Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


the ice cream plant. No one should be allowed to
enter the processing environment who is not familiar
with the required sanitary procedures or who does not
conform to the required dress and personal hygiene
measures. Freedom from chronic contagious diseases
should be confirmed yearly by medical examination.
Provided that the preparation of ice cream is conducted
in a closed processing cycle with modern industrial
equipment of high hygiene standards, the opportunities
for contamination by human contact are few.
0020 Tests carried out during the production process
should be indicative of the standard of hygiene.
Storage (Quality Deterioration)
0021 Microorganisms are unable to grow in ice cream
stored at correct temperatures, although many sur-
vive for extended periods. It is a perfect substrate for
microbe development as far as its composition is
concerned. It has all the essential components (sugar,
proteins, water), oxygen, as well as a relatively high
and suitable pH. High temperature is the only agent
not available. If a rise in temperature does occur, all
the conditions are in place for the development and
proliferation of microbes that may exist. Microbio-
logical considerations, therefore, primarily involve
the elimination of vegetative pathogens by pasteuriza-
tion and the prevention of recontamination due to
insanitary processing and packaging, at all stages up
to point of sale, the microbiological status of ingredi-
ents with particular reference to thermoduric organ-
isms and preformed toxins, and the prevention of
microbial growth before freezing.
0022 Ice cream may be sold direct from the freezer as a
soft-serve product, or it may be further reduced in
temperature and frozen in wind tunnels at 40
C
or in hardening rooms, to produce ‘hard’ ice cream,
which will be stored at a temperature of about
30
C until it is sold. Deep-freezing stabilizes the
microbial content of ice cream: microorganisms
found in it no longer proliferate. Some sensitive
species (Gram-negative) die and their populations
decrease. Even if the period between freezing and
final sale is several months, there will be little change,
if any, in the microbial content of the ice cream.
Extensive research has shown that both Mycobacter-
ium and Salmonella, as well as many other less harm-
ful but often more resistant types, can survive at the
low temperature of storage for very long periods.
They do not multiply provided that the temperature
is low enough for the ice cream to remain hard; in
effect, the microbial quality of ice cream is ‘locked in’
by the hardening process.
0023 Problems of quality deterioration can appear when
there is a delay between pasteurization and freezing,
mainly a delay from heat treatment to cooling of the
mix, or the aging of the mix at a temperature higher
than 4
C due to ignorance or failure of the freezing
system. Spoilage can also occur, in cases of melting
and refreezing of the product resulting from tempera-
ture fluctuations or failure of the freezing systems.
Special care is needed with the mix for soft-serve ice
cream that has to be transported, often for long dis-
tances, in trucks to retail soft-serve stores or stands
where it is kept soft-frozen and dispensed to con-
sumers. Both contamination and temperature abuse
of the mix may easily occur. Furthermore, refriger-
ation space is usually limited, and adequate facilities
for cleaning and sanitizing the freezer and the associ-
ated equipment are often lacking or are, at best,
marginal.
0024Under these conditions, especially when inadequ-
ate practices have preceded storage, the ice cream is
overloaded with microbes which lead to quality de-
terioration or even to cases of food poisoning.
Reference is made in the Further Reading section to
cases of food poisoning following the consumption
of ice cream contaminated by microbes, such as
Staphylococcus, Salmonella, Shigella, Listeria, and
Streptococcus group A organisms. More serious
cases may occur with homemade ice creams, where
a combination of faulty practices, such as the use of
raw milk, eggs contaminated with Salmonella, inad-
equate heat treatment, no rapid cooling following
heat treatment, and contamination by infected per-
sons give rise to products with high microbial loads,
especially of pathogenic bacteria which can survive
many months in contaminated ice creams. (See Lis-
teria: Properties and Occurrence; Shigella; Staphylo-
coccus: Properties and Occurrence.)
Problems at Point of Sale
0025The largest proportion of microbiological problems
with ice cream products, in general, is due to poor
techniques of selling and serving at the final point of
distribution. This plays a vital role in the microbio-
logical load of ice cream. Even when the greatest care
has been taken to produce an ice cream of the highest
quality, it is still liable to contamination at the point
of sale.
0026The method of sale has a major bearing on the level
of contamination to which the product is subjected.
Although much ice cream is retailed in its final pack-
aging, a significant quantity is portioned from bulk
packs at the point of sale.
0027Prepackaged ice cream that is sold in individual
package is considered safer because it has to be
handled only by the consumer in its wrapping and
can be contaminated only by the consumer. Attention
3240 ICE CREAM/Microbiology

must be paid to the right storage temperature without
temperature variations or failure of the freezing
system. Greater degrees of contamination may occur
in ice cream which is portioned from bulk packs in
restaurants or coffee shops, mobile vans (complete
with their own electricity generation equipment),
and kiosks. This process is the weak link from the
hygiene viewpoint since many outlets have only
limited facilities for hand washing and utensil steril-
ization. In order to minimize the possibility of con-
tamination, the equipment (servers, wafer holders,
and so on) has to be kept free of all residues of ice
cream, which might otherwise melt and allow the
growth of bacteria to recommence. Whenever pos-
sible, these items of equipment should be kept in
running cold water. If they have to be kept in a jug
of water, this water must be changed regularly to
avoid it becoming a source of contaminating bacteria.
The personal hygiene of the server is also important.
Persons handling and serving food should be trained
to live up to definite and rigid standards of personal
hygiene. Hair, hands and fingernails, uniforms and
shoes have to be clean. Soap, water, and single-service
towels should be easily accessible.
0028 Soft-serve ice cream sold directly from a dispensing
freezer can become contaminated very easily unless
stringent precautions are taken. The product is usually
manufactured at the point of sale, which may be
a specialist outlet or cafe
´
, a nonfood outlet such as
a gas (petrol) filling station, or a mobile outlet or
kiosk. Soft-serve ice cream may be manufactured
from a conventional mix produced on the premises,
from an ultrahigh-temperature-processed, aseptically
packaged mix, or from a spray-dried powder mix.
