Dinc Ibrahim. Refrigeration systems and applications 2th edition
Подождите немного. Документ загружается.


Heat Pumps 321
Figure 6.20a shows a translucent view of an IsoDie heat spreader used to planarize the uneven
junction temperatures caused by the nonuniform power distribution of an integrated circuit. This
IsoDie has five parts; starting at the bottom is the evaporator sidewall, followed by the evaporator
sidewall microstructure region (actual microstructures not shown for simplicity), planar capillary
form, condenser sidewall microstructure region, and finally, the condenser sidewall. This IsoDie
measures 15 mm
2
and is 2 mm thick. It is constructed entirely from oxygen-free high conductiv-
ity (OFHC) copper and uses water as its working fluid. Thermal analysis suggests that it will
handle over 100 W from a 10 to 100 mm
2
nonuniform power source, including power densities
as high as 3.0 W/mm
2
, with a thermal resistance of less than 0.16
◦
C/W (three times better than
solid copper).
Figure 6.20b shows a custom-made Peltier module (40 × 40 × 3.3 mm) which has a maximum
heat pump capacity of 160 W (10 W/cm
2
), and a maximum temperature differential of 67
◦
C (zero
load), under the following conditions: 16.2 V, 17.6 A, and a hot-side temperature of 50
◦
C.
Figure 6.20c shows a custom-made liquid cooled Peltier (thermoelectric) cooling assembly which
measures 60 mm
2
and is 14 mm high (including copper cold plate), and has a maximum heat pump
capacity of 100 W (2.8 W/cm
2
), with a maximum temperature differential of 63
◦
C (zero load), under
the following conditions: 12.0 V, 11.1 A, and a hot-side liquid input temperature of 50
◦
C. This
Peltier heat pump assembly uses a liquid-cooled copper heat sink, which has a thermal resistance
of 0.031
◦
C/W, with a flow rate of 0.5 L/min, and a pressure drop of 20 kPa. The volumetric thermal
efficiency equals 1.984 W/
◦
C/cm
3
. Total weight is 281 g.
6.21 Resorption Heat Pump Systems
Renewed interest in ammonia has been evident in the wake of the Montreal protocol and mixtures
of ammonia and water appear to be particularly suitable as the working fluid in high-temperature
heat pump applications. The advantages over a single component working fluid are related to the
possibility of matching the temperature glide of the working fluid to that of the source/sink and the
variation of the circulation composition to enable better matching of the source/sink conditions and
load. Furthermore, it is possible to configure the system and working fluid to allow heat rejection
at temperatures in the range of 80–120
◦
C. This temperature range would be typical of a number
of industrial process heating applications, with the heat pump utilizing what might otherwise be
waste heat at temperatures between 40 and 80
◦
C (Mongey et al., 2001). The practical application
of ammonia–water mixtures cannot be achieved using a conventional vapor-compression cycle.
The temperature glide associated with complete phase change is of the order of 100
◦
C, so it
could be expected that only partial phase change will be achieved in any specific application.
Wet compression does not appear to be a realistic proposition, because of the large liquid fraction
remaining after any typical heat exchange process.
A more practical alternative is to separate the phases after the working fluid has come into thermal
contact with the heat source. The vapor passes through the compressor while a solution pump is
used to transfer the liquid to the high side before recombining with the vapor. This approach is
referred to as a resorption cycle, with the desorber and resorber performing the same functions
as the evaporator and condenser in the vapor-compression cycle. The resorption cycle is shown
schematically in Figure 6.21. Changes in the circulating composition can be achieved by varying
the flow ratios of the vapor and liquid phases. Modulation of the flow velocity through the solution
pump is thought to be the most practical means of achieving this end. Because of this, a receiver is
required to store a charge of working fluid that has a much greater volume than that necessary for
circulation purposes. In order to achieve circulating compositions that differ significantly from the
original bulk charge, a considerable proportion of the charge must be removed from circulation.
This excess fluid can be stored at the point where phase separation occurs, since equilibrium liquid
and vapor phases differ markedly.
