Environmental Encyclopedia
Подождите немного. Документ загружается.


Environmental Encyclopedia 3
Center for Science in the Public Interest
O
THER
Earth Ethics. Washington, D.C.: Center for the Respect for Life and
Environment.
O
RGANIZATIONS
Center for Respect of Life and Environment, 2100 L Street, NW,
Washington, D.C. USA 20037 (202) 778-6133, Fax: (202) 778-6138,
Email: info@crle.org, <http://www.crle.org>
Center for Rural Affairs
The Center for Rural Affairs (CRA) is a nonprofit organiza-
tion dedicated to the social, economic and
environmental
health
of rural communities. Founded in 1973, the Center
for Rural Affairs includes among its participants farmers,
ranchers, business people, and educators concerned with the
decline of the family farm.
CRA works to provoke public thought on issues and
government policies that affect rural Americans, especially
in the Midwest and Plains regions of the country. It sponsors
research, education, advocacy, organizing and service proj-
ects aimed to improve the life of rural dwellers. CRA’s
sustainable agriculture
policy is designed to analyze, pro-
pose, and advocate public policies that reward environmental
stewardship, promote family farming, and foster responsible
technology. CRA assists beginning farmers with design and
implement on-site research that helps to make these farms
environmentally sound and economically viable. CRA’s
con-
servation
and education programs address the environmen-
tal problems caused by agricultural practices in the North
Central United States.
Through a rural enterprise assistance program, CRA
teaches rural communities to support self-employment, and
it provides business assistance and revolving loan funds for
the self-employed. It also provides professional farm man-
agement and brokerage service to landowners who are willing
to rent or sell land to beginning farmers. CRA promotes
fair competition in the agriculture marketplace by working
to prevent monopolies, encouraging enforcement of laws
restricting corporate farming in the United States, and advo-
cating for the role of United States farmers in international
markets.
Publications offered by CRA include the Center for
Rural Affairs Newsletter, a monthly report on policy issues
and research findings; the Rural Enterprise Reporter, which
provides information about developing small local enter-
prises; and a variety of special reports on topics such as small
farm technology and business strategy.
[Linda Rehkopf]
227
R
ESOURCES
O
RGANIZATIONS
Center for Rural Affairs, 101 S Tallman Street, P.O. Box 406, Walthill, NE
USA 68067 (402) 846-5428, Fax: (402) 846-5420, Email: info@cfra.org,
<http://www.cfra.org>
Center for Science in the Public
Interest
The Center for Science in the Public Interest (CSPI) was
founded in 1971 by Michael Jacobson, who remains its exec-
utive director. It is a consumer advocacy organization princi-
pally concerned with nutrition and food safety, and its mem-
bership consists of scientists, nutrition educators, journalists,
and lawyers.
CSPI has campaigned on a variety of health and nutri-
tion issues, particularly nutritional problems on a national
level. It is the purpose of the group to address “deceptive
marketing practices, dangerous
food additives
or contami-
nants, conflicts of interests in the academic community, and
flawed science propagated by industries concerned solely
with profits.” It monitors current research on nutrition and
food safety, as well as the federal agencies responsible for
these areas. CSPI maintains an office for legal affairs and
special projects. It has initiated legal actions to restrict food
contaminants and to ban food additives that are either unsafe
or poorly tested. The special projects the group has sponsored
include: Americans for Safe Food, the Nutrition Project,
and the Alcohol Policies Project. The center publishes edu-
cational materials on food and nutrition, and it works to
influence policy decisions affecting health and the na-
tional diet.
CSPI has made a significant impact on food marketing
in the past 10 years, and they have successfully contested
food labeling practices in many sectors of the industry. They
were instrumental in forcing fast-food companies to disclose
ingredients, and they have recently pressed the
Food and
Drug Administration
to improve regulations for companies
which make and distribute fruit juice. Many brands do not
reveal the actual percentages of the different juices used to
make them, and certain combinations of juices are often
misleadingly labeled as cherry juice or kiwi juice, for instance,
when they may be little more than a mixture of apple and
grape juice. The organization has also taken action against
deceptive food advertising, particularly advertising for chil-
dren’s products. It has recently demanded further testing of
a sweetener called sucralose, in the wake of studies that have
suggested that it could cause shrinkage of the thymus, a
gland affecting cellular immune responses.
CSPI is funded mainly by foundation grants and sub-
scriptions to its Nutrition Action Newsletter. The newsletter
is published ten months out of the year and is intended to

Environmental Encyclopedia 3
Centers for Disease Control and Prevention
increase public understanding of food safety and nutrition
issues. It frequently examines the consequences of legislation
and regulation at the state and federal level; it has explored
the controversy over organic and chemical farming methods,
and it has studied how agribusiness has changed the way
Americans eat. CSPI also distributes posters, videos, and
computer software, and it offers a directory of mail-order
sources for organically-grown food. Its brochures and reports
include: Guess What’s Coming to Dinner: Contaminants in
Our Food and Organic Agriculture: What the States Are Doing.
It has a staff of 45, a membership of 800,000, and an annual
budget of $10,000,000.
[Douglas Smith]
R
ESOURCES
O
RGANIZATIONS
Center for Science in the Public Interest, 1875 Connecticut Ave., NW,
Suite 300, Washington, D.C. USA 20009 (202) 332-9110, Fax: (202)
265-4954, Email: cspi@cspinet.org, <http://www.cspinet.org>
Centers for Disease Control and
Prevention
The Centers for Disease Control and Prevention (CDC) is
the Atlanta, Georgia-based agency of the
Public Health
Service
that has led efforts to prevent diseases such as
ma-
laria
, polio, smallpox, tuberculosis, and acquired immuno-
deficiency syndrome (
AIDS
). As the nation’s prevention
agency, the CDC’s responsibilities have expanded, and it
now addresses contemporary threats to health such as injury,
environmental and occupational hazards, behavioral risks,
and chronic diseases.