Powdered mixes may be formulated for reconstitution
in either hot or cold water. Hot-water mixes are prefer-
able with respect to hygiene, but cold-water mixes
are often considered more convenient. Attention must
be paid to the reconstitution of the mixes; this must be
done under satisfactory hygienic conditions to prevent
the proliferation of Salmonella, which may survive if
mixes are not prepared carefully.
0029 Soft-serve ice cream freezers and ancillary equip-
ment should be dismantled, cleaned, and sanitized
daily. It must be recognized that maintenance of the
necessary hygiene standards can be more difficult in
an environment primarily concerned with retailing
than in one wholly concerned with manufacturing.
Particular difficulties may be encountered in outlets
which are predominantly nonfood, such as filling
stations, and those with inherently limited facilities,
such as kiosks. The self-pasteurizing soft-serve freezer
offers at least a partial solution. Such equipment is
designed to heat the mix and machine surfaces in
contact with the mix to an appropriate temperature
and time before cooling rapidly to about 4
C. It is
usual to ‘pasteurize’ daily, usually at the end of the
working day, and restrict full cleaning and sanitiza-
tion to a weekly basis. It must be emphasized that
self-pasteurizing freezers are not intended to process
unpasteurized mix. Generally, special dispensing
freezers should be kept in constant operation, and
placed inside the shop with the taps facing the inter-
ior. In addition, they must not be directly exposed to
the sun, dust, or flies.
0030Probably the most serious and dangerous sources
of contamination are operation and serving. Many
major food-poisoning outbreaks have been caused
by human contamination. Cases of typhoid fever,
including deaths, have been reported to be caused
by ice cream contaminated by the manufacturer
who was a urinary excretor of Salmonella typhi.
There has been a case of Shigella dysentery caused
by an ice cream that was accidentally touched by a
monkey. Also, outbreaks involving salmonellae and
staphylococci have been reported. The personal hy-
giene and habits of vendors at sale points are import-
ant. Education, in addition to medical inspection, is
absolutely necessary and no employee must be
allowed to work without full medical clearance.
0031Finally, birds, rodents, insects, and pet animals
have no place at the retail selling point.
0032Many countries, recognizing the significance of ice
cream in relation to public health, have legislated as
to the conditions and methods to be used for heat
treatment and subsequent storage and sale.
See also: Bacillus: Occurrence; Freezing: Storage of
Frozen Foods; Listeria: Properties and Occurrence;
Mycobacteria; Pasteurization: Principles; Spoilage:
Bacterial Spoilage; Staphylococcus: Properties and
Occurrence; Storage Stability: Mechanisms of
Degradation
Further Reading
Frazier WC and Westhoff DC (1988) Food Microbiology,
4th edn. New York: McGraw-Hill.
Hennessy TW, Hedberg CW, Slutsker L et al. (1996) A
national outbreak of Salmonella enteritidis infection
from ice cream. New England Journal of Medicine
334: 1281
International Commission on Microbiological Specifica-
tions for Food (1974) Microorganisms in Foods 1.
Toronto: ICMSF/University of Toronto Press.
International Commission on Microbiological Specifica-
tions for Food (1980) Microbial Ecology of Foods,
vol. 2. Food Commodities. London: ICMSF/Academic
Press.
Jervis DI (1992) Hygiene in milk product manufacture. In:
Early R (ed.) The Technology of Dairy Products, pp.
272–299. Chichester: John Wiley.
ICE CREAM/Microbiology 3241
Mantis AI (1993) Hygiene and the Technology of Milk and
its Products. Thessaloniki: Kiriakidis.
Marshall R and Arbuckle WS (1996) Ice Cream, 5th edn.
New York: Chapman & Hall.
Miettinen M, Bjo
¨
rkroth J and Korkeala H (1999) Charac-
terization of Listeria monocytogenes from ice
cream plant by serotyping and pulsed field gel electo-
phoresis. International Journal of Food Microbiology
46: 187
Rothwell J (1985) Microbiology of frozen dairy products.
In: Robinson RK (ed.) Microbiology of Frozen Foods,
pp. 209–232. London: Elsevier.
Rothwell J (1990) Microbiology of the ice cream and
related products. In: Robinson RK (ed.) Dairy Micro-
biology, vol. 2, pp. 1–39. London: Elsevier.
Varnam A and Sutherland J (1993) Milk and Milk Products.
Food and Commodities series no. 1. London: Chapman
& Hall.
Immobilized Enzymes See Enzymes: Functions and Characteristics; Uses in Food Processing; Uses in
Analysis
IMMUNOASSAYS
Contents
Principles
Radioimmunoassay and Enzyme Immunoassay
Principles
F S Chu, University of Wisconsin, Madison, WI, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Antibodies are a group of globular proteins called
immunoglobulins (Ig), which are formed in response
to the invasion of foreign substances in vertebrates.
Igs are classified into five groups: IgA, IgG, IgE,
IgD, and IgM. IgG, which has a molecular weight
of 150 kDa and is composed of two heavy chains
(50 kDa) and two light chains (25 kDa), is one of the
most common serum Igs (80% of total Igs). Anti-
bodies are capable of binding with foreign substances
noncovalently with high affinity and, thus, to inacti-
vate them.
0002 Substances that induce the defensive action by pro-
ducing antibodies in the host are called antigens, and
are characterized by their immunogenicity and their
antigenicity. Immunogenicity is the capability of a
molecule to induce antibody formation. Thus, the
term ‘immunogens’ specifically refers to those sub-
stances capable of inducing production of antibodies.