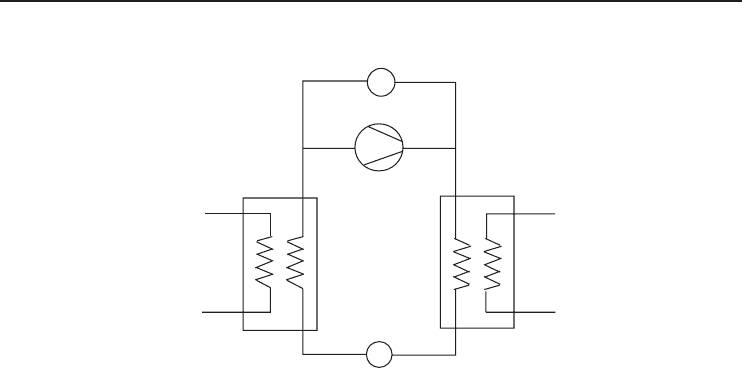
322 Refrigeration Systems and Applications
Solution pump
Compressor
Desorber
Resorber
Source Sink
Expansion valve
Figure 6.21 Schematics of a resorption cycle (Adapted from Mongey et al., 2001).
The temperature of cooling water in the condenser of cold vapor machines (in this category
we have compression and AHPs of conventional type and style) is usually about 5–10
◦
Clower
than the constant condensation temperature, and in the evaporator the temperature of the incom-
ing heat source is higher than the constant temperature of the boiling refrigerant. This results in
irreversibilities, because the absorber has to take in the cold vapor from a temperature which is
lower than the heat source temperature. Similarly, the refrigerant in the expeller is expelled at a
higher temperature than the corresponding heat sink temperature. These losses can be avoided in
part if, according to a recommendation of Altenkirch, the condensation and the evaporation do not
proceed at a constant temperature, but within a given temperature range. This can be obtained by
substituting the branch condenser, refrigerant injection valve, and evaporator by a second working
fluid circuit with resorber (instead of condenser) and desorber (instead of evaporator) in an AHP.
In the resorber a suitable weak solution absorbs the refrigerant set free in the expeller. The solution
temperature in the resorber varies according to the concentration of the solution. The resorption
of the refrigerant vapor does not take place at a constant temperature as in the condenser, but
within a temperature range. The same applies to the desorber which substitutes the evaporator. The
advantages of resorption heat pumps compared with common AHPs are
• no rectification unit (e.g., ammonia/water),
• lower pressure difference,
• reduced losses and increased heat ratio.
However, a high premium has to be paid for the above-mentioned advantages by the higher first
cost (e.g., an additional working fluid pump).
The single-stage resorption heat transformer should be mentioned as the fundamental configu-
ration (Figure 6.22) from which a large number of variations can be developed. The condenser is
replaced by the resorber and the evaporator by the desorber; like the resorption heat pump this
requires the addition of a second solution circuit. This design permits heat transfer at varying
temperature differences. Thereby, lower exergy losses and reduced consumption of cooling water
are attained. Higher heat ratios in comparison with the Type I AHP, and lower electric power
consumption are possible for the resorption heat transformer.
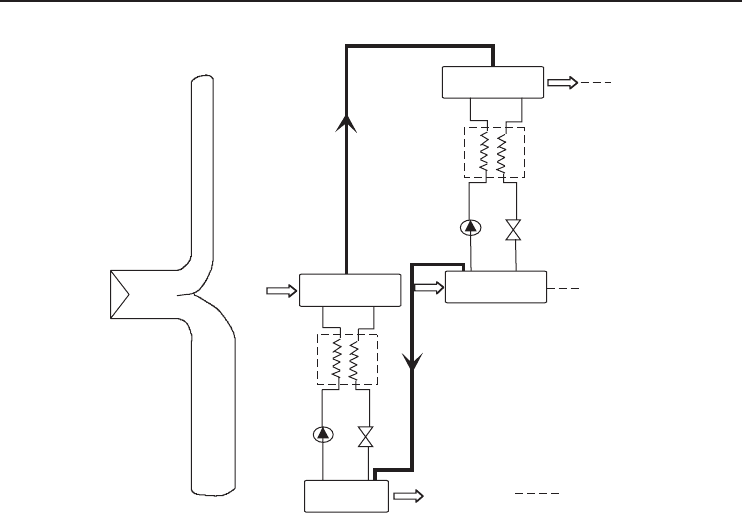
Heat Pumps 323
Resorber
Vapor
Vapor
P1
P2
HE1
HE2
Q
R
Q
R
Q
A
Q
A
Q
D
Generator
Absorber
Desorber
Q
G
Q
D
+ Q
G
T
A
T
M
T
R
Figure 6.22 Principal flow sheet of the resorption heat transformer (Adapted from Podesser, 1984).