Divisions within the CDC use surveillance, epidemio-
logic and laboratory studies, and community interventions
to investigate and prevent public health threats.
The Center for Chronic Disease Prevention and
Health Promotion designs programs to reduce death and
disability from chronic diseases—cardiovascular, kidney,
liver and lung diseases, and
cancer
and diabetes.
The Center for Environmental Health and Injury
Control assists public health officials at the scene of natural
or artificial disasters such as
volcano
eruptions, forest fires,
hazardous
chemical spills
, and nuclear accidents. Scientists
study the effects of
chemicals
and pesticides, reactor acci-
dents, and health threats from
radon
, among others. The
National Institute for Occupational Safety and Health helps
identify chemical and physical hazards that lead to occupa-
tional diseases.
Preventing and controlling infectious diseases has been
a goal of the CDC since its inception in 1946. The Center
for Infectious Diseases investigates outbreaks of infectious
228
disease locally and internationally. The Center for Preven-
tion Services provides financial and technical assistance to
control and prevent diseases. Disease detectives in the
Epide-
miology
Program Office investigate outbreaks around the
world.
Prevention of
tobacco
use is a critical health issue for
CDC because cigarette smoking is the leading preventable
cause of death in this country. The Office on Smoking and
Health conducts research on the effects of smoking, develops
health promotion and education campaigns, and helps health
departments with smoking education programs.
CDC researchers have improved technology for lead
poisoning
screening, particularly in children. CDC evidence
on environmental lead
pollution
was a key in
gasoline
lead
content reduction requirements. The CDC also coordinated
and directed health studies of
Love Canal
, New York, resi-
dents in the 1980s. The director of the CDC administers
the
Agency for Toxic Substances and Disease Registry
,
the public health agency created to protect the public from
exposure to toxic substances in the
environment
. In 1990,
CDC became responsible for energy-related epidemiologic
research for the
U.S. Department of Energy
nuclear facili-
ties. This includes studies of people who have been exposed
to radiation from materials emitted to the air and water from
plant operations.
The CDC today carries out an ever-widening agenda
with studies on adolescent health, dental disease prevention,
the epidemiology of violence, and categorizing and tracking
birth abnormalities and infant
mortality
.
[Linda Rehkopf]
R
ESOURCES
O
RGANIZATIONS
Centers for Disease Control and Prevention, Public Inquiries/MASO,
Mailstop F07, 1600 Clifton Road, Atlanta, GA USA 30333 Toll Free:
(800) 311-3435, , http://www.cdc.gov
CERCLA
see
Comprehensive Environmental
Response, Compensation, and Liability Act
CERES Principles
see
Valdez Principles
Cesium 137
A radioactive
isotope
of the metallic element cesium. Ce-
sium-137 is one of the major products formed when
ura-
nium
undergoes
nuclear fission
, as in a nuclear reactor or
Environmental Encyclopedia 3
Chaparral
A chain reaction. (Illustration by Laurence Lawson. (Reproduced by permission.)
nuclear weapons
. During atmospheric testing of nuclear
weapons after World War II, cesium-137 was a source of
major concern to health scientists. Researchers found that
the isotope settled to the ground, where it was absorbed by
plants and eaten by animals, ultimately affecting humans.
Once inside the human body, the isotope releases beta parti-
cles, which are carcinogens, teratogens, and mutagens. Large
quantities of cesium-137 were released when the Chernobyl
nuclear reactor exploded in 1986.
CFCs
see
Chlorofluorocarbons
CGIAR
see
Consultative Group on International
Agricultural Research
229
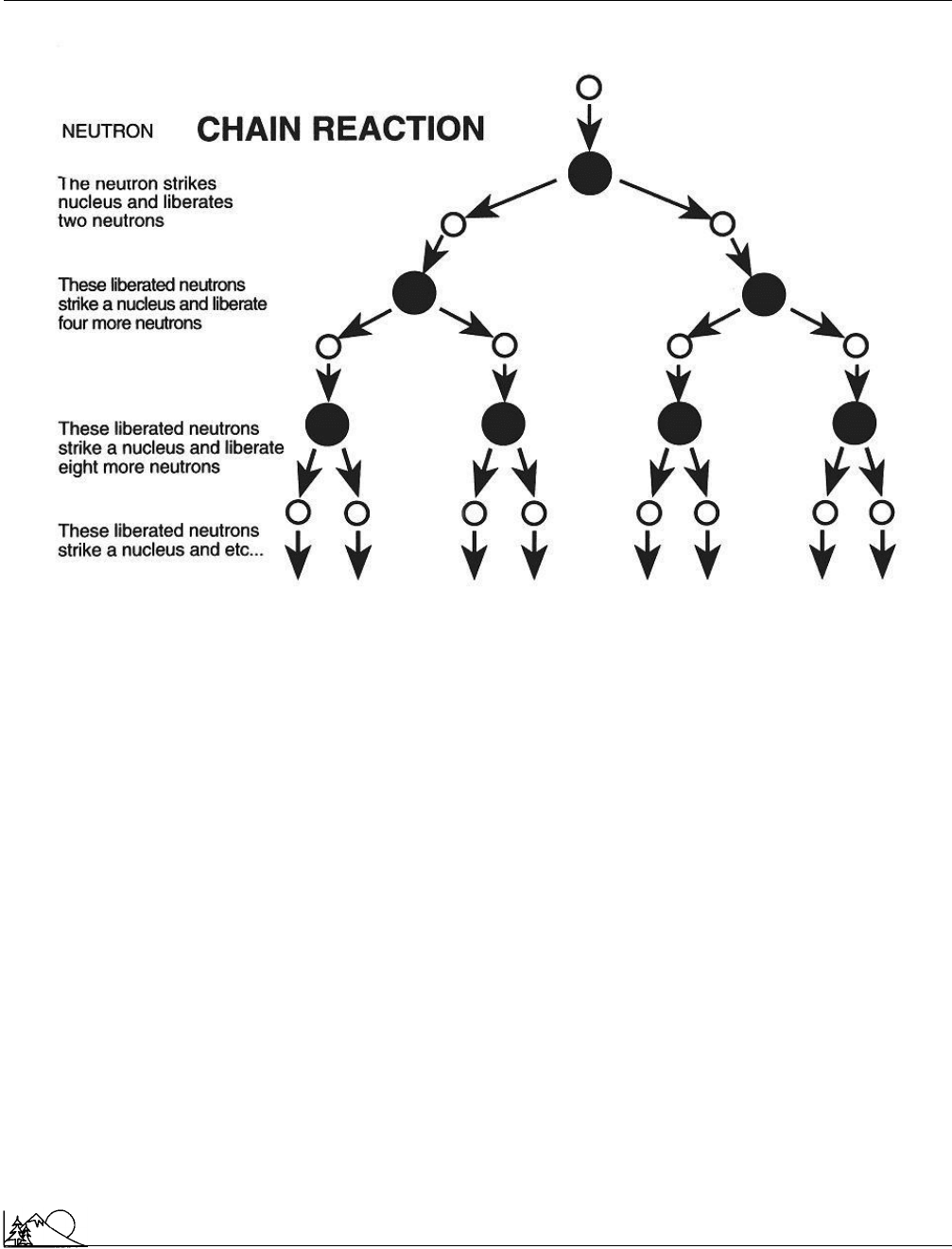
Chain reaction
A situation in which one action causes or initiates a similar