The antigenicity, or antigenic specificity, however, is
the characteristics/capabilities of the interaction of
the antigen with antibodies. The interaction involves
small sites, which are called ‘antigenic determinants’
or ‘epitopes’ on the surface of the antigen molecules.
0003In contrast to antigens, low-molecular-weight com-
pounds (less than 2500 Da) are not immunogenic.
These compounds are called haptens and can only
become immunogenic after conjugation to a high-
molecular-weight protein carrier.
0004Although it has been known for more than a cen-
tury that vertebrates are capable of producing specific
antibodies after invasion by foreign substances, the
complexity of immunoreactions has only been
revealed in the last two decades. With the advances
in understanding of the mechanism of immunoreac-
tions, the specific interaction between antibody and
antigen/hapten has been used as the basis for several
analytical techniques called immunoassays. These
assays, which are characterized by their high sensi-
tivity, specificity, and simplicity, were originally
developed for the analysis of clinical samples. Recent
development has led to the application of immuno-
assays for food and agricultural chemicals, including
both normal and abnormal food components,
additives and contaminants, as well as agricultural
chemical residues.
0005In contrast to clinical samples, immunoassays
for specific substances in foods and feeds are more
3242 IMMUNOASSAYS/Principles

complicated because: (1) specific antibodies must first
be raised against a wide range of food components
and chemicals. Some are antigens, whereas others are
nonimmunogenic haptens, most of which are lipid-
soluble; (2) foods are very complex systems and
sample matrices often interfere with the assay; (3)
changes in physicochemical properties of food com-
ponents/contaminants during processing of foods
often complicate immunoassays because the antibody
may not recognize the denatured species. Neverthe-
less, much progress has been made and numerous
review articles on the immunoassay of food compon-
ents/contaminants have appeared. The reader should
consult these reviews for details.
Principles
0006 All immunochemical techniques are based on the
specific noncovalent interaction between antigens/
haptens and specific antibodies. Earlier applications
of immunochemical methods for analytical purposes
were confined to the interaction between antibodies
and high-molecular-weight antigens that led to the
precipitation of the antigen–antibody complex for
quantitation. However, precipitates are also not
formed between antibody and haptens. Investigations
in the last three decades have led to the development
of more sensitive immunochemical methods. Thus,
the term ‘immunoassay’ now also includes the
modern immunochemical techniques which involve
the use of labeled marker for the antigen or antibody
and innovative approaches for the separation of free
antigen and antigen–antibody complex. As a result of
the development of new instruments and incorpor-
ation of label markers in the assay system, new
innovative precipitation methods with high sensitiv-
ity are now also available.
0007 For simplicity, the binding of antibody with a
simple hapten, which has only one antigenic deter-
minant site (univalent), is used to illustrate the
antigen–antibody interaction. As shown in eqn (1),
the interaction depends on the equilibrium or affinity
constants (K ¼k
a
/k
d
), which is the ratio of the associ-
ation (k
a
) and dissociation rate constants (k
d
) and the
concentrations of the reactants, i.e. [antibody] and
[antigen].
½Antibodyþ½Antigen
k
a
$
k
d
½AntibodyAntigen
0008 In general, the K values for antibody–antigen inter-
action are in the range of 10
6
–10
9
l mol
1
, but they
may vary from 10
3
to 10
14
l mol
1
. Antibody with a
high K value is more preferable for the assay, but any
reaction with a K value above 10
9
l mol
1
would be
feasible. For analytical purposes, either a labeled
antigen or a labeled antibody marker is present in
the system. With an adequate method to separate
the antibody–antigen complex from the free antigen
and antibody, as well as a sensitive method for detec-
tion of the markers in the free or bound form, the
concentration of the analyte can readily be deter-
mined. In general, a standard curve is established
and then used to calculate the concentration of un-
known sample. Thus, it is critical to have a pure
standard from a reliable source which should also
be tested frequently for its stability.
Preparation of Immunochemical
Reagents
0009As indicated above, an effective immunoassay
depends on the availability of: (1) a specific antibody;
(2) a sensitive marker antigen or antibody; and (3) an
effective separation method. Methods for the prepar-
ation of marker antigen or antibody and methods
for the separation of the free and bound species of
antigen and antibody vary considerably with different
immunochemical methods used. Approaches for the
preparation of immunogen and antibody as well as
methods for characterization of antibody can be
generalized as follows.
Preparation of Immunogens
0010Antibodies against major food proteins and naturally
occurring foodborne microbial toxins/contami-
nants have been produced and used in different
types of immunoassays. High-molecular-weight
substances such as proteins and polysaccharides can
be used directly as immunogens. In general, well-
characterized and pure immunogens are used for
generating antibodies. With the development of
monoclonal antibody technology, partially purified
proteins or even crude cellular extracts from patho-
genic bacteria have also been used successfully for
immunization as long as an effective method in
selecting specific hybridoma cell lines is available.
Because of structural changes for some proteins and
other macromolecules during food processing that
alter the immunogenic site, purified proteins are
not necessarily used to generate antibodies from
components in food-related products. Rather, for
determination of such materials in processed foods,
specific antibodies must be raised for corresponding
denatured proteins. Thus, depending on the specific
components to be analyzed, one must consider
selecting an immunogen which has comparable
properties to that actually found in the food.
