaginations
of the basolateral membranes, particularly suited for
antigen uptake and delivery to the closely associated
lymphocytes and macrophages. Other lymphocytes
and dendritic cells are scattered throughout the
lamina propria in anticipation of antigens delivered
through enterocytes. In healthy epithelia, the uptake
of these macromolecules is through active vesicular
transport. When the junctions between gut epithelial
cells become less restrictive, permeability increases
and additional lumen antigens can penetrate the
lamina propria and activate resident immune cells.
The immune response is based on multiple factors.
0014 A dietary antigen that gains access to the GALT has
the potential to induce systemic responses, such as
active immune suppression (oral tolerance), immune
priming, or it can prompt local immune responses
such as the production of sIgA. Oral immune toler-
ance refers to the unresponsiveness of the immune
system, specifically the hyporesponsiveness of mature
lymphocytes in peripheral lymphoid tissue that stems
from prior oral exposure to the antigen. Recent stud-
ies suggest CD4
þ
Th cells are essential for the induc-
tion of oral tolerance because CD8
þ
cytotoxic T
lymphocyte knockout mice can still develop oral tol-
erance. Furthermore, CD4
þ
T cells can transfer oral
tolerance to a naive individual. Immune deviation
from Th1 toward Th2, Th3, or regulatory Tr-1 cells
has been proposed to favor normal production of IgA
with diminished risk of immunopathology. The major
mechanisms, likely acting in concert to establish and
maintain tolerance, are clonal anergy (the lack of
costimulatory molecules), clonal deletion, and sup-
pression by cytokines such as TGF-b. This depends
on the nature of the antigen, dose, maturity of the
host immune system, nature of the flora in the digest-
ive tract, and poorly understood host factors. Under
certain conditions, the immune system is primed for
further immune reaction instead of immune toler-
ance. Moreover, controversy regarding local immune
responses to oral soluble food antigens is not fully
resolved. Some suggest that IgA production and
secretion accompany oral tolerance, while others
contend that healthy individuals do not produce
measurable quantities of food-IgA antibodies. Indeed,
the decision to commit to any of these pathways is the
result of a complex set of cellular interactions and the
summation of many bidirectional messages (Figure 1).
0015Particulate or replicating antigens (e.g., bacteria)
are primarily taken up by M cells and diverted to
Lumen
Suppression
CD8
+
T cell
Enterocytes
Regulatory cells that secrete TGF-β
(e.g., CD4
+
T cells in lamina propria or
mesenteric lymph nodes)
MHC class II presentation of luminal
antigen on enterocytes to CD4
+
T cell
MHC class II presentation
of luminal antigen on
professional APC to
CD4
+
T cell
Tolerance Active immunity
Food
Peyer's patch
CD4
+
T cell
Professional APC
(e.g.,dendritic cells)
fig0001 Figure 1 Routes of antigen transport and mechanisms of immune regulation in the gut. APC, aerobic plate count; TGF-b, transform-
ing growth factor-b; MHC, major histocompatibility complex.
3258 IMMUNOLOGY OF FOOD

the immune cells of Peyer’s patches where they will
most likely prime the immune system for an assault
on these foreign intruders. Most food antigens are
digested in the intestinal lumen, which results in vari-
ous tolerogenic forms of the antigen. The requirement
for tolerance induction will depend on the antigenic
mixture and its breakdown products. Most soluble
antigens induce systemic tolerance.
0016 Oral tolerance has been successfully induced
through different regimens and doses of antigens. It
has been suggested that a single high dose of antigen
(> 0.5 mg g
1
body weight) will abrogate antibody,
cytokine, and cell-mediated responses in naive indi-
viduals as a result of T-cell inactivation. Regulatory T
cells are more likely to be activated following mul-
tiple low doses (< 0.1 mg g
1
body weight). In con-
trast, very low doses of antigen (< 0.005 mg g
1
body
weight) were shown to prime systemic and local
immune responses.
