Environmental Encyclopedia
Подождите немного. Документ загружается.


Environmental Encyclopedia 3
Radioactive waste
release of millions of curies of radioactive material into the
Columbia River, Washington from 1944 through at least
the 1960s. In 1956, 450,000 gal (1.7 million l) of high-
level waste were accidently spilled on the Hanford grounds.
Through the 1950s, additional millions of gallons of waste
were dumped into the ground. Residents in the area and
along the river were never notified about any of the radioac-
tive discharges that took place at Hanford.
In 1988, the
U.S. Department of Energy
reported
that radioactive wastes from Hanford were contaminating
some underground water supplies; Hanford was shut down
a year later. At present, a farm of deteriorating storage tanks
at Hanford containing approximately 57 million gal (216
million l) of radioactive and toxic waste is being monitored
for leaks. Officials are not even sure what
chemicals
lie in
some of these tanks. Federal officials estimate that cleaning
up Hanford and the U.S. government’s other weapons facili-
ties (at Fernald, Ohio; Rocky Flats; Colorado; Savannah
River, Georgia; and other locations) may cost over $200
billion.
Nuclear power plants also contribute to radioactive
pollution. Spent nuclear fuel from these plants, a high-level
waste, must be kept from human contact for hundreds or
thousands of years, yet no completely reliable disposal
method exists. At present, most high-level waste has simply
been left in pools at power plant sites while the government
seeks a location for permanent disposal. Most low-level waste
(anything that is not spent fuel or transuranic waste) gener-
ated by nuclear power plants has been landfilled throughout
the country. Three such
landfill
sites (Maxey Flats, Ken-
tucky; West Valley, New York; and Sheffield, Illinois) have
been shut down due to leakage of radioactive liquid into the
ground.
Nuclear power plants also
discharge
wastes directly,
as a result of malfunctions or intentional dumping. During
the decade from 1979 to 1989, the
Nuclear Regulatory
Commission
recorded 33,000 mishaps at power plants in
the United States, 1,000 of which it classified as “particularly
significant.” Though the vast majority of these incidents
resulted in no release of radioactive material, some accidents,
such as the Three Mile Island partial meltdown and the
Chernobyl explosion, have caused significant discharges of
radioactivity into the
environment
. A sampling of incidents:
O
the Vermont Yankee power plant, a power station with a
poor safety record, was fined $30,000 for dumping 83,000
gal (314,000 l) of radioactive water into the Connecticut
River in July 1976;
O
the accident at Three Mile Island, Pennsylvania in 1979
released 2.5 million curies of radioactive noble gases and
a small quantity of radioactive iodine into the atmosphere;
O
in one of a series of 21 unreported leaks occurring up to
March 1981, radioactive material from the Tsuruga nuclear
1157
power plant in Japan was dumped into Tsuruga Bay after
warning alarms were shut off; operators of the plant later
admitted that they intentionally dumped wastes regularly;
O
in October 1982, heavy rains caused a flood at the Cofrentes
nuclear power plant in Spain which released 154 gal (580
l) of radioactive waste;
O
in December 1985, a power failure at the Rancho Seco,
California plant resulted in a release of a small quantity of
radiation into the air; in 1989 the NRC fined the facility
$100,000 for violating waste disposal regulations from 1983
to 1986;
O
at the Douglas Point, Canada, station in January 1986, it
was discovered that
high-level radioactive waste
had
been leaking from a spent-fuel “storage pond” at a rate of
16 gal (60 l) per hour.
The health effects of radioactive leaks are still debated.
While the harmful effects of Chernobyl are probably beyond
question, analysis of the Three Mile Island incident has not
detected a long-term increase in cancer or diseases as a
result of the discharge there. Studies conducted in England,
however, reveal increased rates of
leukemia
in areas sur-
rounding the nuclear plants at Hinkley Point, Dounreay,
and Windscale.
[Linda Rehkopf and Jeffrey Muhr]
R
ESOURCES
B
OOKS
Brill, A. Bertrand. Low-Level Radiation Effects: A Fact Book. New York:
Society of Nuclear Medicine, 1985.
Caufield, Catherine. Multiple Exposures. New York: Harper & Row, Pub-
lishers, 1989.
Dresser, Peter D., ed. Nuclear Power Plants Worldwide. Detroit: Gale Re-
search, 1993.
Gore, Al. Earth in the Balance. New York: Houghton Mifflin, 1992.
Jagger, J. The Nuclear Lion: What Every Citizen Should Know About Nuclear
Power and Nuclear War. New York: Plenum, 1991.
Jones, R. R., and R. Southwood, eds. Radiation and Health. New York:
Wiley, 1987.
Regenstein, Lewis. How to Survive in America the Poisoned. Washington,
DC: Acropolis Books Ltd., 1986.
Shulman, S. The Threat at Home: Confronting the Toxic Legacy of the U.S.
Military. Boston: Beacon Press, 1992.
P
ERIODICALS
Goldsmith, E., et al. “Chernobyl: The End of Nuclear Power?” The Econo-
mist 16 (1986): 138–209.
Radioactive waste
Radioactive waste is the “garbage” left as a result of the use
of nuclear materials by human societies. Such waste can be
categorized as low-level, intermediate-level, or high-level
waste. The term transuranic waste is also used to describe

Environmental Encyclopedia 3
Radioactive waste management
materials consisting of elements heavier than
uranium
in
the periodic table.
The term
low-level radioactive waste
usually refers
to materials that contain a small amount of
radioactivity
dispersed in a large volume of material. Such materials are
produced in a great variety of industrial, medical, and re-
search procedures. A common practice is to store these mate-
rials in sealed containers until their level of radioactivity is
very low and then to dispose of them by shallow burial or
in other traditional
solid waste
disposal systems.
The assumption is that the level of radiation released
by these wastes is too low to cause any harmful environmental
effects. That assumption has been challenged by some scien-
tists who believe that enough is not yet known about the
long-term effects of radiation. They suggest that safer meth-
ods of disposal for such wastes need to be developed.
Intermediate-level wastes, as the name suggests, con-
tain a higher level of radioactivity than low-level wastes, but
a lower level than high-level wastes. These materials cannot
be discharged directly into the
environment
. An important
source of such wastes is the re-processing of nuclear fuels.
At one time, large quantities of intermediate-level wastes
were dumped into the deepest parts of the Atlantic Ocean.
That practice has been discontinued and intermediate-level
wastes are now being stored on land until a permanent
disposal system is developed.
High-level radioactive wastes consist of materials that
contain a large amount of radioactivity that will remain at
dangerous levels for hundreds or even thousands of years.