action. In a nuclear chain reaction, for example, a
neutron
strikes a uranium-235 nucleus, causing the nucleus to un-
dergo fission, which in turn produces a variety of products.
Among these products is one or more neutrons. Thus, the
particle needed to initiate this reaction (the neutron) is itself
produced as a result of the reaction. Once begun, the reaction
continues as long as uranium-235 nuclei are available. Nu-
clear chain reactions are important sources of fission and
fusion energy.
Chaparral
Chaparral is an ecological community consisting of drought-
resistant evergreen shrubs and small trees that are adapted
to long, hot, dry summers and mild, rainy winters. The
chaparral is found in five places on earth where there is a
warm land mass and a cool ocean: southern California, the

Environmental Encyclopedia 3
Chelate
Cape Town area of South Africa, the western tip of
Austra-
lia
, the west coast of South America, and the coastal areas
of the Mediterranean in southern Europe. Total annual pre-
cipitation ranges between 15 and 40 inches per year, while
annual temperatures range from 50–64.4 °F (10–18 °C).
Droughts and fires, which are often set by lightning during
the summer/autumn dry season, are common in the chapar-
ral. In fact, because the release of minerals occurs as a result
of fire, many chaparral plants grow best after a fire.
The chaparral may have many types of terrain, includ-
ing flat plains, rocky hills, and mountain slopes. The word
chaparral comes from the Spanish word chaparro, meaning
a dry thicket of oak shrubs.
The plants and animals that live in the chaparral are
adapted to the characteristic hot and dry climatic conditions.
Most of the plants are less than 10 ft tall and have leathery
leaves with thick cuticles that hold moisture. Many of the
shrub
flora
are aromatic, contain flammable oils, and are
adapted to periodic burns. Examples of chaparral plants
include poison oak, scrub oak, pine, manzanita, chamise,
yucca, and cacti. The animals are mainly grassland and
de-
sert
types, including coyotes, jack rabbits, mule deer, rattle-
snakes, mountain lions, kangaroo rats, foxes, bobcats, lizards,
horned toads, praying mantis, honey bees, and ladybugs.
[Judith L. Sims]
R
ESOURCES
B
OOKS
Collins, Barbara. Key to Coastal and Chaparral Flowering Plants of Southern
California. Third Edition. Dubuque, IA: Kendall/Hunt Publishing Com-
pany, 2000.
Ricciuti, Edward. R. Chaparral (Biomes of the World. Tarrytown, NY: Bench-
mark Books, 1996.
O
THER
Collins, Barbara J. Wildflowers of Southern California: Photographs of the
Chaparral. California Lutheran University, February 23, 2002. [cited May
27, 2002]. <http://ww1.clunet.edu/wf/>
Chelate
A chemical compound in which one atom is enclosed within
a larger cluster of atoms that surrounds it like an envelope.
The term comes from the Greek word chela, meaning claw.
Chelating agents—compounds that can form chelates with
other atoms—have a wide variety of environmental applica-
tions. For example, the compound ethylenediaminetetraace-
tic
acid
(EDTA) is used to remove
lead
from the blood.
EDTA molecules surround and bind to lead atoms, and the
chelate is then excreted in the urine. EDTA can also be
used to soften hard water by chelating the calcium and
magnesium ions that cause hardness.
230
Chelyabinsk, Russia
Chelyabinsk is the name of a province and its capital city
in west-central Russia. It covers an area of about 34,000 mi
2
(88,060 km
2
) and has a population of about 3.6 million.
Chelyabinsk city lies on the Miass River on the eastern side
of the Ural Mountains. Its population in 1990 was about
1.2 million.
Chelyabinsk is best known today as the home of
Mayak, a 77-mi
2
(200-km
2
) complex where
nuclear weap-
ons
were built for the former Soviet Union. Because of
intentional policy decisions and accidental releases of radio-
active materials, Mayak has been called the most polluted
spot on Earth.
