Fowler A. Mathematical Geoscience
Подождите немного. Документ загружается.


420 7 Groundwater Flow
The local block equation (7.165)
1
rewritten in the block variables
X =
x
ε
,T=
t
ε
, (7.169)
is then
εPe
∂c
m
∂T
+u
m
.∇
X
c
m
=∇
2
X
c
m
−ε
2
PeΛc
m
. (7.170)
What is obvious is that all the terms cannot balance in both local and global prob-
lems, and this leads to simplifications. The simplest case is where the macroscopic
Péclet number and reaction number Λ are both taken to be O(1); then (7.167) sug-
gests that in (7.170) we put
c
m
=¯c(x) +ε
2
˜c
m
(X) (7.171)
(and thus also c
f
=¯c(x) +ε
2
˜c
f
(X)); both inner and outer problems are well scaled,
and the advective term can be neglected in solving the inner problem, which in fact
reduces approximately to Poisson’s equation. Specifically, using multiple scales in
both x and t, we have at leading order in ε,
∇
2
X
˜c
m
=Pe
∂ ¯c
∂t
+u
m
.∇
x
¯c
+PeΛ ¯c −∇
2
x
¯c (7.172)
subject to ˜c
m
=˜c
f
on ∂M and conditions of periodicity on M. (If we integrate
(7.172) over M, we regain the averaged equation (7.164) as an integrability condi-
tion for (7.172), as expected via homogenisation.)
In a similar way, the leading order local equation for the fracture perturbed con-
centration ˜c
f
is
∇
2
X
˜c
f
−
(1 −φ
f
)
φ
f
∂ ˜c
m
∂N
∂M
=Pe
∂ ¯c
∂t
+u
f
.∇
x
¯c
+PeΛ ¯c −∇
2
x
¯c (7.173)
(note that a term
(1−φ
f
)
εφ
f
∂ ¯c
∂n
|
∂M
∝∇
x
¯c.n|
∂M
is identically zero, because the normals
n on the two faces of a fracture cancel each other). ˜c
f
satisfying (7.173) is subject to
periodicity in ∂M and zero mean. As for the fluid flow, we can solve (7.172)using
a Green’s function for the Laplacian in M, so that the boundary derivative term
in (7.173) becomes an integral convolution in terms of ˜c
f
. We then solve (7.173)
using a Green’s function for the Laplacian on ∂M. This allows us to determine the
homogenised equation for ¯c (see Question 7.13).
In fact, it is rarely the case that Pe and Λ are O(1): more commonly they are both
large. Suppose for example that ε = 10
−4
, Pe = 10
4
, Λ = 10
4
: not unreasonable
values (as we shall see below). Putting
p =εPe,λ=εΛ, (7.174)

7.7 Environmental Remediation 421
it seems natural to take λ ∼ p ∼O(1). The block problem becomes (in terms of T
and X only)
p
∂c
m
∂T
+u
m
.∇
X
c
m
=∇
2
X
c
m
−λp c
m
, (7.175)
which implies that reaction occurs on the block scale. This is a linear equation for c
m
which can in principle be solved to give the boundary flux term in the c
f
equation
as a convolution integral in terms of c
f
. In a strict sense, this distinguished limit
describes the structure of a reaction front in which both reaction and dispersion are
important. Outside this front, reaction and dispersion are negligible, and the reactant
simply advects with the flow.
7
Importantly, it implies that the speed of the front is
determined by the local diffusion and reaction within the block.
If Λ is even larger than this, so that λ 1, then the reaction is fast at the block
scale, and occurs in a thin rind within the block; for a single species, as here, this
rind must be on the boundary, as the interior reactant concentration reaches zero
rapidly. Bearing in mind that the normal coordinate n at ∂M points into the fracture,
the boundary layer solution for c
m
is just
c
m
≈c
f
exp
(λp)
1/2
n
, (7.176)
and thus the flux term in the local fracture equation derived from (7.165)
2
is
−
1
φ
f
∂c
m
∂n
∂M
=−
1
φ
f
(λp)
1/2
c
f
. (7.177)
Hence c
f
satisfies the local equation
∇
2
X
c
f
−
1
φ
f
(λp)
1/2
c
f
=p
∂c
f
∂T
+u
f
.∇
X
c
f
+λp c
f
. (7.178)
As is perhaps obvious, the reaction is fast in the fractures also and c
f
rapidly ap-
proaches zero.
7.7 Environmental Remediation
Environmental pollution is now an endemic phenomenon. All over every industri-
alised country, spills of hydrocarbons, effluents, and industrial waste have caused
pollution of underlying groundwater. The resulting ‘plumes’ move slowly with the
prevailing background groundwater flow, and in course of time will enter streams,
rivers and lakes, with consequent health risk via the contamination of drinking wa-
ter. Short of more drastic measures such as pumping out polluted groundwater and
7
Actually, this only makes sense for multi-species reactions of the form A +B →P ,forthenthe
reaction rate ∝AB, and can be zero by virtue of A = 0 on one side of the front, and B =0onthe
other.