These materials pose the most difficult disposal problem of
all since they must be completely isolated and stored for
very long periods of time. The primary sources of high-
level wastes are nuclear
power plants
and research and
development of
nuclear weapons
.
A number of methods for the storage of high-level
wastes have been suggested. Among these are burial in large
chunks of concrete, encapsulation in glass or ceramic, projec-
tion of them inside rockets into outer space, and burial in
the Antarctic ice sheet. Various countries around the world
have developed a variety of methods for storing their high-
level wastes. In Canada, such wastes have been stored in
water-filled pools for more than 25 years. France, with one
of the world’s largest
nuclear power
establishments, has
developed no permanent storage system but plans to build
a large underground vault for its wastes by the early twenty-
first century.
In the United States, Congress passed the Nuclear
Waste Policy Act in 1982, outlining a complete program
for the construction of a high-level waste repository in the
early twenty-first century. In 1987,
Yucca Mountain
, Ne-
vada, was selected as the location for that site. Current plans
call for a huge vault 1,000 ft (305 m) underground as the
1158
site for long-term, high-level waste storage at this location.
See also Nuclear fission; Nuclear Regulatory Commission
(NRC); Ocean dumping; Office of Civilian Radioactive
Waste Management (OCRWM); Radioactive decay; Radio-
active waste management
[David E. Newton]
R
ESOURCES
B
OOKS
Bartlett, D. L., and J. B. Steele. Forevermore: Nuclear Waste in America.
New York: W. W. Norton, 1985.
Carter, L. J. Nuclear Imperatives and Public Trust: Dealing With Radioactive
Waste. Baltimore: Resources for the Future, 1987.
League of Women Voters. The Nuclear Waste Primer. Washington, DC:
League of Women Voters, 1985.
Managing the Nation’s Nuclear Waste. Washington, DC: Office of Civilian
Radioactive Waste Management, March 1990.
Resnikoff, M. Deadly Defense: Military Radioactive Landfills. New York:
Radioactive Waste Campaign, 1988.
P
ERIODICALS
Hunt, C. B. “Disposal of Radioactive Wastes.” Bulletin of the Atomic Scien-
tists (April 1984): 44–46.
Radioactive waste management
Radioactive waste
materials are produced as by-products of
research,
nuclear power
generation, and
nuclear weapons
manufacture. Radioactive waste is classified by the U.S. gov-
ernment into five groups: high-level, transuranic (chemical
elements heavier than
uranium
), spent fuel, uranium mill
tailings
, and
low-level radioactive waste
.
The management and disposal of radioactive waste
receives attention at all levels of state and local governments,
but the regulations sometimes conflict or are confusing. The
U.S. Congress passes relevant legislation, the
Environmen-
tal Protection Agency
(EPA) sets applicable environmental
standards, and the
Nuclear Regulatory Commission
(NRC) develops regulations to implement the standards.
For
high-level radioactive waste
, the
U.S. Department
of Energy
(DOE) is responsible for the design, construction
and operation of suitable disposal facilities. And courts have
recently gotten into the act, ordering states to arrange to
have necessary disposal facilities designed, constructed and
operated for management of low-level wastes.
The principal federal laws related to the management
and disposal of radioactive waste include the Atomic Energy
Act (1954), the Uranium Mill Tailings Radiation Control
Act (1978), the Low-Level Radioactive Waste Policy Act
(1980), the Nuclear Waste Policy Act (1982), the Low-
Level Radioactive Waste Policy Amendments Act (1985),
and the Nuclear Waste Policy Amendments Act (1987). In
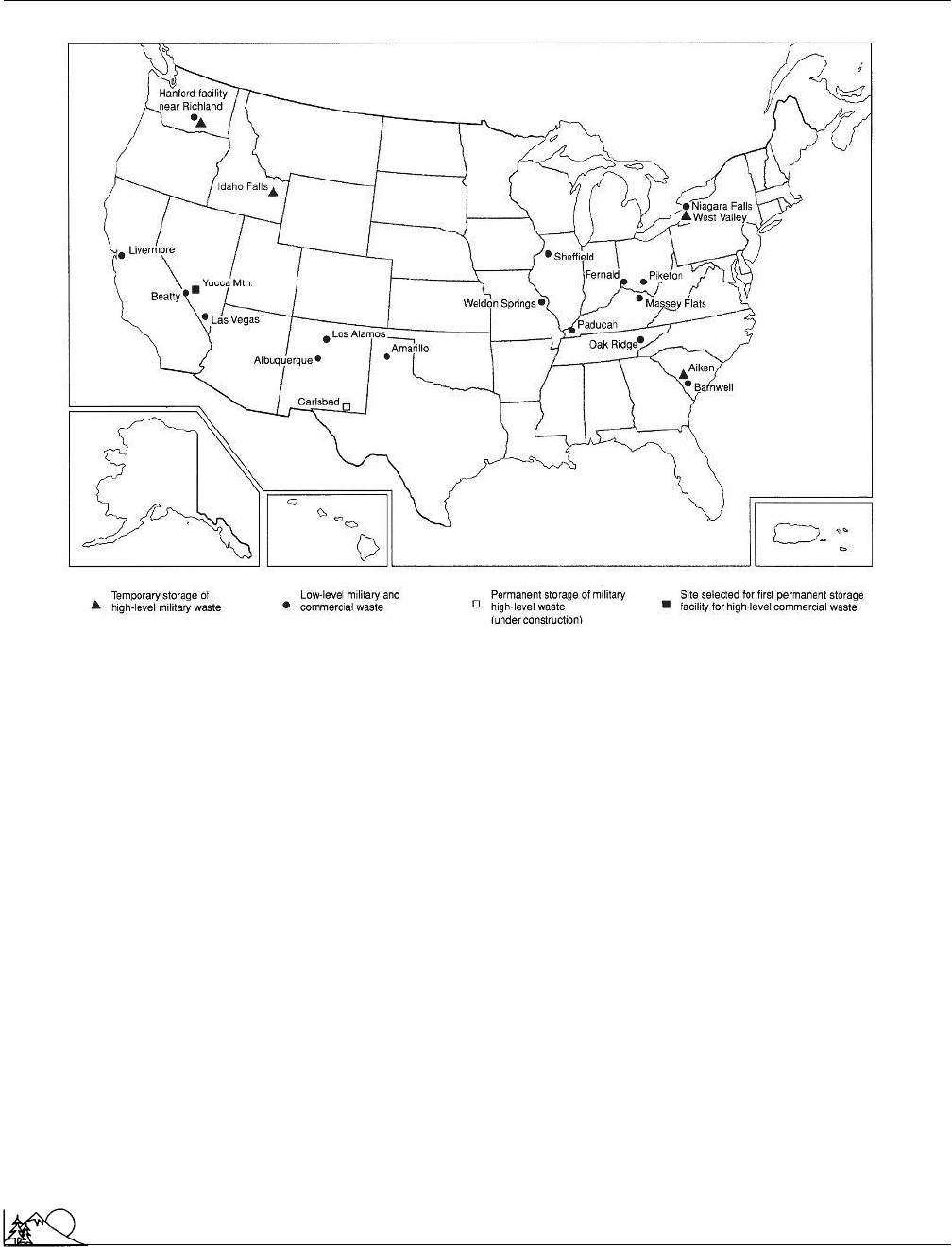
Environmental Encyclopedia 3
Radioactive waste management
A map of the United States showing radioactive waste disposal site locations. (McGraw-Hill Inc. Reproduced by
permission.)