Virtually nothing was known about Mayak by the
outside world, the Russian people, or even the residents of
Chelyabinsk themselves until 1991. Then, under the new
philosophy of glasnost, Soviet president Mikhail Gorbachev
released a report on the complex. It listed 937 official cases of
chronic
radiation sickness
among Chelyabinsk residents.
Medical authorities believe that the actual number is many
times larger.
The report also documented the secrecy with which
the Soviet government shrouded its environmental problems
at Mayak. Physicians were not even allowed to discuss the
cause or nature of the radiation sickness. Instead, they had
to refer to it as the “ABC disease.”
Chelyabinsk’s medical problems were apparently the
result of three major “incidents” involving the release of
radiation at Mayak. The first dated from the late 1940s
to the mid-1950s, when
radioactive waste
from nuclear
weapons research and development was dumped directly into
the nearby Techa River. People downstream from Mayak
were exposed to radiation levels 57 times greater than those
at the better-known Chernobyl accident in 1986. The Gor-
bachev report admitted that 28,000 people received radiation
doses of “medical consequence.” Astonishingly, almost no
one was evacuated from the area.
The second incident occurred in 1957, when a nuclear
waste dump at Mayak exploded with a force equivalent to
a five to 10 kiloton atomic bomb. The site had been con-
structed in 1953 as an alternative to simply disgorging radio-
active wastes into the Techa. When the automatic cooling
system failed, materials in the dump were heated to a temper-
ature of 662°F (350°C). In the resulting explosion, 20 million
curies of radiation were released, exposing 270,000 people
to dangerous levels of
radioactivity
. Neither the Soviet
Union nor the United States government, which had de-
tected the accident, revealed the devastation at Mayak.
The third incident happened in 1967. In their search
for ways to dispose of radioactive waste, officials at Mayak
decided in 1951 to use Lake Karachay as a repository. They

Environmental Encyclopedia 3
Chemical oxygen demand
realized that dumping into the Techa was not a satisfactory
solution, and they hoped that Karachay—which has no natu-
ral outlet—would be a better choice.
Unfortunately, radioactive materials began
leaching
into the region’s water supply almost immediately. Radiation
was eventually detected as far as 2 mi (3 km) away. The
1967 disaster occurred when an unusually dry summer di-
minished the lake significantly. A layer of radioactive mate-
rial, deposited on the newly exposed shoreline, was spread
by strong winds that blew across the area. This released
radiation equivalent to the amount contained in the first
atomic bomb explosion over Hiroshima.
[David E. Newton]
R
ESOURCES
P
ERIODICALS
Cochran, T. B., and R. S. Norris. “A First Look at the Soviet Bomb
Complex.” Bulletin of the Atomic Scientists 47 (May 1991): 25-31.
Hertsgaard, M. “From Here to Chelyabinsk.” Mother Jones 17 (January-
February 1992): 51-55+.
Perea, J. “Soviet Plutonium Plant ’Killed Thousands’.” New Scientist 134
(20 June 1992): 10.
Wald, M. L. “High Radiation Doses Seen for Soviet Arms Workers.” New
York Times (16 August 1990): A3.
Chemical bond
A chemical bond is any force of attraction between two
atoms strong enough to hold the atoms together for some
period of time. At least five primary types of chemical bonds
are known, ranging from very strong to very weak. They are
covalent, ionic, metallic, and
hydrogen
bonds, and London
forces.
In all cases, a chemical bond ultimately involves forces
of attraction between the positively-charged nucleus of one
atom and the negatively-charged electron of a second atom.
Understanding the nature of chemical bonds has practical
significance since the type of bonding found in a substance
explains to a large extent the macroscopic properties of that
substance.
An ionic bond is one in which one atom completely
loses one or more electrons to a second atom. The first atom
becomes a positively charged
ion
and the second, a negatively
charged ion. The two ions are attracted to each other because
of their opposite electrical charges.
In a covalent bond, two atoms share one or more pairs
of electrons. For example, a hydrogen atom and a fluorine
atom each donate a single electron to form a shared pair
that constitutes a covalent bond between the two atoms.
Both electrons in the shared pair orbit the nuclei of both
atoms.
231
In most cases, covalent and ionic bonding occur in
such a way as to satisfy the Law of Octaves. Essentially that
law states that the most stable configuration for an atom is
one in which the outer energy level of the atom contains
eight electrons or, in the case of smaller atoms, two electrons.
Ionic and covalent bonds might appear to represent
two distinct limits of electron exchange between atoms, one
in which electrons are totally gained and lost (ionic bonding)
and one in which electrons are shared (covalent bonding).
In fact, most chemical bonds fall somewhere between these
two extreme cases. In the hydrogen-fluorine example men-
tioned above, the fluorine nucleus is much larger than the
hydrogen nucleus and, therefore, exerts a greater pull on the
shared electron pair. The electrons spend more time in the
vicinity of the fluorine nucleus and less time in the vicinity
of the hydrogen nucleus. For this reason, the fluorine end
of the bond is more negative than the hydrogen end, and
the bond is said to be a polar covalent bond. A non-polar
covalent bond is possible only between two atoms with equal
attraction for electrons as, for example, between two atoms
of the same element.
Metallic bonds are very different from ionic and cova-
lent bonds in that they involve large numbers of atoms. The
outer electrons of these atoms feel very little attraction to
any one nucleus and are able, therefore, to move freely
throughout the metal.
Hydrogen bonds are very weak forces of attraction
between atoms with partial positive and negative charges.
Hydrogen bonds are especially important in living organisms
since they can be broken and reformed easily during bio-
chemical changes.
London forces are the weakest of chemical bonds.