0011Haptens must first be conjugated to a protein/poly-
peptide carrier before immunization. Methods for
IMMUNOASSAYS/Principles 3243

the conjugation of various low-molecular-weight nat-
urally occurring toxins, including antibiotics, myco-
toxins, phycotoxins (marine toxins), pesticides, and
other agricultural chemicals, to protein carriers have
been established. Proteins, including bovine serum
albumin (BSA), modified BSA, and keyhole limpet
hemocyanin, are most commonly used. In general, if
the hapten has a reactive group such as a carboxylic
or an amino group, it can be conjugated to the protein
directly via one of the following approaches: water-
soluble carbodiimide method, mixed anhydride
method, condensation method in the presence of
formaldehyde (Mannich reaction), cross-linking
with glutaraldehyde, activated ester method through
the formation of N-hydroxysuccinimide (NHS)
esters, 1,1
0
-carbonylimidazole and m-maleimidoben-
zoyl-N-hydroxysuccinimide ester, and many other in-
novative methods. Derivatization is necessary if no
reactive group is present. Before derivatization or
conjugation, one must select appropriate strategies
to insure that certain portions of the hapten molecule
are exposed. Not only the amount of hapten conju-
gated to the protein molecule is important in deter-
mining the effectiveness of the conjugates as an
immunogen, but also the position of the side chain
in the hapten where conjugation is made, as well as
the space between the hapten and protein molecule
are critical. However, a conjugate containing a large
amount of hapten may not necessarily be a better
immunogen. Antibodies have been obtained from
rabbits immunized with immunogen containing only
2 mol of hapten per mole of carrier protein. The
optimum amount of hapten coupled to a protein
that yields a good immunogen should be experimen-
tally determined, but generally is in the range of 10–
20 mol per mole of protein.
0012 Rapid progress in cloning genes for many proteins/
enzymes/toxins in food and agricultural commodities
has led to another approach for the preparation of
immunogens. By knowing the DNA sequence of a
gene, the amino acid sequence for a specific gene
product in which we are interested could be deduced.
Through structural analysis, the highly immunogenic
fragment in this protein could be identified and
chemically synthesized. These peptides could then be
conjugated to a branched-chain lysine to form a mul-
tiple-chain immunogenic polypeptide for immuniza-
tion. Using this approach, it is possible to generate an
antibody for a protein even before it is purified and
characterized.
Preparation of Antibodies
0013 Two approaches have been used for the production of
antibodies. The classic method involves immuniza-
tion of animals and then obtaining the antibodies
from the sera of the immunized animals. Antibodies
obtained by this approach are polyclonal anti-
bodies (PAbs) because they are derived from different
B lymphocyte clones. In this method, immunogens
are mixed with adjuvant and then injected into
animals, most commonly rabbits, intradermally or
subcutaneously through the thigh or on the back at
multiple sites. For production of a large quantity of
antiserum, large animals such goats and horses are
used. Other animals, such as pigs and mice, have
also been used. Production of antibody has been
optimized by immunization of chickens and then
isolation of antibody from eggs.
0014The amount of immunogen used in the immuniza-
tion varies considerably. In general, 1–2 mg protein is
used for each rabbit. To avoid toxicity of some food-
borne bacterial toxins, a smaller amount of toxins or
toxoids are used in the initial immunization (e.g.,
5 mg) and this is followed by a gradual increase in
dose for subsequent booster injections. For haptens,
200–500 mg of conjugate is generally used in each
injection. Although antiserum obtained from animals
could be used directly in the immunoassay, simple
purification such as ammonium sulfate precipitation
is generally necessary. Further purification of anti-
serum through various chromatographic methods
can improve the efficacy of immunoassays.
0015If the immunogen is highly immunogenic, antibody
titers, as determined by different approaches such
as immunodiffusion, radioimmunoassay (RIA) or
enzyme immunoassay (EIA), usually start to increase
at 4–7 weeks. Subsequent booster injections are gen-
erally performed once a month. Since PAbs are het-
erogeneous, it is advisable that the purified antigen be
used in the titer determination. For haptens, a specific
hapten-marker must be used. Use of inadequate
antigen or hapten-markers usually fails to provide
information regarding whether the immunogen and
immunization protocols are effective.
0016Although production of PAbs is relatively simple
and the affinity of Pabs is generally high, they are
heterogeneous, and their supply is limited. With in-
creasing demand for large amounts of antibodies with
homogeneous properties for immunoassays, as well
as advances in hybridoma technology, specific mono-
clonal antibodies (MAbs) for a number of food com-
ponents and contaminants in foods have also been
produced. In this method, spleen cells obtained from
mice immunized with immunogen or hapten–protein
conjugates are fused with myeloma cells, followed by
propagation of the hybrid cells, and selection and
characterization of the clones, as well as isolation
and characterization of the antibody. Production of
mAbs can either be carried out in tissue culture or in
mice, in which ascites fluids are collected. Thus,
3244 IMMUNOASSAYS/Principles

mAbs are derived from a single B lymphocyte clone
and are immortal. Once a hybridoma cell line is
obtained, there is an unlimited supply of homoge-
neous antibodies with unique properties. Similar to
the production of PAbs, the key to success in
obtaining a useful hybridoma cell line lies in the
effectiveness of immunogens as well as the immuno-
gen-marker or the hapten-marker. The importance of
an effective immunoassay protocol in selecting the
positive clones cannot be overemphasized. Many
new stable hybridoma cell lines for production of
antibodies against various food components, contam-
inants, naturally occurring toxicants, and agricultural
residuals are now available. Rapid progress in mo-
lecular cloning of MAbs has also led to the applica-
tion of this technology for the production of antibody
important to agricultural and food scientists and
several laboratories have succeeded in this regard.
Antiidiotype Antibodies
0017 The development of immunochemical methods for
agriculture and food analysis has led to a great
demand for specific antibodies and related immuno-
chemical reagents for the assay. An alternative ap-
proach for preparing immunochemical reagents is
through generating antiidiotype antibodies (Ab2),
which have gained wide application in diagnostic
and therapeutic areas for large molecules. In the last
few years, Ab2 against small-molecular-weight
haptens, including insecticides, herbicides, hormones,
mycotoxins, and phycotoxins, have been successfully
developed. Some Ab2 have not only been shown
effectively to mimic the biological functions of the
haptens, but have also been used as the immunogen
to generate antiantiidiotype antibodies (Ab3) with
specificity similar to the original antibody (Ab1).