addition, the Federal Facility Compliance Act (1992) forces
the military to clean up its waste sites.
One of the more difficult aspects of the regulatory
quagmire is the problem of dealing with waste, especially
spent nuclear fuel and contaminated material such as worn-
out reactor parts. Today, the official policy objective for
nuclear waste is to dispose of it so that it will never do any
appreciable damage to anyone, under any circumstances, for
all time. However, for some radioactive waste, “for all time”
is measured in thousands of years.
Although radioactive waste carries some hazard for
many years,
radioactive decay
removes most of the hazard
after a few hundred years. A well-designed waste storage
system can be made safe for a long time, some scientists
and policy-makers insist, provided that engineers ensure that
erosion
,
groundwater
, earthquakes, and other unpredict-
able natural or human activities do not breach safety barriers.
Disposal methods for radioactive wastes have varied.
Over the past 50 years, low-level wastes have been flushed
down drains, dumped into the ocean, and tossed into land-
fills. Uranium mill tailings have been mounded into small
1159
hills at sites throughout the western United States. Storage
tanks and barrels at DOE sites hold millions of gallons of
radioactive waste and toxic
chemicals
, the by-products of
plutonium
production for nuclear weapons.
Over 80% of the total volume of radioactive waste
generated in the United States is considered low-level. There
is now a shift away from the most common disposal method
of shallow land burial. Because of public demand for disposal
methods that provide the greatest safety and security, dis-
posal methods now include above- and below-ground vaults
and earth-mounded concrete bunkers.
However, the country’s 17,000 laboratories, hospitals,
and nuclear
power plants
that produce low-level radioactive
byproducts could become de facto disposal sites. The federal
low-level radioactive waste policy enacted in 1980 was de-
signed to remedy the inequity of shipping all the nation’s
low-level radioactive waste to three states (Illinois, Kentucky,
and New York) which never agreed to serve as the nation’s
sole disposal facilities. Its intent was to require every state
to either build its own low-level repository or compact with
other states to build regional facilities by 1986. The 1980

Environmental Encyclopedia 3
Radioactive waste management
federal act was amended in 1985, when it became clear that
the 1986 target would not be met, and the deadline was
postponed to 1993. As of late 1992, however, few states had
even determined sites for such facilities. And after 1996, the
institutions generating low-level wastes will be held liable
for all low-level wastes they produce.
The principal sources of high-level radioactive waste
are nuclear power plants, and programs of the U. S. Depart-
ment of Defense and DOE, especially those dealing with
nuclear weapons. These wastes include spent fuel removed
from nuclear plants, and fission products separated from
military fuel that has been chemically processed to reclaim
unused uranium and plutonium. In the United States, com-
mercial nuclear power plant fuel removed from those facili-
ties is stored on-site until a geologic repository is completed.
The expected completion date for the repository is 2010.
Typically, waste fuel is stored at the power station in
a pressurized
buffer
storage tube for 28 days, then the fuel
rod is broken down to component parts. Irradiated fuel
elements then are stored for 80 days in a water-filled pond
to allow for further decay. For the nonreusable radioactive
waste that remains, about 2.5%, there is no totally safe
method for disposal.
Radioactive waste from one of the first nuclear bomb
plants is so volatile that the clean-up problems have stymied
the experts. The plant at the DOE’s
Hanford Nuclear
Reservation
near Richland, Washington, is the nation’s
largest repository of nuclear waste. There, 177 tanks contain
more than 57 million gal (26 million l) of radioactive waste
and toxic chemicals, byproducts of plutonium production for
nuclear weapons. The DOE and Westinghouse Corporation
(the contractor in charge of Hanford’s clean-up) are still not
sure exactly what mixture of chemicals and radioactive waste
each tank contains, but a video taken inside one tank shows
the liquid bubbling and roiling from chemical and nuclear
reactions. Corrosive, highly radioactive liquids have eaten
through Hanford’s storage tanks and are being removed to
computer-monitored, carbon-steel storage tanks. The clean-
up at Hanford could cost as much as $57 billion. The only
other country known to have had a waste problem as large
as the one at Hanford is the former Soviet Union, where a
nuclear waste dump exploded in the nuclear complex at
Chelyabinsk in 1957, contaminating thousands of square
miles of land.
As the military starts massive clean-up efforts, it has
identified 17 nuclear facilities as among the worst of the
more than 20,000 suspected toxic sites. For example, since
the 1970s, about 77,000 barrels of low-level radioactive
wastes have been stored at the
Oak Ridge, Tennessee
nu-
clear reservation. Today, some barrels are rusting and leaking
radioactivity
. Other identified radioactive waste sites in-
clude California’s Lawrence Livermore National Laboratory
1160
and Sandia National Laboratory, Colorado’s
Rocky Flats
nuclear plant
, and New Mexico’s Los Alamos National
Laboratory. The Savannah River nuclear plant in South
Carolina has been called especially dangerous. Both the
Sa-
vannah River site
and the Hanford site pose the risk of
the kind of massive nuclear waste explosion that occurred
at Chelyabinsk.
One new technology in development for the treatment
of hazardous and radioactive waste is in situ vitrification
(ISV), which utilizes electricity to melt contaminated mate-
rial in place. This technology was developed primarily to treat
radioactive waste, but it also has applications to hazardous
chemical wastes. The end-result glass formed by ISV is
unaffected by extremes of temperature, is not biotoxic,
should remain relatively stable for one million years, and
passes government tests that measure the speed of contami-
nant
leaching
over time. The process permits treatment of
mixtures of various wastes including organic, inorganic, and
radioactive. The fact that it is not necessary to excavate the
waste prior to treatment is seen as a major advantage of
the technology, since excavation along with
transportation
increases health risks. The technology is currently being used
in the United States at Superfund sites.