6.22 Absorption Heat Pump (AHP) Systems
The beginning of the absorption cooling technology dates back to the middle of the nineteenth
century. At that time, the brothers Ferdinand and Edmund Carre were building a periodic and,
some years later, a continuously working absorption cooling machine for ice production. As a
result, names such as E. Altenkirch, R. Planck, F. Merkel, F. Bosnjakovit, W. Niebergall, and some
others are inseparably connected with the research and development of the absorption cooling
machine. Altenkirch, in particular, made various suggestions for different methods and measures
for the reduction of irreversible losses, and, as early as 1911, suggested a central heating system
using AHP. In 1959, Niebergall gave a comprehensive presentation of absorption technology.
The heat pump is one of the two classical processes to generate large amounts of low- and
medium-temperature energy by means of relatively small amounts of exergy (available energy),
the other process being the cogeneration of heat and power. The implementation of the heat pump
process requires mechanical (or thermal) equipment, increasing first cost but decreasing energy
cost. It is not surprising that increased cost of conventional fuel and decreasing first cost of heat
pumps (by mass production) has greatly improved the economy of the heat pump.
The main topics of research and development on heat pump units are technical improvements
of the units on the one hand, and, on the other hand, reduction of their production cost by large-
scale, partly or fully automated production and by a design geared to it. Generally, technical
improvements will cause higher production cost, but the actual cost increase may be small if
modern production techniques are applied. Some of the many ways to improve the heat pump units
are increased motor efficiency, lower heat losses, speed control by pole changing and by thyristors,
new improved compressor types, double cycle, economizers, nonazeotropic refrigerant mixtures,
improved heat exchangers and expansion valves, and use of waste heat from the compression side
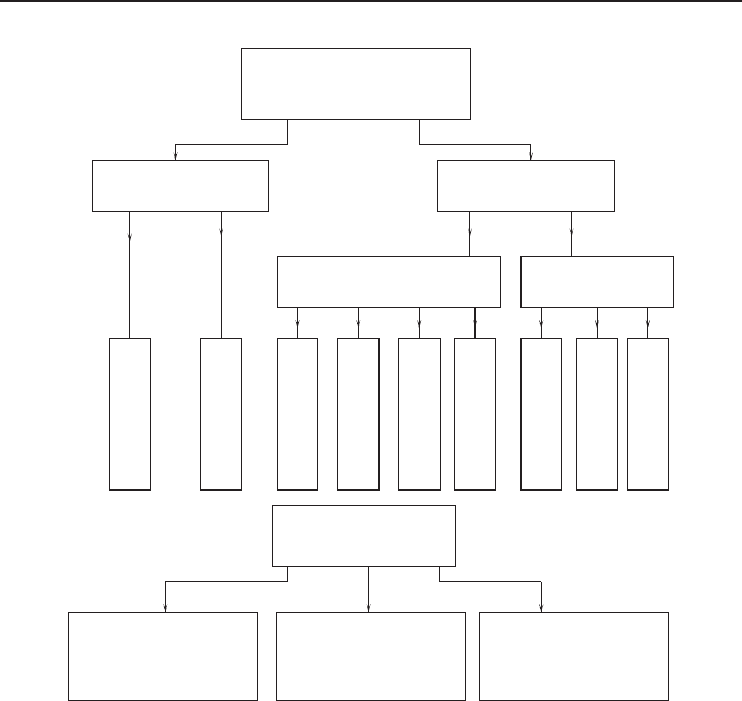
324 Refrigeration Systems and Applications
Special types
Sorption heat pumps
(SHP)
Periodically working
SHP
Continuously working
SHP
Absorption heat pumps
(AHP) Type1
Heat transformer
AHP Type2
Absorption heat pump
with
booster compressor
Compression heat pump
with
working pair circuit
Other combinations
(compression systems
combined with absorption
system)
Adsorption - HP
Single and multistage
absorption - HP
AHP with
auxillary gas
Single stage
AHP,Type1
Multistage
AHP,Type 1
Resorption - Hr
(RHP)
Single stage
AHP,Type2
Multistage
AHP,Type 2
Combinations
Figure 6.23 Classification of diffusion heat pumps (Adapted from Podesser, 1984).