Both Ab2 and Ab3 have been shown to be effective
in immunoassays.
Characterization of Antibodies
0018 Antibodies obtained from the above methods should
be well-characterized before they are used in the
immunoassay. In general, both the type of immuno-
globulin and the specificity of the antibody to various
analogs of the immunogen/hapten should be deter-
mined. The typing of immunoglobulin is generally
done by using commercially available kits. Informa-
tion on the immunoglobulin type is important in
selecting adequate markers as well as an appropriate
method for separation of free antigen or antibody
from the antigen–antibody complex in the immuno-
assay. Information on the specificity will provide an
accurate assessment as to whether structurally related
compounds will interfere in the immunoassay. They
could also be used to estimate the affinity of the
antibody to the immunogen/hapten and its structur-
ally related analogs.
0019The specificity of the antibody, usually expressed as
the cross-reactivity of an antibody, is primarily deter-
mined by the type of immunogen that has been used.
A minor change in the immunogen structure could
generate a different antibody. Some antibodies are
very specific, whilst others show a broad specificity.
The apparent cross-reactivity of the antibody also
varies with the type and concentrations of the marker
antibody or antigen and the format used in the
immunoassay. Thus, in addition to knowing the
specificity of the antibodies generated by a specific
immunogen, the conditions under which that specifi-
city was determined should be stated. Preferably, the
‘apparent cross-reactivity’ should be reevaluated
under the conditions that are to be used for the
assay. The specificity of the polyclonal antibodies
can be improved by removal of nonspecific antibodies
through immunoabsorption.
0020For haptens, the site in the hapten molecule linking
to the carrier protein is important. The degree of
cross-reactivity of these antibodies with structural
analogs of the hapten used in the conjugation should
be determined. The accuracy of immunoassay of
hapten in naturally contaminated samples is generally
affected by both the specificity of the antibody and
the possible presence of structurally related analogs
of the hapten in the sample as well as by some sub-
stances with similar structures of the hapten.
0021Experimentally, the cross-reactivity of antibody is
determined by an immunoassay where various struc-
turally related analogs of immunogen/haptens at a
wide range of concentrations are used to compete
with the binding of the marker ligand with the anti-
body in the assay. The concentration at 50% inhib-
ition (I
50
) of the binding is generally used as the basis
to calculate the relative cross-reactivity for each
analog. A typical example of such a competitive RIA
and enzyme-linked immunosorbent assay (ELISA) for
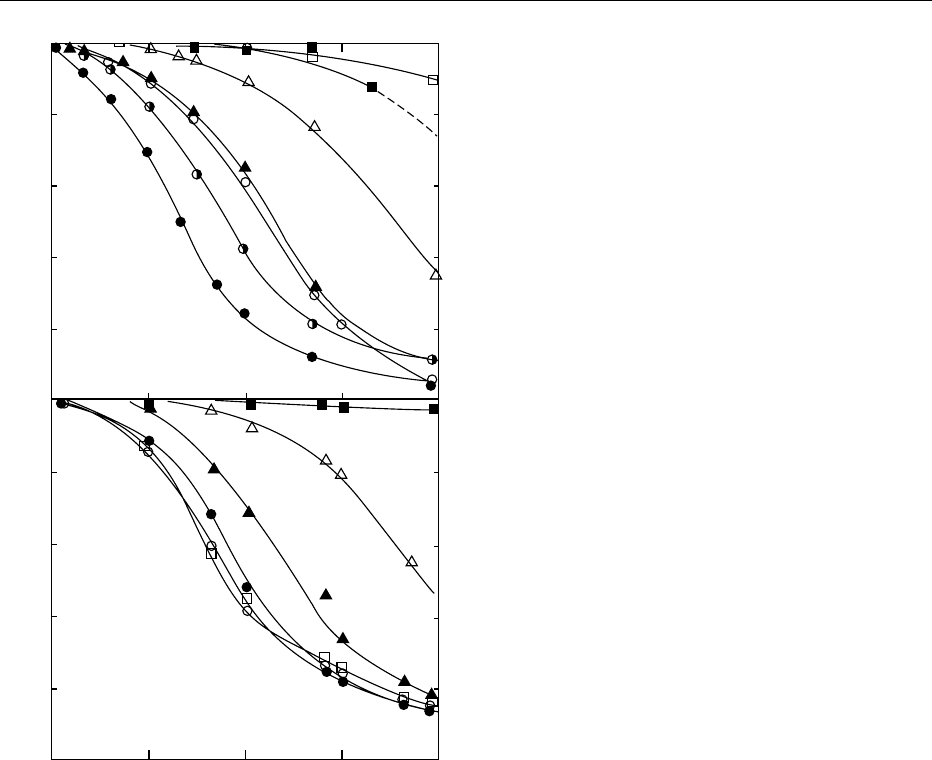
aflatoxins is shown in Figure 1. The specificity of the
antibody is generally documented with data describ-
ing antibody production and characterization for a
particular immunogen.
Immunoassays other than RIA and EIA
used in Food Analysis
0022With the availability of different antibodies against vari-
ous food components and contaminants, many types of
immunoassays have been developed. Before modern
immunoassays such as RIA and EIA were developed,
several classic methods, most of which are based on
the precipitation of the immunocomplex, were used
widely for the analysis of high-molecular-weight
IMMUNOASSAYS/Principles 3245
immunogens. Considerable improvement has been
made over the years to some of these methods. With
the availability of new instruments and enhancement of
the particle size, both the precision and sensitivity have
been improved in recent years. Thus, they are still fre-
quently used for the analysis of food constituents.
Immunochemical methods other than RIA and ELISA
in food analysis are briefly described here.