Incineration
is also used for disposal of low-level ra-
dioactive waste, but incomplete
combustion
can produce
dioxins and other toxic ash and aerosols. Although finding
a site for ash disposal is difficult, the ash is considered a
better form for burial than the original waste. It is biologically
and structurally more stable, and many of the compounds
it contains are insoluble. The residual waste produced by
incineration also is less susceptible to leaching by rain and
groundwater.
Although progress is slow and the situation is often
chaotic in the United States, the state of radioactive
waste
management
in much of the rest of the world is even
less advanced. The situation is particularly acute in Eastern
Europe and the former Soviet Union, since these countries
have generated huge quantities of radioactive waste. In many
cases the information-gathering necessary to plan clean-up
efforts has not begun or is barely underway, and most, if
not all, of the actual clean-up remains. See also Eastern
European pollution; Ocean dumping; Radiation exposure;
Radiation sickness; Radioactive pollution; Waste Isolation
Pilot Plant; Yucca Mountain, Nevada
[Linda Rehkopf]
R
ESOURCES
B
OOKS
Deadly Defense: Military Radioactive Landfills. New York: Radioactive
Waste Campaign, 1988.
Moeller, D. W. Environmental Health. Cambridge: Harvard University
Press, 1992.

Environmental Encyclopedia 3
Radioactivity
Shulman, S. The Threat at Home: Confronting the Toxic Legacy of the U.S.
Military. Boston: Beacon Press, 1992.
The Nuclear Waste Primer. Washington, DC: League of Women Voters,
1985.
P
ERIODICALS
Hammond, R. P. “Nuclear Wastes and Public Acceptance.” American Scien-
tist 67 (March–April 1979): 146–50.
Shulman, S. “Operation Restore Earth: Cleaning Up After the Cold War.”
E Magazine 4 (March–April 1993): 36–43.
Radioactivity
In 1896, the French physicist Henri Becquerel accidentally
found that an ore of
uranium
, pitchblende, emits an invisible
form of radiation, somewhat similar to light. The phenome-
non was soon given the name radioactivity and materials like
pitchblende were called radioactive.
The radiation Becquerel discovered actually consists
of three distinct parts, called alpha, beta, and gamma rays.
Alpha and beta rays are made up of rapidly moving parti-
cles—helium nuclei in the case of alpha rays, and electrons
in the case of beta rays. Gamma rays are a form of electro-
magnetic radiation with very short wavelengths.
Alpha rays have relatively low energies and can be
stopped by a thin sheet of paper. They are not able to
penetrate the human skin and, in most circumstances, pose
a relatively low health risk. Beta rays are more energetic,
penetrating a short distance into human tissue, but they can
be stopped by a thin sheet of
aluminum
. Gamma rays are
by far the most penetrating form of radiation, permeating
wood, paper, plastic, tissue, water, and other low-density
materials in the
environment
. They can be stopped, how-
ever, by sheets of
lead
a few inches thick.
Radioactivity is a normal and ubiquitous part of the
environment. The most important sources of natural radio-
activity are rocks containing radioactive isotopes of uranium,
thorium, potassium, and other elements. The most common
radioactive
isotope
in air is carbon-14, formed when neu-
trons from cosmic ray showers react with
nitrogen
in the
atmosphere
. Humans, other animals, and plants are con-
stantly exposed to low-level radiation emitted from these
isotopes, and they do suffer to some extent from that expo-
sure. A certain number of human health problems—cancer
and genetic disorders, for example—are attributed to damage
caused by natural radioactivity.
In recent years, scientists have been investigating the
special health problems related to one naturally occurring ra-
dioactive isotope, radon-226. This isotope is produced when
uranium decays, and since uranium occurs widely in rocks,
radon-226 is also a common constituent of the environment.
Radon-226 is an alpha-emitter, and though the isotope does
have a long
half-life
(1,620 years), the alpha particles are not
1161
energetic enough to penetrate the skin. The substance, how-
ever, is a health risk because it is a gas that can be directly
inhaled. The alpha particles come into contact with lung tis-
sue, and some scientists now believe that radon-226 may be
responsible for a certain number of cases of lung
cancer
. The
isotope can be a problem when homes are constructed on land
containing an unusually high concentration of uranium. Ra-
don-226 released by the uranium can escape into the base-
ments of homes, spreading to the rest of a house. Studies by
the
Environmental Protection Agency
(EPA) have found
that as many as eight million houses in the United States have
levels of radon-226 that exceed the
maximum permissible
concentration
recommended by experts.
Though Becquerel had discovered radiation occurring
naturally in the environment, scientists immediately began
asking themselves whether it was possible to convert normally
stable isotopes into radioactive forms. This question became
the subject of intense investigation in the 1920s and 1930s,
and was finally answered in 1934 when Ire
`
ne Curie and Fre
`
d-
e
`
ric Joliot bombarded the stable isotope aluminum-27 with
alpha particles and produced phosphorus-30, a radioactive
isotope. Since the Joliot-Curie experiment, scientists have
found ways to manufacture hundreds of artificially radioactive
isotopes. One of the most common methods is to bombard a
stable isotope with gamma rays. In many cases, the product
of this reaction is a radioactive isotope of the same element.
Highly specialized techniques have recently been de-
vised to meet specific needs. Medical workers often use
radioactive isotopes with short half-lives because they can be
used for diagnostic purposes without remaining in a patient’s
body for long periods of time. But the isotope cannot have
such a short half-life that it will all but totally decay between
its point of manufacture and its point of use.
One solution to this problem is the so-called “molyb-
denum cow.” The cow is no more than a shielded container
of radioactive molybdenum-99. This isotope decays with a
long half-life to produce technetium-99, whose half-life is
only six hours. When medical workers require technetium-
99 for some diagnostic procedure, they simply “milk” the
molybdenum cow to get the short-lived isotope they need.
Artificially radioactive isotopes have been widely em-
ployed in industry, research, and medicine. Their value lies
in the fact that the radiation they emit allows them to be
tracked through settings in which they cannot be otherwise
observed. For example, a physician might want to know if
a patient’s thyroid is functioning normally. In such a case,
the patient drinks a solution containing radioactive iodine,
which concentrates in the thyroid like stable iodine. The
isotope’s movement through the body can be detected by a
Geiger counter or some other detecting device, and the speed
as well as the extent to which the isotope is taken up by the
thyroid is an indication of how the organ is functioning.