for superheating suction gas. The technical development potential is large, even if it cannot be fully
utilized for economic reasons.
The sorption heat pump process differs fundamentally from the compression heat pump process
in that the mechanical compressor is replaced by a thermal compressor. The thermal compressor
consists of expeller (generator), where the drive energy (heat) is supplied, solution heat exchanger,
absorber, working fluid pump, and expansion valve. It is powered by heat. The entire group of pos-
sible heat pumps with thermal compressors is, according to Niebergall, called sorption heat pumps,
because the procedure of “sucking in” can occur both through absorption into liquid or nonliq-
uid substances and through adsorption from nonliquid substances. Figure 6.23 shows a possible
categorization of the better known types of sorption machines.
So far, only the periodically working absorption systems and, especially, the periodic AHP
systems with a liquid working pair, show significance. Basically, the single-stage periodic AHP
consists of two parts, one of which acts as both evaporator and condenser, and the other as both
expeller and absorber, but in a shift of time. The single-stage periodically working absorption
system has been applied many times to cooling machines, but as an AHP has not become very
significant, till date.

Heat Pumps 325
The advantage of the multistage type in comparison to the single-stage type lies in the attain-
ment of low evaporation temperatures or better heat ratio (the ratio between available heat and
driving heat).
The majority of plants built to date fall into this group of AHPs. In most cases the experience
from absorption refrigeration technology could be used in their design and construction. In general,
heat requirements of from at least 15 kW up to several megawatts are required for the application
of AHPs. In this connection, a COP as high as possible for the capacity of the AHP should be
aimed at. For this reason, AHPs with an auxiliary gas and no fluid pumps are scarcely used because
of the low heat ratio of approximately 1.25, all in spite of the captivating simplicity of the system.
The continuously working, single-stage AHP has become the most famous type of AHP through
applications in experimental plants of small capacities (up to 50 kW) for heating houses or large
capacities up to several megawatts. All the experience from absorption refrigeration technology
can be adapted directly to the design of such AHPs up to output temperatures of about 60
◦
C.
Mostly used working pairs are LiBr/H
2
O, LiBr/CH
3
OH, and H
2
O/NH
3
. The limitations to the
application of the working pairs mentioned are determined by the danger of recrystalization, of
chemical decomposition, and of increased susceptibility to corrosion at high working temperatures.
Absorption refrigeration machine, AHP (Type I), and heat transformer (Type II) are shown. In the
above-mentioned summer and winter operations a LiBr/H
2
O AHP and a LiBr/CH
3
OH AHP work
together, the only connection being by means of the external water circuits. In summer this system
is used for ice (−13
◦
C) production and for supplying the air-conditioning system (+6
◦
C) while
in winter it works as a heat transformer with temperatures of +90
◦
C for space heating. Waste heat
with a temperature of 60
◦
C is used to drive the system. As an AHP (Type I), this plant achieves
a heat ratio of 1.67, and as heat transformer (Type II), the heat ratio is 0.51. All main elements of
these machines are taken directly from absorption refrigeration technology. The COP ranges from
1.45 to 1.55 for single-stage machines at design conditions, because of the irreversible losses caused
by dephlegmation and rectification (increase of concentration of the expelled working vapors).
The conventional application of the absorption refrigeration machine has been clearly abandoned.
It is worth mentioning that the working pair NH
3
/H
2
O in the expeller is operated nearly at the limit
temperature of about 180
◦
C, where the pressure reaches almost 40 bar.