Precipitation
0023 This method is based on the formation of precipitate
as a result of antibody–antigen interaction. Several
formats, which require several minutes to several
days to perform and have a sensitivity range from
0.1 to 10 mgml
1
, have been used.
0024Precipitation in solution or quantitative precipitin
method In general, an aliquot of different concen-
trations of antigen or antibody is added to a series of
tubes containing a constant amount of antibody or
antigen. A precipitate is formed after incubation. The
concentration of the unreacted antigen or antibody in
the supernatant solution or the weight of the precipi-
tate of the antigen–antibody aggregate is determined
after centrifugation. Maximum precipitate is only
formed at the equivalent zone where none of the
reactants is at excess. This approach is very tedious
and subject to error, and is not commonly used at
present. Instead, turbidimeter and nephelometer are
used in most laboratories. Recent developments have
led to measuring the light-scattering effect of the
particles instrumentally; the term ‘light-scattering
immunoassay’ is used for this type of assay.
0025Methods based on simple diffusion of reactants
and subsequent precipitation of immunocomplex in
agar gel These methods, which are based on the
formation of precipitin bands or lines in agar
gel, were originally developed by Oudin and Ouch-
terlony. Three formats are generally used: simple
gel diffusion, double diffusion, and radial immuno-
diffusion (RID).
0026Simple gel diffusion This method involves the use of
a small tube (about 0.5 8 cm; Oudin tube) partially
filled with agar containing antibody (occasionally
antigen). The test solution is placed on top of the
agar column. After antigen diffuses into the agar, a
precipitation band is formed at the interface as a
result of the antigen–antibody interaction.
0027Double diffusion (double immunodiffusion) This
method involves the use of an agar gel plate (some-
times called an Ouchterlony plate) where cups or
wells containing test antibody and antigen solutions
are placed in such an order that the antigen and
antibody solutions diffuse toward each other through
the gel. Most commonly, the antibody well is placed
in the center, with the antigen wells around it. After
incubation, a band is formed between the antibody
and test antigen solution. The intensity of the preci-
pitin band is related to the concentration of the react-
ants. With appropriate arrangement of the wells for
the antigen and antibody, the cross-reactivity of the
antibody with structurally related antigen could also
be determined. If the precipitin band of the antigen is
completely connected with the band of the next well,
these two antigens are identical. If they are partially
connected to form a spur (arc) type of band, then they
are partially identical. However, if both bands cross
each other, these two antigens are not identical.
0
20
40
60
80
100
20
40
60
80
100
(b)
(a)
R
0
B
2
B
1
B
1
B
1
B
2
oxi.
G
1
G
2
G
1
G
2
M
1
ST
M
1
Percentage of binding
Percentage of max. absorbance (414 nm)
0 1.0 10 100 1000
Concentration (n
g
mI
−1
)
fig0001 Figure 1 Cross-reactivity of polyclonal antibody against afla-
toxin B
1
with various aflatoxins as determined by (a) radioimmu-
noassay and (b) enzyme-linked immunosorbent assay. (d),
Aflatoxin B
1
;(g), aflatoxin B
1
-carboxymethyl-oxime; (s), afla-
toxin B
2
;(m), aflatoxin G
1
;(n), aflatoxin G
2
;(&), aflatoxin M
1
;
(h), sterigmamatocystin.
3246 IMMUNOASSAYS/Principles

0028 Radial immunodiffusion In this assay, antibody is
distributed throughout the gel matrix. The test anti-
gen is placed in wells punched into the gel. After
incubation, the antigen is diffused into the gel to
form an opaque zone where the immunocomplex
occurs. The diameter or area of the circular zone is
proportional to concentrations of the test solutions.
0029 Combination of other separation methods with
immunodiffusion and precipitation reaction One of
the most common approaches is immunoelectro-
diffusion (IED) or immunoelectrophoresis, which
involves the separation of antigen electrophoretically,
followed by immunodiffusion. In practice, a trough
parallel to the direction of electrophoresis in a gel is
cut immediately after the electrophoresis. The trough
is then filled with specific antibody. After incubation,
precipitin bands/arcs are formed in the gel.
0030 Several variations of this method have been de-
veloped. For example, the IED or so-called ‘rocket’
immunoelectrophoresis method involves the combin-
ation of electrophoresis with RID. In this assay, the
antibody is distributed in the gel and antigen or ana-
lyte is placed in the well at the end of the gel. Electro-
phoresis is carried out under conditions which do not
permit the migration of antibody. After electro-
phoresis and diffusion, rocket-shaped precipitates
are formed. The height of the rocket is generally
related to the concentrations of the test solution.
Other variations such as counterimmunoelectrophor-
esis or immunoosmophoresis, or electrosyneresis and
zone immunoassay (ZIA), have been reported, but
have not been widely used.
Agglutination Assay/Particle Immunoassay
0031 The unique ability of immunoglobulin to agglutinate
cells with multiple antigens has been used in an assay
system to determine antigen through competition.
Different from the precipitation methods, either an
antigen or the corresponding antibody is attached to
an inert particle via direct binding or covalent inter-
action, which serves as the label. Whereas both red
blood cells (RBCs) and latex particles have been used
for this purpose for some years, recent developments
have led to the use of other particles. Thus, a general
term of ‘particle immunoassay’ is also used for this
type of assay.
0032 Hemagglutination In hemagglutination (or hemag-
glutination inhibition assay, HIA, or passive hemag-
glutination assay, PHA), RBCs (generally sheep),
which have been coated or coupled with antigen
(thus called sensitized RBCs), are incubated with anti-
body and sample. After incubation, the degree of
agglutination is determined. In this assay, antigen in
the sample competes with the antigen coated on the
RBCs for their interaction with antibody. Another
variation of the HIA is reverse passive hemagglutina-
tion assay, in which the purified antibody is coupled
to the RBCs. Agglutination is observed when antigen
or sample extract is added to the system. Although
HIA is relatively simple, food extracts often interfere
with the assay and a large amount of antiserum is
needed. Only semiquantitative information can be
obtained.