Environmental Encyclopedia 3
Radiocarbon dating
Artificially radioactive isotopes can pose a hazard to
the environment. The materials in which they are wrapped,
the tools with which they are handled, and the clothing
worn by workers may all be contaminated by the isotopes.
Even after they have been used and discarded, they may
continue to be radioactive. Users must find ways of disposing
of these wastes without allowing the release of dangerous
radiation into the environment, a relatively manageable
problem. Most materials discarded by industry, medical
facilities, and researchers are
low-level radioactive waste
.
The amount of radiation released decreases quite rapidly,
and after isolation for just a few years, the materials can be
disposed of safely with other non-radioactive wastes.
The same cannot be said for the
high-level radioac-
tive waste
produced by nuclear
power plants
and defense
research and production. Consisting of radioactive isotopes,
such wastes are produced during fission reactions and release
dangerously large amounts of radiation for hundreds or thou-
sands of years.
Nuclear fission
was discovered accidentally in the
1930s by scientists who were trying to produce artificial
radioactive isotopes. In a number of cases, they found that
the reactions they used did not result in the formation of
new radioactive isotopes, but in the splitting of atomic nuclei,
a process that came to be known as nuclear fission.
By the early 1940s, nuclear fission was recognized as
an important new source of energy. That energy source was
first put to use for destructive purposes, in the construction
of
nuclear weapons
. Later, scientists found ways to control
the release of energy from nuclear fission in nuclear reactors.
The most serious environmental problem associated
with fission reactions is that their waste products are largely
long-lived radioactive isotopes. Attempts have been made
to isolate these wastes by burying them underground or
sinking them in the ocean. All such methods have proved
so far to be unsatisfactory, however, as containers break open
and their contents leak into the environment.
The United States government has been working for
more than four decades to find better methods for dealing
with these wastes. In 1982, Congress passed a Nuclear Waste
Policy Act, providing for the development of one or more
permanent burial sites for high-level wastes. Political and
environmental pressures have stalled the implementation of
the act and a decade after its passage, the nation still has no
method for the safe disposal of its most dangerous radioactive
wastes. See also Ecotoxicology; Hazardous waste; Nuclear
fusion; Nuclear winter; Radiation exposure; Radiation sick-
ness; Radioactive fallout; Radioactive pollution; Radioactive
waste management
[David E. Newton]
1162
R
ESOURCES
B
OOKS
Gofman, J. W. Radiation and Human Health. San Francisco: Sierra Club
Books, 1981.
Inglis, D. R. Nuclear Energy: Its Physics and Social Challenge. Reading, MA:
Addison-Wesley, 1973.
Jones, R. R., and R. Southwood, eds. Radiation and Health. New York:
Wiley, 1987.
Wagner, H. N., and L. E. Ketchum. Living With Radiation. Baltimore:
Johns Hopkins University Press, 1989.
Radiocarbon dating
Radiocarbon dating is a technique for determining the age
of very old objects consisting of organic (carbon-based) ma-
terials, such as wood, paper, cloth, and bone. The technique
is based on the fact that both stable and radioactive isotopes
of
carbon
exist. These isotopes behave almost identically
in biological, chemical, and physical processes.
Carbon-12, a stable
isotope
, makes up about 99% of
all carbon found in
nature
. Radioactive carbon-14 is formed
in the
atmosphere
when neutrons produced in cosmic ray
showers react with
nitrogen
atoms.
Despite the fact that it makes up no more than 0.08%
of the earth’s crust, carbon is an exceedingly important ele-
ment. It occurs in all living materials and is found in many
important rocks and minerals, including limestone and mar-
ble, as well as in
carbon dioxide
. Carbon moves through
the atmosphere, hydrosphere, lithosphere, and
biosphere
in a series of reactions known as the
carbon cycle
. Stable
and radioactive isotopes of the element take part in identical
reactions in the cycle. Thus, when green plants convert car-
bon dioxide to carbohydrates through the process of
photo-
synthesis
, they use both stable carbon-12 and radioactive
carbon-14 in exactly the same way. Any living material con-
sists, therefore, of a constant ration of carbon-14 to car-
bon-12.
In the mid-1940s, Willard F. Libby realized that this
fact could be used to date organic material. As long as that
material was alive, he pointed out, it should continue to take
in both carbon-12 and carbon-14 in a constant ratio. At its
death, the material would no longer incorporate carbon in
any form into its structure. From that point on, the amount
of stable carbon-12 would remain constant. The amount
of carbon-14, however, would continuously decrease as it
decayed by beta
emission
to form nitrogen. Over time, the
ratio of carbon-14 to carbon-12 would grow smaller and
smaller. That ratio would provide an indication of the length
of time since the material had ceased being alive.
Radiocarbon dating has been used to estimate the age
of a wide variety of objects ranging from charcoal taken from
tombs to wood found in Egyptian and Roman ships. One

Environmental Encyclopedia 3
Radioisotope
of its most famous applications was in the dating of the
Shroud of Turin. Some religious leaders had claimed that
the Shroud was the burial cloth in which Jesus was wrapped
after his crucifixion. If so, the material of which it was made
would have to be nearly 2,000 years old. Radiocarbon dating
of the material showed, however, that the cloth could not
be more than about 700 years old.
Radiocarbon dating can be used for objects up to
30,000 years of age, but it is highly reliable only for objects
less than 7,000 years old. These limits result from the fact
that eventually carbon-14 has decayed to such an extent that
it can no longer be detected well or, eventually, at all in a
sample. For older specimens, radioisotopes with longer half-
lives can be used for age determination. See also Half-life;
Radioactive decay
[David E. Newton]
R
ESOURCES
B
OOKS
Taylor, R. E., et al., eds. Radiocarbon Dating: An Archaeological Perspective.
Orlando: Academic Press, 1987.
———. Radiocarbon After Four Decades: An Interdisciplinary Perspective.
New York: Springer Verlag, 1992.
Radioisotope
The term radioisotope is shorthand for radioactive
isotope
.
Isotopes are forms of an element whose atoms differ from
each other in the number of neutrons contained in their
nuclei and, hence, in their atomic masses. Hydrogen-1, hy-
drogen-2, and hydrogen-3 are all isotopes of each other.
Isotopes may be stable or radioactive. That is, they
may exist essentially unchanged forever (stable), or they may
spontaneously emit an
alpha particle
or
beta particle
and/
or a
gamma ray
, changing in the process into a new sub-
stance. Hydrogen-1 and hydrogen-2 are stable isotopes, but
hydrogen-3 is radioactive.