AHPs are thermally driven, which means that heat rather than mechanical energy is supplied
to drive the cycle. AHPs for space conditioning are often gas fired, while industrial installations
are usually driven by high-pressure steam or waste heat. Most attention presently is going to the
AHPs in industry. The phenomenon of vapor absorption in a liquid has been applied successfully
in refrigeration equipment. However, only very few AHPs are presently available on the market.
The transition from cooling device to heating device, in contrast with vapor-compression systems,
has not been achieved thus far.
As with vapor-compression cycle heat pumps, there are many varieties of AHPs, using different
working fluids or different cycles. The AHPs use a combination of a refrigerant and an absorbent,
called the working fluid.
Absorption systems utilize the ability of liquids or salts to absorb the vapor of the working fluid.
The most common working pairs for absorption systems are
• water (working fluid) and lithium bromide (absorbent) and
• ammonia (working fluid) and water (absorbent).
A profound understanding of the operation of an AHP requires acquaintance with the thermody-
namics of binary mixtures. Based on the properties of these mixtures, thermodynamic absorption
cycles are developed and discussed. Distinction here is made with heat transformer cycles, the
latter being characterized by the utilization of low-temperature heat as driving heat source. Special
attention is devoted to the requirements to be filled by the working pairs suited for AHPs and
transformers.
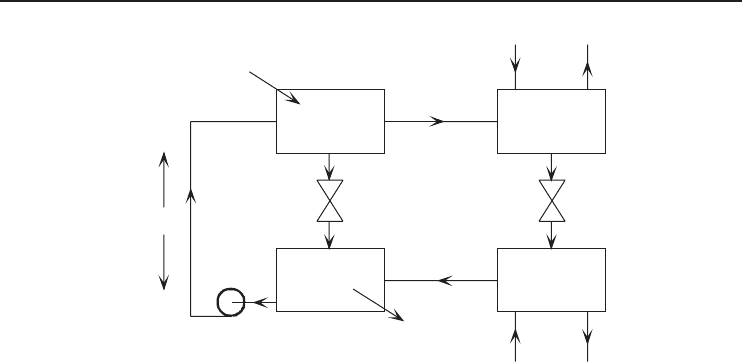
326 Refrigeration Systems and Applications
Generator
Absorber
Condenser
Evaporator
Expansion
valve
Expansion
valve
Source
Sink
Heat out to sink
Heat in
NH
3
vapor
NH
3
vapor
NH
3
–H
2
O
solution
High
pressure
Low
pressure
Figure 6.24 An ammonia–water absorption cycle heat pump.
AHPs, in addition to condensers and evaporators, require components such as: generators, rec-
tifiers, and absorbers. The principles underlying the operation of each of these components are
discussed in detail. It is shown that design and optimization of these items are very complex and
require extensive knowledge of the thermodynamic and thermal properties of the working pairs.
Such knowledge is often lacking. Furthermore, it will become apparent as to how a number of
limitations originating from the working pair influence the performance of the heat pump. It is felt
that more suitable working pairs have to be developed.
A schematic diagram of a basic ammonia–water AHP is shown in Figure 6.24. It can be seen
in Figure 6.24 that the absorption cycle is similar to the vapor-compression cycle. In the vapor-
compression cycle, there is a vapor-compression part (including the compressor). In the absorption
cycle there is an absorption part (including the absorber, solution pump, and generator). Therefore,
the difference is that the compressor is replaced with an absorber, a solution pump, and a generator.
As can be seen in Figure 6.24, the ammonia refrigerant absorbs heat in the evaporator and rejects
heat in the condenser in the same way as in vapor-compression heat pump systems. In the absorber,
the water, which is the absorbent fluid, absorbs the cool ammonia vapor, creating a strong solution.
This solution enters a solution pump and is pumped to the high-pressure side, to the generator. From
the generator, hot ammonia vapor is then condensed, expanded, and returned to the evaporator in
the normal way.