They are forces of attraction between two uncharged mole-
cules. The force appears to arise from the temporary shift
of electrical charges within each molecule.
[David E. Newton]
R
ESOURCES
B
OOKS
Giddings, J. Calvin. Chemistry, Man, and Environmental Change: An Inte-
grated Approach. San Francisco: Canfield Press, 1973.
Chemical oxygen demand
Chemical oxygen demand (COD) is a measure of the ability
of chemical reactions to oxidize matter in an aqueous system.
The results are expressed in terms of oxygen so that they can
be compared directly to the results of
biochemical oxygen
demand
(BOD) testing. The test is performed by adding
the oxidizing solution to a sample, boiling the mixture on

Environmental Encyclopedia 3
Chemical spills
a refluxing apparatus for two hours and then titrating the
amount of dichromate remaining after the refluxing period.
The titration procedure involves adding ferrous ammonium
sulfate (FAS), at a known normality, to reduce the remaining
dichromate. The amount of dichromate reduced during the
test—the initial amount minus the amount remaining at the
end—is then expressed in terms of oxygen. The test has
nothing to do with oxygen initially present or used. It is a
measure of the demand of a solution or suspension for a
strong oxidant. The oxidant will react with most organic
materials and certain inorganic materials under the condi-
tions of the test. For example, Fe
2+
and Mn
2+
will be oxidized
for Fe
3+
and Mn
4+
, respectively, during the test.
Generally, the COD is larger than the BOD exerted
over a five-day period (BOD
5
), but there are exceptions in
which
microbes
of the BOD test can oxidize materials that
the COD reagents cannot. For a raw, domestic
waste-
water
, the COD/BOD
5
ratio is in the area of 1.5–3.0/1.0.
Higher ratios would indicate the presence of toxic, non-
biodegradable or less readily
biodegradable
materials.
The COD test is commonly used because it is a rela-
tively short-term, precise test with few interferences. How-
ever, the spent solutions generated by the test are hazardous.
The liquids are acidic, and contain chromium, silver,
mer-
cury
, and perhaps other toxic materials in the sample tested.
For this reason laboratories are doing fewer or smaller COD
tests in which smaller amounts of the same reagents are used.
[Gregory D. Boardman]
R
ESOURCES
B
OOKS
Corbitt, R. A. Standard Handbook of Environmental Engineering. New York:
McGraw-Hill, 1990.
Davis, M. L., and D. A. Cornwell. Introduction to Environmental Engi-
neering. New York: McGraw-Hill, 1991.
Peavy, H. S., D. R. Rowe, and G. Tchobanoglous. Environmental Engi-
neering. New York: McGraw-Hill, 1985.
Tchobanoglous, G., and E. D. Schroeder. Water Quality. Reading, MA:
Addison-Wesley, 1985.
Viessman, W., Jr., and M. J. Hammer. Water Supply and Pollution Control.
5th ed. New York: Harper Collins, 1993.
Chemical spills
Chemical spills are any accidental releases of synthetic
chem-
icals
that pose a risk to the
environment
.
Spills occur at any of the steps between the production
of a chemical and its use. A railroad tank car may spring a
leak; a pipe in a manufacturing plant may break; or an
underground storage tank may corrode allowing its contents
to escape into
groundwater
. These spills are often classified
into four general categories: the release of a substance into
232
a body of water; the release of a liquid on land; the release
of a solid on land; and the release of a gas into the
atmo-
sphere
. The purpose of this method of classification is to
provide the basis for a systematic approach to the control
of any type of chemical spill.
Some of the most famous chemical spills in history illus-
trate these general categories. For example, seven cars of a
train carrying the
pesticide
metam sodium fell off the tracks
near Dunsmuir, California, in August 1991, breaking open
and releasing the chemicals into the Sacramento River. Plant
and aquatic life for 43 mi (70 km) downriver died as a result
of the accident. The pesticide eventually formed a band 225
ft (70 m) wide across Lake Shasta before it could be contained.
In 1983, the
Environmental Protection Agency
(EPA) purchased the whole town of
Times Beach
, Missouri,
and relocated more than 2,200 residents because the land
was so badly contaminated with highly toxic dioxins. The
concentration of these compounds, a by-product of the pro-
duction of herbicides, was more than a thousand times the
maximum recommended level.
In December 1984, a cloud of poisonous gas escaped
from a Union Carbide chemical plant in
Bhopal, India
. The
plant produced the pesticide Sevin from a number of chemi-
cals, many of which were toxic. The gas that accidentally es-
caped probably contained a highly toxic mixture of phosgene,
methyl isocyanate (MIC),
chlorine
,
carbon monoxide
, and
hydrogen
cyanide, as well as other hazardous gases. The
cloud spread over an area of more than 15 mi
2
(40 km
2
),
exposing more than 200,000 people to its dangers.
Chemists have now developed a sophisticated approach
to the treatment of chemical spills, which involves one or more
of five major steps: containment, physical treatment, chemical
treatment, biological treatment, and disposal or destruction.
Soil
sealants, which can be used to prevent a liquid from sink-
ing into the ground, are an example of containment. One of
the most common methods of physical treatment is activated
charcoal, because it has the ability to adsorb toxic substances
on its surface, thus removing them from the environment.
Chemical treatment is possible because many hazardous ma-
terials in a spill can be treated by adding some other chemical
that will neutralize them, and biological treatment usually in-
volves
microorganisms
that will attack and degrade a toxic
chemical. Open burning,
deep-well injection
, and burial in
a
landfill
are all methods of ultimate disposal.
[David E. Newton]
R
ESOURCES
B
OOKS
Unterberg, W., et al. How to Respond to Hazardous Chemical Spills. Park
Ridge, N.J.: Noyes Data Corporation, 1988.