The first naturally occurring radioisotopes were dis-
covered in the late 1890s. Scientists found that all isotopes
of the heaviest elements—uranium, radium,
radon
, tho-
rium, and protactinium, for example—are radioactive. This
discovery raised the question as to whether stable isotopes
of other elements could be converted to radioactive forms.
By the 1930s, the techniques for doing so were well
established, and scientists routinely produced hundreds of
radioisotopes that do not occur in
nature
. As an example,
when the stable isotope carbon-12 is bombarded with neu-
trons, it may be converted to a radioactive cousin, carbon-
13. This method can be used to manufacture radioisotopes
of nearly every element.
1163
Naturally occurring radioisotopes are responsible for
the existence of
background radiation
. Background radia-
tion consists of alpha and beta particles and gamma rays
emitted by these isotopes. In addition to the heavy isotopes
mentioned above, the most important contributors to back-
ground radiation are carbon-14 and potassium-40.
Synthetic radioisotopes have now become ubiquitous
in human society. They occur commonly in every part of
nuclear power
plant operations. They are also used exten-
sively in the health sciences, industry, and scientific research.
A single example of their medical application is
cancer
therapy. Gamma rays emitted by the radioisotope cobalt-60
have been found to be very effective in treating some forms
of cancer. Other gamma-emitting radioisotopes can also be
used in this procedure.
The potential for the release of radioisotopes to the
environment
is great. For example, medical wastes might
very well contain radioisotopes that still emit measurable
amounts of radiation. Stringent efforts are made, therefore,
to isolate and store radioisotopes until their radiation has
reached a safe level.
These efforts have two aspects. Some radioisotopes
have short half-lives. The level of radiation they emit drops
to less than 1% of the original amount in a matter of hours
or days. These isotopes need only be stored in a safe place
for a short time before they can be safely discarded with
other solid wastes.
Other radioisotopes have half-lives of centuries or mil-
lennia. They will continue to emit harmful radiation for
thousands of years. Safe disposal of such wastes may require
burying them deep in the earth, a procedure that still has
not been satisfactorily demonstrated. In spite of the potential
environmental hazard posed by radioisotopes, they do not
presently pose a serious threat to plants, animals, or humans.
The best estimates place the level of radiation from artificial
sources at less than five percent of that from natural sources.
See also Half-life; Radioactive decay; Radioactive waste man-
agement; Radioactivity
[David E. Newton]
R
ESOURCES
B
OOKS
Baker, P., et al. Radioisotopes in Industry. Washington, DC: U.S. Atomic
Energy Commission, 1965.
Corless, W. R., and R. L. Mead. Power from Radioisotopes. Washington,
DC: U.S. Atomic Energy Commission, 1971.
Kisieleski, W. E., and R. Baserga. Radioisotopes and Life Processes. Washing-
ton, DC: U.S. Atomic Energy Commission, 1967.
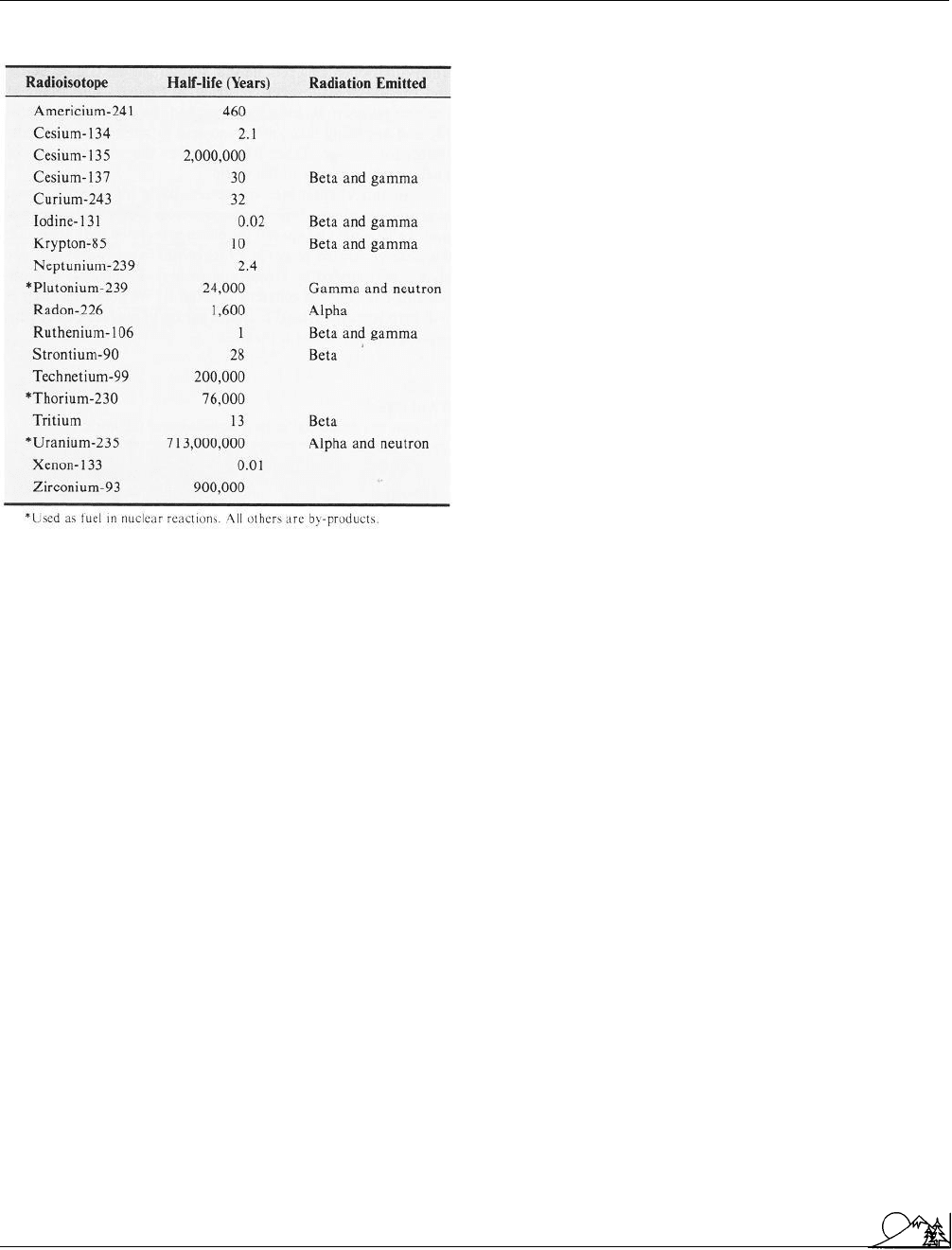
Environmental Encyclopedia 3
Radiological Emergency Response Team
Some radioisotopes associated with nuclear
power. (McGraw-Hill Inc. Reproduced by permission.)