In absorption systems, compression of the working fluid is achieved thermally in a solution circuit
which consists of an absorber, a solution pump, a generator, and an expansion valve as shown in
Figure 6.25. Low-pressure vapor from the evaporator is absorbed in the absorbent. This process gen-
erates heat. The solution is pumped to high pressure and then enters the generator, where the working
fluid is boiled off with an external heat supply at a high temperature. The working fluid (vapor)
is condensed in the condenser while the absorbent is returned to the absorber via the expansion
valve. Heat is extracted from the heat source in the evaporator. Useful heat is given off at medium
temperature in the condenser and in the absorber. In the generator high-temperature heat is supplied
to run the process. A small amount of electricity may be needed to operate the solution pump.
For heat transformers, which, through the same absorption processes, can upgrade waste heat
without requiring an external heat source. The details of the heat transformers are given in
Section 6.21.
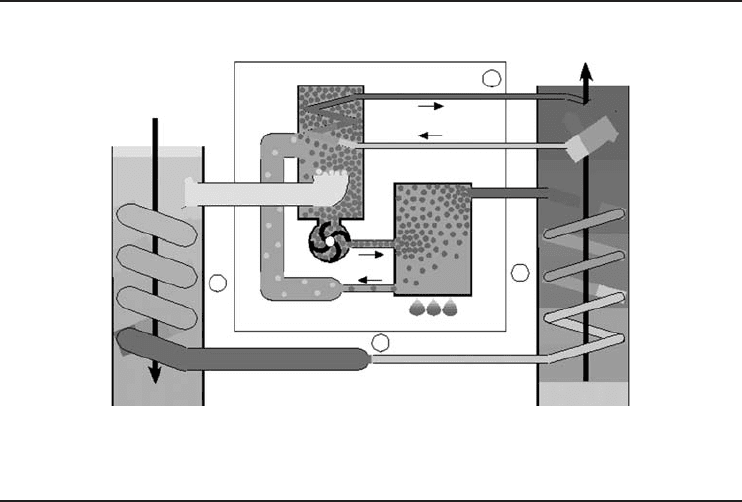
Heat Pumps 327
Heat in
1
4
3
2
Evaporator
Expansion valve
Heater
Generator
Pump
Absorber
Heat out
Condenser
Expansion valve
Figure 6.25 An absorption heat pump (Courtesy of IEA-HPC ).
Example 6.3
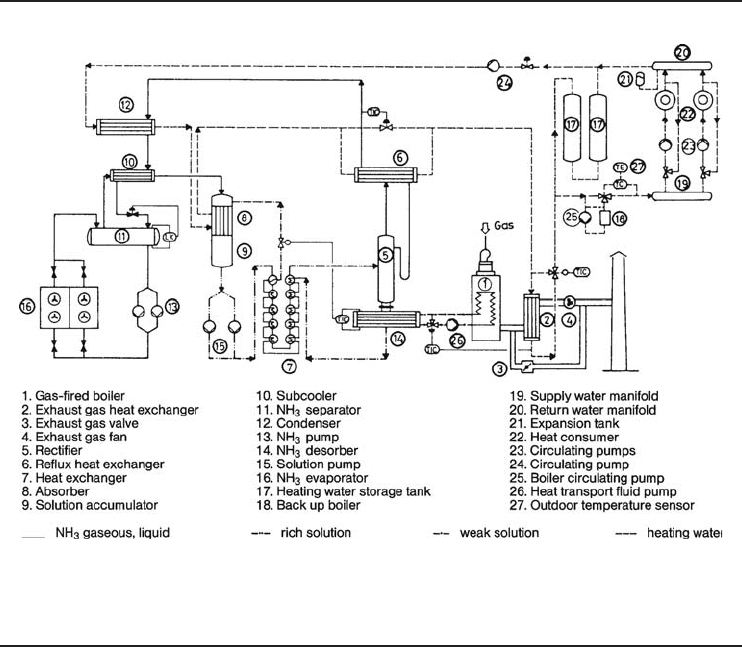
Figure 6.26 shows a schematic diagram of a single-stage, ammonia–water AHP which started
operating at the Technische Werke der Stadt Stuttgart AG. The general and technical features and
details of this system are as follows (Lehmann, 1986):
• Heat use: space heating, ventilating system, local heaters
• Refrigerant: ammonia
• Absorbent: water
• Fuel: natural gas
• Heat source: ambient air
• Operation mode: parallel
• Heat output: 310 kW
• Fuel consumption in boiler: 235 kW
• Heat ratio (COP): 1.32
• Cooling capacity: 88 kW
• Heating water temperature supply and return: 50 and 41.5
◦
C
• Electric power demand for pumps and fan: 12 kW
In Figure 6.26, the operating principle of the AHP is described: in two outdoor air heat exchangers
ammonia is evaporated at a low pressure extracting heat from outdoor air (16). This vapor is
dissolved in the poor water–ammonia solution in the absorber (8) releasing heat to the heating
system. The resulting rich solution is pumped (15) through a heat exchanger (7) and the rectifier
(5) into the generator (14). The generator is indirectly heated via an intermediate heating circuit by
a gas-fired boiler (1). Thermal oil is used as heat transport fluid in this intermediate circuit. In the
generator the working fluid ammonia is evaporated from the ammonia–water solution. This vapor
still containing some water is purified in a rectifier (5) and a reflux heat exchanger (6) before being
condensed in the condenser (12).