Environmental Encyclopedia 3
Chemicals
Chemicals
The general public often construes the word “chemical” to
mean a harmful synthetic substance. In fact, however, the
term applies to any element or compound, either natural or
synthetic. The thousands of compounds that make up the
human body are all chemicals, as are the products of scientific
research. A more accurate description, however, can be found
in the dictionary. Thus, aspirin is a chemical by this defini-
tion, since it is the product of a series of chemical reactions.
The story of chemicals began with the rise of human
society. Indeed, early stages of human history, such as the
Iron,
Copper
, and Bronze Ages reflect humans’ ability to
produce important new materials. In the first two eras, peo-
ple learned how to purify and use pure metals. In the third
case, they discovered how to combine two to make an alloy
with distinctive properties.
The history of ancient civilizations is filled with exam-
ples of men and women adapting
natural resources
for
their own uses. Egyptians of the eighteenth dynasty (1700–
1500
B.C.
), for example, knew how to use cobalt compounds
to glaze pottery and glass. They had also developed tech-
niques for making and using a variety of dyes.
Over the next 3,000 years, humans expanded and im-
proved their abilities to manipulate natural chemicals. Then,
in the 1850s, a remarkable breakthrough occurred. A discov-
ery by young British scientist William Henry Perkin led to
the birth of the synthetic chemicals industry.
Perkin’s great discovery came about almost by accident,
an occurrence that was to become common in the synthetics
industry. As an 18-year-old student at England’s Royal Col-
lege of Chemistry, Perkin was looking for an artificial com-
pound that could be used as a quinine substitute. Quinine,
the only drug available for the treatment of
malaria
, was
itself in short supply.
Following his teacher’s lead, Perkin carried out a num-
ber of experiments with compounds extracted from
coal
tar,
the black, sticky
sludge
obtained when coal is heated in
insufficient air. Eventually, he produced a black powder
which, when dissolved in alcohol, created a beautiful purple
liquid. Struck by the colorful solution, Perkin tried dyeing
clothes with it.
His efforts were eventually successful. He went on to
mass produce the synthetic dye—mauve, as it was named—
and to create an entirely new industry. The years that fol-
lowed are sometimes referred to as The Mauve Decade
because of the many new synthetic products inspired by
Perkin’s achievement. Some of the great chemists of that
era have been memorialized in the names of the products
they developed or the companies they established: Adolf von
Baeyer (Bayer aspirin), Leo Baekeland (Baekelite plastic),
Eleuthe
`
re Ire
´
ne
´
e du Pont (DuPont Chemical), George East-
233
man (Eastman 910 adhesive and the Eastman Kodak Com-
pany), and Charles Goodyear (Goodyear
Rubber
).
Chemists soon learned that from the gooey, ugly by-
products of coal tar, a whole host of new products could be
made. Among these products were dyes, medicines, fibers,
flavorings,
plastics
, explosives, and
detergents
. They found
that the other fossil fuels—petroleum and natural gas—could
also produce synthetic chemicals.
Today, synthetic chemicals permeate our lives. They
are at least as much a part of the
environment
, if not more,
than are natural chemicals. They make life healthier, safer,
and more enjoyable. People concerned about the abundance
of “chemicals” in our environment should remember that
everyone benefits from anti-cancer drugs, pain-killing anes-
thetics, long-lasting fibers, vivid dyes, sturdy synthetic rubber
tires, and dozens of other products. The world would be a
much poorer place without them.
Unfortunately, the production, use, and disposal of
synthetic chemicals can create problems because they may
be persistent and/or hazardous. Persistent means that a sub-
stance remains in the environment for a long time: dozens,
hundreds, or thousands of years in many cases. Natural prod-
ucts such as wood and paper degrade naturally as they are
consumed by
microorganisms
. Synthetic chemicals, how-
ever, have not been around long enough for such microor-
ganisms to evolve.
This leads to the familiar problem of
solid waste
disposal. Plastics used for bottles, wrappings, containers, and
hundreds of other purposes do not decay. As a result, landfills
become crowded and communities need new places to dump
their trash.
Persistence is even more of a problem if a chemical
is hazardous. Some chemicals are a problem, for example,
because they are flammable. More commonly, however, a
hazardous chemical will adversely affect the health of a plant
or animal. It may be (1) toxic, (2) carcinogenic, (3) terato-
genic, or (4) mutagenic.
Toxic chemicals cause people, animals, or plants to
become ill, develop a disease, or die. DDT,
chlordane
,
heptachlor, and aldrin are familiar, toxic pesticides. Carcino-
gens cause
cancer
; teratogens produce
birth defects
. Muta-
gens, perhaps the most sinister of all, inflict genetic damage.
Determining these effects can often be very difficult.
Scientists can usually determine if a chemical will harm or
kill a person. But how does one determine if a chemical
causes cancer twenty years after exposure, is responsible for
birth defects, or produces genetic disorders? After all, any
number of factors may have been responsible for each of
these health problems.
As a result, labeling any specific chemical as carcino-
genic, teratogenic, or mutagenic can be difficult. Still, envi-

Environmental Encyclopedia 3
Chemosynthesis
ronmental scientists have prepared a list of synthetic chemi-
cals that they believe fall into these categories. Among them
are
vinyl chloride
, trichloroethylene,
tetrachloroethylene
,
the nitrosamines, and chlordane and heptachlor.
Another class of chemicals are hazardous because they
may contribute to the
greenhouse effect
and
ozone layer
depletion
. The single most important chemical in determin-
ing the earth’s annual average temperature is a naturally-
occurring compound,
carbon dioxide
. Its increased produc-
tion is believed to be responsible for a gradual increase in
the planet’s annual average temperature.