Radiological Emergency Response
Team
The Radiological Emergency Response Team (RERT) of
the federal
Environmental Protection Agency
(EPA) re-
sponds to emergencies that involve the release of radioactive
materials. The team responds to emergencies such as acci-
dents at nuclear
power plants
, accidents involving the ship-
ment of radioactive material, and acts of nuclear terrorism.
RERT works with the EPA Superfund Program, as well as
federal, state, and local agencies to develop and enforce
strategic plans.
RERT is based in the EPA Office of Radiation and
Indoor Air in Washington, D.C., and at two national labora-
tories.
Environmental monitoring
and assessment is per-
formed by employees at the National Air and Radiation
Environmental Laboratory in Montgomery, Alabama, and
the Radiation and Indoor Environments National Labora-
tory in Las Vegas, Nevada. Approximately 75 RERT mem-
bers were stationed in Washington and at the laboratories
in the spring of 2002.
In an emergency, an RERT field team goes to the site
where radioactive material was released. The team’s duties
include taking environmental measurements and doing labo-
ratory work. In addition, RERT works with state and local
1164
authorities to protect the public from exposure to harmful
radiation levels. Team equipment ranges from a mobile radi-
ation laboratory to the personal dosimeter used to measure
the radiation dose in an individual.
[Liz Swain]
Radionuclides
Radionuclides are radioactive elements.
Radioactivity
can
be defined as the release of
alpha and beta
particles from
atoms, and/or
gamma rays
that takes place when the nuclei
of certain unstable substances spontaneously disintegrate. It
is during this disintegration process that they emit radiation.
The two main types of radiation released during such pro-
cesses are termed
ionizing
, or of sufficient strength to forc-
ibly eject electrons from their orbit around an atoms nucleus,
producing ions, and
non-ionizing
, of less strength and inca-
pable of displacing electrons and forming ions.
Ionizing
radiation
is highly significant because when it occurs within
the atoms of molecules in living things, it is capable of
causing biological damage such as the death of cells or the
unnatural reproduction of cells. We term this unnatural re-
production of cells
cancer
.
Radionuclides occur in the
environment
both natu-
rally and as a result of human industry. Some naturally-
occurring radionuclides exist all over the earth and have been
present since its formation 4.5 billion years ago. But most
elements can be made artificially radioactive by bombarding
them with high-energy particles such as neutrons. Each
radionuclides forms and behaves in unique ways, with its
own method and rate of decay. Such decay is measured by
what is called
half-life
, or the time it takes for a group of
atoms to decay to half of their original number.
One of the substances that occurs naturally in the
earth’s crust is
uranium
, a radionuclide used often as fuel
to produce
nuclear power
. Uranium is the heaviest element
found on earth with the exception of tiny amounts of an
element called neptunium. When the German chemist Mar-
tin H. Klaproth discovered uranium in 1789, he named
it in honor of the recently discovered planet Uranus. The
scientific community showed little interest in uranium until
1896, when Henri Becquerel discovered radioactivity, and
uranium was named as one of only two known radioactive
elements of the time. In 1938, scientists discovered
nuclear
fission
, a reaction that produces energy. In fact, uranium
can produce energy at nearly three million times that of
coal
(1 lb of uranium can produce the same amount of energy
produced by 3 million lb [1.4 million kg] of coal).
The most abundant form (
isotope
) of uranium boasts
a
half-life
nearly identical to that of the earth itself. This
allows scientists to use the substance’s disintegration to date

Environmental Encyclopedia 3
Radionuclides
other geological features of the earth by comparison. How-
ever, uranium use also has many drawbacks. Most notably,
use of the substance produces nuclear waste that must be
carefully transported and stored. In addition, easily obtain-
able supplies of uranium on Earth are limited, therefore the
costs of locating and refining uranium can be extremely high.
Radium occurs as a result of uranium disintegration.
Marie Curie, the first woman to win a Nobel Prize, and
one of the few scientists to ever receive the Nobel prize twice,
along with her husband Pierre, discovered the substance. She
had followed up on Becquerel’s observations on uranium’s
radioactive properties for her doctoral dissertation in the late
1890s. The Curies began refining pitchblende, a waste ore
that was commonly found around uranium mines, known
to emit radiation. The Curies first discovered the radioactive
salt they named polonium, and then radium, found to be
thousands of times more radioactive than any other substance
discovered to date.
Radium is found in water,
soil
, plants, and food, but
at low concentrations. Drinking water holds the highest
potential for human exposure to radium. When humans are
exposed to radium orally, they are at risk for lung, bone,
brain and nasal passage tumors. Chronic exposure has led
to acute
leukemia
and other complications. Radium has
been classified by the
Environmental Protection Agency
(EPA) as a human
carcinogen
. Today, radium is still con-
sidered among the most radioactive metals on Earth, and
requires careful attention and handling. Its use has been
limited in many products, but the substance still helps treat
cancers through radiation therapy and aids in some forms
of research. As evidence of the validity of the EPA’s finding,
its discoverer, Marie Curie died at the age of 67 of leukemia,
caused by her prolonged exposure to radiation.
Radon
is a gas that can diffuse out of uranium (from
radium) in the ground from uranium and thorium present
in minerals, ores and rocks. A German physics professor
named Friedrich Dorn discovered radon in 1900, after he
followed the experiments of Marie Curie. He found that
radium emitted a radioactive gas he termed
radium emana-
tion
. Dorns discovery was important because it showed that
an element could be transmuted from metal to gas as part
of the
radioactive decay
process. The hazards of radon
were not discovered until late in the twentieth century, and
since that time, testing houses for the gas has become an
important precaution. Radon has shown the capability of
seeping into
groundwater
and contaminating public drink-
ing supplies.
As Radon is colorless and odorless, it can prove partic-
ularly dangerous. Radon exposure occurs mostly through
inhalation of the gas in indoor locations such as schools,
homes, or office buildings. Chronic exposure produces seri-
ous respiratory effects, and smokers are at particular risk for
1165
lung cancer, estimated at 10–20 times the risk of nonsmok-
ers. Although hazardous, radon has been useful in predicting
earthquakes. In 1918, the discovery by a group of Chinese
scientists that radon levels in groundwater rise just before
earthquakes led to the prediction of several earthquakes by
monitoring radon concentrations in well water. Radon has
also proves useful in detecting leaks, inspecting metal welds,
and measuring flow rates. However, radons high risk status
as a carcinogen outweighs its beneficial uses.