0033Latex agglutination Instead of using sheep RBCs,
latex particles coated with antigen have been used as
the agglutinating agent in the antigen/antibody reac-
tion. Both regular inhibition and reversed type of
agglutination methods have been used.
0034Other particles and new intrumentation methods for
particle counting Several other particles, including
microcapsule gelatin, silicate, gold, and emulsified
fluorocarbon, have also been introduced as labels in
recent years. Among these, the microcapsule gelatin is
used most often. Instruments to measure the agglutin-
ated particles are now available and are termed ‘par-
ticle-counting immunoassay’. Both photometric and
light-scattering methods have also been used. The
availability of sophisticated instruments has not
only improved the sensitivity to a level in the sub-
nanograms per milliliter, but has also led to an
automated system that can handle large volumes
of samples with good precision.
Other Immunochemical Approaches
0035Several other immunochemical methods that have
gained wide applications in recent years are cited
below. Immunoadsorption methods are based on
temporary immobilization of immunocomplexes on
a solid support; immunoaffinity chromatography is a
means of removing undesirable antibodies from an
immune serum using the corresponding antigens.
Conversely, immunoaffinity chromatography is also
used for retaining a given antigen from a complex
matrix by using the antiantigen antibodies. Usually,
the antigens or the antibodies are immobilized on a
support and packed in a small column for immunoaf-
finity chromatography, which is then used in different
types of immunoassays and automation. Some rapid-
screening test kits are based on this principle and have
gained wide applications. In addition to this tech-
nique for haptens, immunomagnetic beads have been
used in affinity enrichment and isolation of patho-
genic bacteria, including Escherichia coli O157:H7.
The separation is quick and convenient, requires no
elaborate equipment, and can be used instead of pre-
cultivation as a precursor for other tests to extract the
IMMUNOASSAYS/Principles 3247
target organism. Another powerful immunochemical
method is the Western blot, which involves the
separation of food components electrophoretically,
followed by transfer of the separated components on
to nitrocellulose membrane and then staining immu-
nochemically with either enzyme-labeled or radio-
labeled antibody. Using a similar principle and in
combination with microscopy, a rapid antibody-
direct epifluorescent filter technique (antibody-
DEFT) for screening of E. coli O157:H7 in beef at a
level of 0.1 CFU g
1
was developed recently. The
antibody-DEFT involved membrane filtration, fluor-
escent antibody staining, and epifluorescence micro-
scopy and was accomplished in less than 1h.
Seealso: Immunoassays:Radioimmunoassayand
EnzymeImmunoassay
Further Reading
Chu FS (1997) Recent development and application of
immunochemistry in agriculture and food analysis. In:
Dewarti-Hariyadi R and Zakaria F (eds) Proceedings of
the International Symposium of Biomolecular Reactions
and Industrial Applications of Immunology in Foods
and Agriculture, pp. 21–40. Jakarta: IPB, Bogor and
the French Embassy.
Chu FS (2000) Immunoassays for mycotoxins. In: Hui YH,
Smith RA and Spaerke DG et al. (eds) Foodborne Disease
Handbook, vol. 3, pp. 683–713. New York: Dekker.
Fung DYC and Pabon R (1998) Rapid methods and auto-
mation in microbiology. Food Testing and Analysis 6:
20–26.
Kurtz DA, Skerritt JH and Stanker JH (1995) New Fron-
tiers in Agrochemical Immunoassay. Arlington, VA:
AOAC International.
Langone JJ and Van Vunakis H (1980–1983) Immuno-
chemical techniques, parts B–F. Monoclonal antibodies
and general immunoassay methods. In: Methods in En-
zymology, vols. 73, 74, 84, 92, 93 . New York: Academic
Press.
Nakamura RM, Kasahara Y and Rechnitz GA (1992) Immu-
nochemical Assays and Biosensor Technology for 1990s.
Washington, DC: American Society for Microbiology.
Perkin-Elmer www.perkinelmer.com.
Rittenburg JH (1990) Development and Application of
Immunoassay for Food Analysis. London: Elsevier
Applied Science.
Tijssen P (1985) Practice and Theory of Enzyme Immuno-
assays. Amsterdam: Elsevier Scientific.
Vanderlaan M, Stanker LH, Watkins BE and Roberts DW
(1991) Immunoassays for Trace Chemical Analysis:
Monitoring Toxic Chemicals in Humans, Foods and
the Environment. ACS symposium series 451. Washing-
ton, DC: American Chemical Society.
Van Vunakis H and Langone JJ (1980) Immunochemical
techniques, part A. In: Methods in Enzymology, vol 70.
New York: Academic Press.
Radioimmunoassay and
Enzyme Immunoassay
F S Chu, University of Wisconsin, Madison, WI, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Immunochemical methods discussed in the previous
article generally involve no labeled antigen or labeled
antibody. Concentration of the antigen–antibody
complex is estimated from the secondary reaction
that leads to precipitation or agglutination. These
methods are not sensitive, are subject to nonspecific
interference, and are primarily used for analysis of
high-molecular-weight proteins. However, the devel-
opment of radioimmunoassay (RIA) of insulin by
Yalow and her colleagues in the late 1950s has
widened the scope of immunoassays. This method
revolutionized the classic approaches by combining
the unique properties of specific antibody–antigen
interaction and the use of a radioactive labeled
marker to monitor complex formation. Thus, RIA
provides specificity, sensitivity, and simplicity, and
can be used for analysis of both antigen and haptens.