But synthetic compounds may also play a role in global
warming.
Chlorofluorocarbons
(CFCs) are widely used in
industry because of their many desirable properties, one of
which is their chemical
stability
. This very property means,
however, that when released into the
atmosphere
, they
remain there for many years. Since they capture heat radiated
from the earth in much the way
carbon
dioxide does, they
are probably important contributors to global warming.
These same chemicals, highly unreactive on earth, de-
compose easily in the upper atmosphere. When they do so,
they react with the
ozone
in the
stratosphere
, converting
it to ordinary oxygen. This may have serious consequences,
since stratospheric ozone shields the earth from harmful
ultraviolet radiation
.
There are two ways to deal with potentially hazardous
chemicals in the environment. One is to take political or
legal action to reduce the production, limit the use, and/or
control the disposal of such products. A treaty negotiated
and signed in Montreal by more than forty nations in 1987,
for example, calls for a gradual ban on CFC production. If
the treaty is honored, these chemicals will eventually be
phased out of use.
A second approach is to solve the problem scientifi-
cally. Synthetic chemicals are a product of scientific research,
and science can often solve the problems these chemicals
produce. For example, scientists are exploring the possibility
of replacing CFCs with related compounds called fluorocar-
bons (FCs) or hydrochloroflurocarbons (HCFCs). Both are
commercially appealing, but they have fewer harmful effects
on the environment.
[David E. Newton]
R
ESOURCES
B
OOKS
Giddings, J. Calvin. Chemistry, Man, and Environmental Change: An Inte-
grated Approach. San Francisco: Canfield Press, 1973.
Joesten, M. D., et al. World of Chemistry. Philadelphia: Saunders College
Publishing, 1991.
Newton, David E. The Chemical Elements. New York: Franklin Watts, 1994.
234
Chemosynthesis
Chemosynthesis is a metabolic pathway used by some bacte-
ria to synthesize new organic compounds such as carbohy-
drates by using energy derived from the oxidation of inor-
ganic molecules—hydrogen sulfide (H
2
S) or ammonia
(NH
3
). Chemosynthesis can occur in environments such as
the deep ocean around
hydrothermal vents
, where sunlight
does not penetrate, but where
chemicals
like
hydrogen
sulfide are available. Chemosynthesis is also a critical part
of the
nitrogen cycle
, where bacteria that live in the
soil
,
or in special plant structures called heterocysts, utilize ammo-
nia for energy and produce
nitrates and nitrites
which can
subsequently be used as nutrients for plants. Some bacteria
can also utilize hydrogen gas (H
2
) and
carbon dioxide
(CO
2
) in a chemosynthetic pathway that results in the pro-
duction of new organic compounds and
methane
(CH
4
).
[Marie H. Bundy]
Chernobyl nuclear power station
On April 26, 1986, at precisely 1:24
A.M.
, the Chernobyl
nuclear power
plant exploded, releasing large amounts of
radioactivity
into the
environment
. The power station is
located 9 mi (14.5 km) northwest of the town of Chernobyl,
with a population of 12,500, and less than 2 mi (3.2 km)
from the town of Pripyat, which contains 45,000 inhabitants.
The explosion and its aftermath, including the manner in
which the accident was handled, have raised questions about
the safety and future of nuclear power.
The Chernobyl accident resulted from several factors:
flaws in the engineering design, which were compensated
by a strict set of procedures; failure of the plant management
to enforce these procedures; and finally the decision of the
engineers to conduct a risky experiment. They wanted to
test whether the plant’s turbine generator—from its rotating
inertia—could provide enough power to the reactor in case
of a power shutdown. This experiment required discon-
necting the reactor’s emergency core cooling pump and other
safety devices.
The series of critical events, as described by Richard
Mould in Chernobyl, The Real Story, are as follows: At 1:00
A.M.
on April 25, power reduction was started in preparation
for the experiment. At 1:40
A.M.
the reactors’s emergency
core cooling system was turned off. At 11:10
P.M.
power
was further reduced, resulting in a nearly unmanageable
situation. At 1:00
A.M.
on April 26, power was increased in
an attempt to stabilize the reactor; however, cooling pumps
were operating well beyond their rated capacity, causing a
reduction in steam generation and a fall in stream pressure.
By 1:19
A.M.
, the water in the cooling circuit had approached

Environmental Encyclopedia 3
Chernobyl nuclear power station
the boiling point. At 1:23
A.M.
, the operators tried to control
the reaction by manually pushing control rods into the core;
however, the rods did not descend their full length into the
reactor since destruction of the graphite core was already
occurring. In 4.5 seconds, the power level rose two thou-
sandfold. At 1:24
A.M.
, there was an explosion when the hot
reactor fuel elements, lacking enough liquid for cooling,
decomposed the water into
hydrogen
and oxygen. The
generated pressures blew off the 1,000-ton concrete roof of
the reactor, and burning graphite, molten
uranium
, and
radioactive ashes spilled out to the
atmosphere
.
The explosion that occurred was not a nuclear explo-
sion such as would occur with an atomic bomb but its effects
were just as devastating. In order to put the expulsion of
radioactive material from the Chernobyl reactor into per-
spective, almost 50 tons of fuel went into the atmosphere
plus an additional 70 tons of fuel, and 700 tons of radioactive
reactor graphite settled in the vicinity of the damaged unit.
Some 50 tons of nuclear fuel and 800 tons of reactor graphite
remained in the reactor vault, with the graphite burning up
completely in the next several days after the accident. The
amount of radioactive material that went into the atmosphere
was equivalent to 10 Hiroshima bombs.