Plutonium
is an example of an artificial radioactive
substance. It was discovered around the early 1940s during
experiments on nuclear fission conducted by a number of
scientists. Plutonium is used in breeder reactors when ura-
nium undergoes nuclear fission to produce energy and occurs
as a waste product of uranium. Like uranium, plutonium
produces large amounts of energy and its properties were
put to the test in the first
nuclear weapons
test in New
Mexico in 1945.
Knowledge of the properties and risks of all radionu-
clides have made it necessary for people all over the world
to become more aware of the inherent dangers they possess
and the necessary preventative measures needed for protec-
tion. People that work in factories that process uranium or
with phosphate fertilizers are at increased risk of exposure
to radionuclides, as are those living in close proximity to
uranium mines. Tests can measure radioactivity levels in the
body and elimination of radium and radon in exhaled breath.
Uranium, radon, and radium levels can be measured in
the urine.
One of the greatest challenges facing scientists and
policymakers throughout the world is the safe transport and
disposal of the
hazardous waste
resulting from radionu-
clides. The subject has been steeped in controversy for many
years, as no place seems safe enough to handle storage of a
substance that lives for millions of years. However, scientists
continue to develop new methods for storage of nuclear
byproducts. In early 2001, a Los Alamos National Labora-
tory team announced that certain ceramic materials held up
against radiation damage and could potentially offer new
solutions to resist
leaching
and radiation for thousands of
years.
A consortium of international agencies promotes in-
ternational cooperation in managing nuclear waste, since
nearly every developed country has some sort of nuclear
power or weaponry project in place. Most of these countries
have developed plans to safely dispose of nuclear waste early
in the twenty-first century. The United States leads most
countries in storage efforts, with the 1982 passage of the
Nuclear Waste Policy Act. The act identified objectives for
developing geologic repositories for high-level nuclear waste.
The federal government identified
Yucca Mountain
in Nevada for deep underground storage of wastes to replace

Environmental Encyclopedia 3
Radiotracer
storage that now lies at commercial nuclear
power plants
and research reactor sites in 43 states. The EPA continues
to develop public health and environmental standards to set
safe limits for the long-term storage of highly
radioactive
waste
. However, a coalition of environmental groups also
continues to watch and question the agency to ensure that
the radiation standard remains protective enough. Like the
Waste Isolation Pilot Plant
(WIPP) storage facility in
southern New Mexico, the Yucca Mountain site will no
doubt remain controversial for some time.
The EPA provides printed fact sheets on radionuclides
that warn of environmental and occupational exposure to
the substances and acceptable levels of contact for humans.
According to the agency, uranium is present in rocks and
soil and throughout the environment, and although exposure
can occur through air, higher levels generally occur in food
or drinking water. Chronic long-term exposure to uranium
and radon has been linked to both lung and kidney diseases.
The EPA is currently in the process of promulgating new
drinking water standards (the first update since 1976) regard-
ing (non-Radon) radionuclides. These standards will become
effective December 8, 2003, and will affect only Community
Water Systems (CWSs), or those systems that serve more
than 25 residents regularly all year.
[Joan M. Schonbeck]
R
ESOURCES
B
OOKS
Alexander, D. E., and R. W. Fairbridge. Encyclopedia of Environmental
Science. Dordrecht, The Netherlands: Kluwer Academic Publishers, 1999.
Clayman, Charles. The American Medical Association Home Medical Encyclo-
pedia. New York: Random House, 1998.
Emsley, J. The Elements. New York: Clarendon Press, 1998.
O
THER
Environmental News Network. [cited July 2002]. <http://wwww.enn.com>.
O
RGANIZATIONS
Alliance for Nuclear Accountability, 1914 N. 34th St., Ste. 407, Seattle,
WA USA 98103 (206) 547-3175, Fax: (206) 547-7158, Email:
ananuclear@earthlink.net, <http://www.ananuclear.org>
Environmental Protection Agency Office of Air and Radiation, Ariel Rios
Building, 1200 Pennsylvania Ave. NW, Washington, DC USA 20460
(202) 564-7400, <http://www.epa.gov/air/oarofcs.html>
Radiotracer
A radioactive
isotope
progressing through a biological or
physical system can be followed by several tracking proce-
dures. For example,
fertilizer
containing radioactive
phos-
phorus
can be added to
soil
. Plants grown in this soil then
take up the radioactive phosphorus just as they do non-
radioactive phosphorus. If one of these plants is placed on
1166
a photographic plate, radiation from the radioactive phos-
phorus exposes the plate. The plant “takes its own picture,”
showing where the phosphorus concentrates in the plant.
Radiotracers are a highly desirable research technique as they
do not require the destruction of an organism for its study.
Radon
Although it has received attention as an environmental haz-
ard only recently, radon is a naturally occurring radioactive
gas that is present at low concentrations everywhere in the
environment
. Colorless and odorless, radon is a decay prod-
uct of radium; radium is a decay product of the radioactive
element
uranium
, which occurs naturally in the earth’s crust.
Radon continues to break down into products called radon
progeny. Radon is measured in units called picocuries per
liter (pCi/L), and it becomes a health concern when people
are exposed to concentrations higher than normal back-
ground levels. Some geologic formations, such as the Read-
ing Prong in New Jersey, are naturally very high in radon
emissions.
During their normal decay process, radioactive ele-
ments emit several kinds of radiation, one of which is alpha
radiation. The health effects of radon are associated with
these alpha particles. These particles are too heavy to travel
far and they cannot penetrate the skin, but they can enter
the body through the lungs during inhalation. Studies of
miners exposed to high concentrations of radon have shown
an increased risk of lung
cancer
, and this is the health effect
most commonly associated with radon. Background levels
are usually estimated at 1 pCi/L. It is estimated that a person
exposed to this concentration for 18 hours a day for five
years increases their risk of developing cancer to one in 1000.
At radon levels of 200 pCi/L, the increased risk of lung
cancer after five years of exposure at 18 hours per day rises
to 60 in 1,000. Because cancer is a disease that is slow to
develop, it may take five to 50 years after exposure to radon
to detect lung cancer.
In the outdoor environment, radon gas and its decay
products are usually too well-dispersed to accumulate to
dangerous levels. It is indoors without proper ventilation, in
places such as basements and ground floors, where radon
can seep from the
soil
and accumulate to dangerous concen-
trations. The most common methods of reducing radon
buildup inside the home include installing blowers or simply
opening windows. Plugging cracks and sealing floors that
are in contact with soil also reduces the concentration. In
the United States, environmental and public health agencies
have instituted free programs to test for radon concentra-
tions, and they also offer assistance and guidelines for reme-