328 Refrigeration Systems and Applications
Figure 6.26 Schematic diagram of a single-stage, ammonia–water absorption heat pump (Courtesy of IEA-
HPC ).
6.22.1 Diffusion Absorption Heat Pumps
In the past, attempts made to solve the problems of AHPs have almost all been with absorption
refrigeration machines using mechanical solution pumps. These pumps are used successfully on
large absorption cooling machines of 50–1000 kW and more. Miniaturizing such machines presents
difficulties which involve the small inefficient solution pump. This led to the evaluation of the
diffusion-type absorption cycle. Absorption cooling units working with ammonia–water and hydro-
gen as auxiliary gas are well known. Millions have been built and are mainly used in domestic,
camping, and caravan refrigerators. They are powered electrically, by LPG, natural gas, or kerosene.
The cooling performance of such a cooling unit is in the order of 20–50 W, in contract to 1000 W
required for a 3-kW heat pump. Contrary to the miniaturizing problems mentioned above, one now
faces enlargement problems of the diffusion absorption units.
6.22.2 Special-Type Absorption Heat Pumps
In this group, we find different systems combining vapor compression with AHP elements. Special
types of AHP-like combinations of elements originating from the compression and absorption cycle
promise competitive solutions. Test plants of systems with a compressor solution circuit show that
at high output temperatures comparatively low process pressures can be achieved compared with
one-substance compression heat pumps.
Heat Pumps 329
Absorber
P
Generator
Condenser
Evaporator
Booster
compressor
Q
C
Q
O
Q
A
Q
G
P
el.
SHE
P
A
P
O
P
G
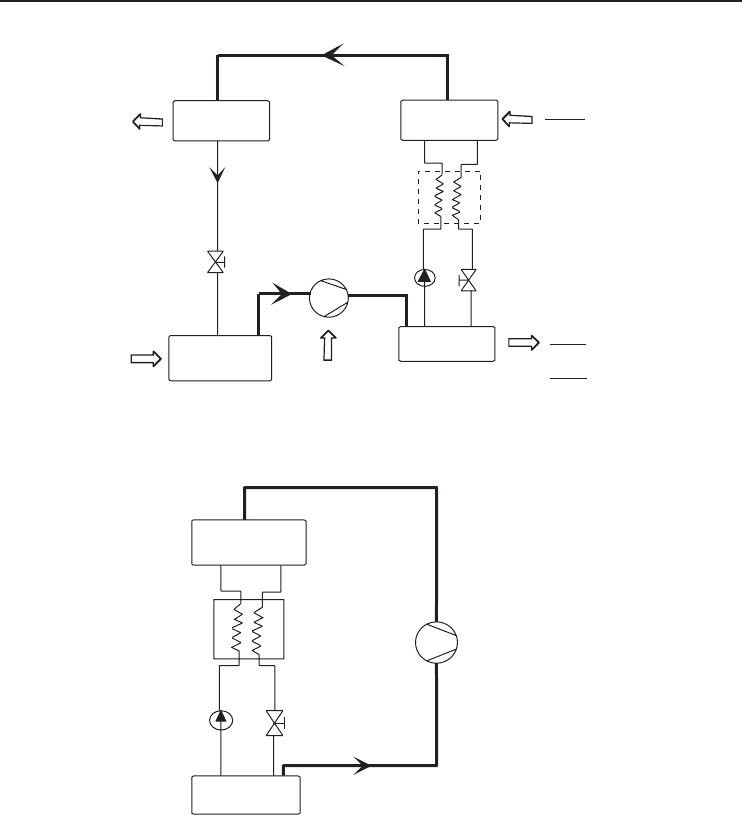
Figure 6.27 An AHP with booster compressor (Adapted from Podesser, 1984).