With the introduction of enzymes as markers in
the assay, the sensitivity is further amplified. Using
different labeled markers, a variety of immunoassays,
including fluorescence immunoassay (FIA), time-
resolved FIA, FIA polarization immunoassay, enzyme
immunoassay (EIA), luminescent immunoassay (LIA),
metalloimmunoassay (MIA), and viroimmunoassay
(VIA) have been developed. Since RIA and EIA are
most commonly used in food analysis, only these two
and immunochemical biosenors or immunosensors,
which have recently been developed, will be discussed
in detail.
Radioimmunoassays
Principles
0002RIA involves the use of a radioactive marker in the
assay, which competes with an analyte in the sample
for binding to an antibody. For RIA of high-molecu-
lar-weight antigen, either the antigen or antibody
molecules can be labeled. It is also common to use a
radiolabeled second antibody, i.e., antibody against
the primary antibody. In contrast, labeled hapten is
typically used in RIA for low-molecular-weight sub-
stances. For simplicity, RIA is explained in eqns (1)
and (2).
Antibody þ Antigen* ÐAntibody Antigen*
þ Antigen* ð1Þ
3248 IMMUNOASSAYS/Radioimmunoassay and Enzyme Immunoassay

Antibody þ Antigen* þ Analyte ÐAntibody
Antigen* þ Antibody Analyte þ Antigen* ð2Þ
0003 At a constant amount of antibody and labeled anti-
gen (denoted by an asterisk), the presence of analyte
in the sample (eqn 2) will result in a decrease in
radioactivity in the bound form and an increase
in radioactivity in the free form because the analyte
is also bound with antibody. In practice, antibody is
incubated simultaneously with a solution of un-
known sample or known standard, and a constant
amount of labeled antigen or hapten. After separation
of the free and bound forms, the radioactivity in those
fractions is determined. The concentration of the sub-
stances to be analyzed in the unknown sample is
determined by comparing the results to a standard
curve, which is established by plotting the ratio of
radioactivities in the bound fraction and free fraction
versus the logarithm of the concentration of un-
labeled standard analyte.
0004 Although RIA is simple and sensitive, it is limited
by the need for a marker with high specific radioactiv-
ity, instruments for measuring radioisotopes, and li-
censes for using radioactive materials and disposal of
radioactive materials. Consequently, enzyme-linked
immunosorbent assay (ELISA) has become more
popular. Nevertheless, because the radioactive
marker has the same structural features as the com-
pound to be analyzed, RIA provides good accuracy
and is an effective method in the initial phase for
screening of antibodies.
Preparation of Radioactive Ligands
0005 The specific activity of the radioactive ligand plays an
essential role in the sensitivity of RIA in addition to
the affinity constant of antibody and antigen inter-
action. Because of the availability of high-specificity
radioactive iodine, immunoglobulin and protein
antigen are generally labeled with
125
I. Reagents for
iodination such as Bolton-Hunter reagent, chlora-
mine T, iodo-gen, lactoperoxidase, and iodo-beads
are commercially available. Thus, most laboratories
can prepare their own labeled marker. Since the half-
life of
125
I is short, iodinated markers generally
should not be stored very long. For haptens such
as mycotoxins or antibiotics, both tritiated and iodin-
ated derivatives are used. Occasionally,
14
C-labeled
compounds have also been used. Some high-specific-
activity compounds are made commercially by a trit-
ium exchange method. Reduction of a hapten with
high-specific-activity-tritiated sodium borohydride is
commonly used. For hapten containing hydroxyl
groups, the secondary hydroxyl group is first oxidized
and then reduced with tritiated sodium borohydride.
Iodinated hapten markers are prepared by direct
iodination or by iodination of hapten derivatives con-
taining a linker such as tyrosine, histamine, tyramine,
or others. For example, 3
0
-[
125
I]tyramine-aflatoxin
B
1
-O-carboxymethyl oxime (2300 Ci nmol
1
) was
used in the RIA of both aflatoxins B
1
and M
1
with
high sensitivity (10 pg per assay).
Separation of Antigen–Antibody Complex from Free
Antigen
0006To assess the amount of complex formation, effective
methods for the separation of free and bound antigen/
antibody must be established. Approaches for the
separation of high-molecular-weight antigen and
immunocomplex generally can also be used for RIA
of hapten as well as for EIA; however, the converse is
not always true.
0007Separation of hapten and hapten–antibody com-
plex Methods such as equilibrium dialysis, ammo-
nium sulfate precipitation, precipitation with organic
solvent, polyethylene glycol 6000, membrane filtra-
tion, dextran-coated charcoal, and albumin-coated
charcoal have been used. Except for the coated char-
coal methods, all these methods are based on the
separation of molecular weight.
0008Separation using solid-phase matrix In this ap-
proach, antibody is coated to a solid matrix such as
polystyrene tubes/beads, microtiter plate, or modified
nylon tubes or beads noncovalently, or conjugated to
various matrices such as acetylbromocellulose, vari-
ous types of sepharose gel or agarose gel, and con-
trolled-size fine-particle magnetic gels. Separation is
achieved by filtration or centrifugation after the reac-
tion.
0009Separation using a secondary antibody or other
reagents In this approach, a second antibody is
added to the reaction mixture to separate the free
antigen and immunocomplex. Although the forma-
tion of precipitate of the primary antibody with
second antibody was used in earlier studies, a solid
matrix coupled with the second antibody is now more
commonly used. Because of their high affinity to im-
munoglobulin G (IgG), staphylococcal protein A and
protein G coupled to a solid-phase matrix are also
commonly used for separation in both hapten and
macromolecule assays.
Enzyme Immunoassay
General Considerations and Assay Configurations
0010EIA is a general term for immunoassays involving
use of an enzyme as a marker for the detection of
IMMUNOASSAYS/Radioimmunoassay and Enzyme Immunoassay 3249