0017 The age at first encounter with the antigen is an
important determinant of the type of immune re-
sponse that ensues – oral tolerance or immune
priming. In general it has been observed that it is
easier to tolerize young mice than adult mice. The
mechanisms leading to oral tolerance also vary with
age. Anergy and/or deletion may be predominant in
the immature gut immune system, whereas in adults,
regulatory mechanisms have been established and
tolerance is more likely the result of active immune
suppression. These characteristics of the neonatal
immune system may be ascribed to the less restrictive
absorption in the neonatal gut, less competent or
‘immature’ GALT, or differences in the regulation of
neonatal lymphocyte responses. A breakdown or fail-
ure to establish oral tolerance results in various forms
of food hypersensitivity.
Abnormal Immune Reactions to Food
0018 In some individuals, ingestion of certain foods results
in a disproportionate immune reaction or allergic
reaction. Allergenic foods are commonly resistant to
proteolysis, undergo posttranslational glycosylation,
and may exhibit enzymatic activity. A distinction
should be made between allergy, which is immune-
mediated, and intolerance. Intolerance refers to a
nonimmunologically mediated reaction stemming
from abnormal metabolism (e.g., enzyme deficiency)
or impaired excretion of a food constituent or prod-
uct. Allergy or immediate type 1 hypersensitivity is
usually the result of sufficient numbers of IgE mol-
ecules binding to some dietary macromolecules, thus
initiating the complement cascade and inducing mast
cell degranulation. The release of preformed immune
mediators stored in the mast cells instigates the
immediate onset of symptoms ranging from cutaneous,
and gastrointestinal to respiratory, as well as systemic
anaphylactic reactions. The severity of the allergic
response is associated with the number of immune
cells being activated. An allergen that is distributed
by the circulatory system has the potential to stimu-
late a greater number of mast cells, releasing large
quantities of immune mediators. Studies of intestinal
biopsies have suggested that food allergy in animals
and humans is characterized by an increased number
of intraepithelial lymphocytes, mucosal mast cells,
and eosinophils.
0019Food allergies and other inflammatory disorders of
the intestine are associated with a dysfunction of the
epithelial barrier. However, whether the epithelial
barrier dysfunction predisposes an individual to aller-
gic disease or is a consequence of a proinflammatory
state is controversial. One hypothesis that has sought
to explain food allergy suggests that excessive hygiene
may be preventing the gut immune system from learn-
ing to differentiate between harmful and harmless
macromolecules, leading to an inappropriate inflam-
matory response. Another theory suggests that expos-
ure to high levels of antigen in childhood in
individuals with an inherent dysfunction of the gut
mucosa may promote allergic diseases. IgE cross-
reactivity between food allergens and pathogens is
another possible mechanism that may explain the
observation that risk of allergy may be proportional
to the rate of infection and vaccination. However, the
pathogenesis of food hypersensitivity reactions
remains unknown.
Diet–Gene Regulation
0020Food components can influence directly or indirectly
the transcription of genes. A case in point is curcumin,
which directly influences acute-phase immune re-
sponses. Curcumin, a component of turmeric powder
from the rhizomes of the Curcuma longa L. plant,
has antiinflammatory properties that stem from its
capacity to inhibit the expression of proinflammatory
genes. The transcription of these genes is regulated by
NFkB, which in turn is regulated by an endogenous
inhibitor called IkBa. Curcumin prevents the degrad-
ation of IkBa, which blocks NFkB from translocating
to the nucleus to initiate transcription of kB-dependent
genes such as TNF-a, IL-6, IL-8, Cox-2 (cyclooxygen-
ase), and nitric oxide synthase. Similarly, some
nutrients, such as vitamins A and D, fatty acids, sterols,
and zinc, bind to regulatory elements of genes. For
example, the metallothionein gene promoter requires
the binding of zinc–protein complexes in order to initi-
ate transcription. In addition to the metallothionein
gene, 47 other potential zinc-regulated genes have
IMMUNOLOGY OF FOOD 3259