Desorber
P
Resorber
SHE
Vapor
Compresso
r
Figure 6.28 Compression heat pump with solution circuit (Adapted from Podesser, 1984).
In refrigeration technology, designs of absorption refrigeration machines using a booster com-
pressor are known. These are used to achieve the features of dual and multistage absorption plants
by means of single-stage, continuously working absorption machines. A booster compressor as
shown in Figure 6.27 is connected either between the expeller and the condenser or between the
evaporator and the absorber. Good performance figures can be attained by a compression heat pump
that includes a sorption circuit (Figure 6.28). This can be achieved by designing the resorber and
desorber in such a way that the irreversible losses can be kept low by operation according to the
Lorenz process (large temperature differences between the outlet and inlet of the mass flow and
comparatively small temperature differences between both mass flows). A further advantage is the
lower pressure difference compared to compression heat pumps, resulting in a reduced required
drive power.

330 Refrigeration Systems and Applications
A comparison of COPs shows that an adapted compression heat pump with a solution circuit
brings slight improvements over a compression heat pump with a nonazeotropic working fluid
mixture. When used instead of a compression heat pump with a one-substance refrigerant (e.g.,
R-22), it brings marked improvements. This category of heat pump has interesting application
possibilities. However, before reaching market fitness, considerable development work has still to
be carried out.
6.22.3 Advantages of Absorption Heat Pumps
In recent years, AHPs have received considerable interest because of a number of features. Absorp-
tion cycle heat pumps, depending somewhat on the cycle and the particular design configuration
employed, have the following major advantages:
• Inherently simple and potentially highly reliable equipment.
• No compressor needed.
• Possibility for direct firing.
• High primary energy efficiency.
• Long life expectancy due to the lack of components with moving parts.
• No vibration or noise problems.
• High efficiencies in the heating mode due to the possibility of heat transfer from on-site com-
bustion into the absorption cycle.
• Refrigerant–absorbent fluids can be used which are chemically nonreactive with atmospheric
ozone, although some of the promising working pairs include CFCs.
• Cycle reversal or hot refrigerant bypass is practicable for evaporator defrost.
The cost-effectiveness of an AHP is very much a function of the fluids used. The gas-fired AHP
benefits from having the fuel combustion take place on-site just as a thermal engine heat pump
does, but the former is much simpler insofar as lack of reciprocating or rotating machinery is
concerned. The on-site waste heat is available as supplementary heat and, for evaporator defrost,
this aspect is important not only from the standpoint of efficiency and reliability but also because
refrigerant flow reversal is not as easily handled as with Rankine cycle machines.
6.22.4 Disadvantages of Absorption Heat Pumps
Absorption cycle heat pumps also have a number of disadvantages:
• Comparatively large heat-exchanger areas (at attendant high first cost) required for realization of
acceptable performance (expensive rectifiers may also be needed).
• Usually lower cooling-mode performance than obtainable with Rankine refrigeration machinery
(including electric heat pumps).
• Toxicity of some refrigerants, for example, ammonia, where used, requires that direct refrigerant-
conditioned air contact in water/ammonia systems must be avoided (requiring the use of either
a water loop or other intermediate means of heat transfer).
• Corrosiveness of some working fluids, for example, water–ammonia systems requires the use of
steel rather than aluminum or copper in the manufacture of the heat pump (the organic absorption
system uses aluminum).
• Diameter limitation of the absorber requires the use of a tall unit with attendant bulkiness of
equipment.