Officials at first denied that there had been a serious
accident at the power plant. The government in Moscow
was led to believe for several hours after the explosion and
fire at Chernobyl that the reactor core was still intact. This
delayed the evacuation for a critical period during which
local citizens were exposed to high radiation levels. The
evacuation of Chernobyl and local villages was spread out
over eight days. A total of 135,000 persons were evacuated
from the area, with the major evacuation at Pripyat starting
at 2:00
P.M.
, the day after the explosion. Tests showed that
air, water, and
soil
around the plant had significant contami-
nation. Children, in particular, were a matter of concern and
were evacuated to the southern Ukraine, the Crimea, and
the Black Sea coast.
At the time of the accident, and for several days there-
after, the winds carried the
radioactive waste
to the north.
The radioactive cloud split into two lobes, one spreading
west and then north through Poland, Germany, Belgium,
and Holland, and the other through Sweden and Finland.
By the first of May, the wind direction changed and the
radioactive fallout—at a diminished rate—went south over
the Balkans and then west through Italy. Large areas of
Europe were affected, and many farmers destroyed their
crops for fear of contamination. Forests have been cleared
and large amounts of earth were removed in order to clean
up radioactivity. Plastic film has been laid in some areas in
an effort to contain radioactive dust.
Officially 31 persons were reported to have been killed
at the reactor site by a combination of the explosion and
235
radiation exposure
; another 174 were exposed to high
doses of radiation which resulted in
radiation sickness
and long-term illnesses. The maximum permissible dose of
radiation for a nuclear power operator is 5 roentgens per
year and for the rest of the population, 0.5 roentgens per
year. At the Chernobyl plant, the levels of radiation ranged
from 1,000 to 20,000 roentgens per hour. One British report
estimates that worldwide, the number of persons afflicted
with
cancer
which can be attributed to the Chernobyl acci-
dent will be about 2,300. Others argued that the number
will be much higher. In Minsk, the rate of
leukemia
has
more than doubled from 41 per million in 1985 to 93 per
million in 1990.
Many heroic deeds were reported during this emer-
gency. Fire fighters exposed themselves to deadly radiation
while trying to stop the inferno. Every one eventually died
from radiation exposure. Construction workers volunteered
to entomb the reactor ruins with a massive concrete sarcoph-
agus. Bus drivers risked further exposure by making repeated
trips into contaminated areas in order to evacuate villagers.
Over 600,000 people were involved in the decontamination
and clean up of Chernobyl. The health effects on them from
their exposure are not completely known. The Chernobyl
accident focused international attention on the risks associ-
ated with operating a nuclear reactor for the generation of
power. Public apprehension has forced some governments
to review their own safety procedures and to compare the
operation of their nuclear reactors with Chernobyl’s. In a
review of the Chernobyl accident by the Atomic Energy
Authority of the United Kingdom, an effort was made to
contrast the design of the Chernobyl reactor and manage-
ment procedures with those in practice in the United States
and the United Kingdom.
Three design drawbacks were noted of the Chernobyl
nuclear power plant:
O
The reactor was intrinsically unstable below 20% power
and never should have been operated in that mode. (U.S.
and UK reactors do not have this design flaw).
O
The shut-down operating system was inadequate and con-
tributed to the accident rather than terminating it. (U.S.
and UK control systems differ significantly).
O
There were no controls to prevent the staff from operating
the reactor in the unstable region or preventing the disa-
bling of existing safeguards.
In addition, the Chernobyl management had no effec-
tive watchdog agency to inspect procedures and order closure
of the facility. Also in years prior to the accident there
was a lack of information given the public of prior nuclear
accidents, typical of the press censorship and news manage-
ment occurring in the period before glasnost. The operators
were not adequately trained nor were they themselves fully
aware of prior nuclear power accidents or near accidents

Environmental Encyclopedia 3
Chesapeake Bay
Chernobyl Nuclear Power Station. Lighter areas of the buidling are part of the original structure, while darker
areas are the steel and concrete “sarcophagus” that was added to contain radioactivity leaking from the faulty
reactor. (Corbis-Bettmann. Reproduced by permission.)
which would have made them more sensitive to the dangers
of a runaway reactor system.
Unfortunately in the former Soviet block nations there
are several nuclear reactors that are potentially as hazardous
as Chernobyl but which must continue operation to maintain
power requirements; however, the operational procedures
are under constant review to avoid another accident. Clearly
the Western world will have to assist the former Soviet block
to bring reactor operating equipment and standards up to a
much higher level of safety to avoid a similar and possibly
more disastrous accident.
[Malcolm T. Hepworth]
R
ESOURCES
B
OOKS
Feshbach, M., and A. Friendly Jr. Ecocide in the USSR. New York: Basic
Books, 1992.
Fusco, Paul, and Magdalena Caris. Chernobyl Legacy. de.MO, 2001.
Medvedev, G. No Breathing Room: The Aftermath of Chernobyl. New York:
Basic, 1993.
236
Mould, R. E. Chernobyl: The Real Story. New York: Pergamon, 1988.
Chesapeake Bay
The Chesapeake Bay is the largest
estuary
(186 mi [300
km] long) in the United States. The Bay was formed 1500
years ago by the retreat of glaciers and the subsequent sea
level rise that inundated the lower Susquehanna River valley.
The Bay has a
drainage
basin of 64,076 square miles
(166,000 sq km) covering six states and running through
Pennsylvania, Maryland, the District of Columbia, and Vir-
ginia before entering the Atlantic Ocean. While 150 rivers
enter the Bay, a mere eight account for 90% of the freshwater
input, with the Susquehanna alone contributing nearly half.
Chesapeake Bay is a complex system, composed of numerous
habitats and environmental gradients.
Chesapeake Bay’s abundant
natural resources
at-
tracted native Americans, first settling on its shores. The
first European record of the Bay was in 1572 and the area