422 7 Groundwater Flow
treating it, natural bioremediation anticipates that microbial action will eventually
break down most pollutants, rendering them harmless. The issue for the environ-
mental scientist is to predict the future movement of the plume, and the likelihood
of microbial breakdown before it reaches drinking water sources.
In so doing, groundwater flow modelling is essential, because of its ability to
predict into the future, and also because accurate monitoring of subsurface contam-
ination is expensive and not straightforward. Against this, subsurface soil and rock
is usually an extremely heterogeneous medium, both physically and chemically, and
the validation of computational results is difficult.
7.7.1 Reactive Groundwater Flow
The general context of many subsurface pollution problems of concern is similar,
and we therefore begin with some generalities. Contaminants may be aqueous, in
which case they mix with the groundwater, or non-aqueous, in which case they
do not. Hydrocarbons, for example, are non-aqueous. Amongst the non-aqueous
phase liquids (NAPLs), one distinguishes dense liquids (DNAPLs) from light ones
(LNAPLs). DNAPLs will sink into the saturated zone, whereas LNAPLs, such as
hydrocarbons, will sink to the base of the unsaturated zone, and there sit on the
water table, from where their constituents may diffuse downwards.
The contaminant plume will typically consist of a cocktail of different chemicals,
which flow with the local groundwater flow, disperse within it, and react with oxy-
gen and other substances in the soil via the agency of microbial action. The typical
sort of model of concern is thus the reaction-advection-dispersion equation
R
∂c
∂t
+∇.(cu) =∇.(D .∇c) +S, (7.179)
where c is one of a sequence of reactants, u is the local groundwater flux (given by
Darcy’s law), D represents dispersion, and is typically anisotropic, in the sense that
dispersion in the longitudinal direction is larger than lateral dispersion. Dispersion
itself is partly due to molecular diffusion, but more importantly (at high grain scale
Péclet number) is due to grain scale shear-induced distortion of the fluid associated
with Taylor dispersion, together with remixing at pore junctions. Typically, the lon-
gitudinal dispersion coefficient D
∼ d
p
|u|, where d
p
is grain size, while lateral
dispersion D
⊥
is a factor of 10–100 smaller.
The coefficient R is the retardation factor, and it is a slowing rate due to the ad-
sorption of aqueous phase concentration on solid particles. Specifically, we actually
have two separate conservation laws for solid and aqueous concentrations c
s
and c
l
,
respectively, thus
∂c
l
∂t
+∇.(c
l
u) =∇.(D.∇c
l
) +S +k
d
c
s
−k
a
c
l
,
∂c
s
∂t
=−k
d
c
s
+k
a
c
l
,
(7.180)

7.7 Environmental Remediation 423
where k
d
and k
a
are desorption and adsorption rates, respectively. (7.180) specifi-
cally assumes that the reaction occurs only in the aqueous phase, by way of example.
Now the point is that if the sorption rates are very large (and constant), then the
second equation in (7.180) tells us that
c
s
≈
k
a
c
l
k
d
, (7.181)
and the sum of the two equations then gives us (7.179), with the retardation factor
R =1 +
k
a
k
d
. (7.182)
The source term S represents reaction driven sources and sinks. It is typically
the case that there are many, many reactions and reactants. Equally typically, many
of the reaction rates are not well known, and the rates may be widely disparate. In
general this will imply that many reactions can be taken to be in equilibrium, with
only the slowest (rate-controlling) reaction being of dynamical importance.
7.7.2 Biomass Modelling
The reaction terms S in (7.180) are mediated by bacteria within soil, which consume
the various nutrients provided through metabolic reactions. Like all living things,
microbes survive by generating energy from nutrients through a variety of such re-
actions. This process involves a network of redox (oxidation–reduction) reactions,
and involves the overall exchange of electrons between two distinct chemical fuels
which are consumed in the reactions; the metabolic process is in this case called
respiration. While there may be a number of such fuels, there is a hierarchy in their
use. Dissolved oxygen is commonly the terminal electron acceptor (as the exter-
nally sourced oxidant is typically referred to), while an organic carbon compound
is the electron donor. When these preferred substrates are absent or depleted, other
compounds can be used instead. When the same organic compound is used as both
donor and acceptor, the metabolic process is called fermentation.
Many bacteria are able to use several reaction pathways independently, giving
them a degree of flexibility to differing conditions. This capability is very species-
dependent, and competition ensures that the species which are best adapted to
local conditions become dominant. Microbial growth depends heavily on energy
metabolism but also requires the uptake of other substrates needed to generate new
biomass. Growth rate is generally limited by the supply of one or more substrates,
but saturates to a maximum growth rate in conditions of ample supply. The depen-
dence of bacterial growth rate is commonly taken, by analogy with simple enzyme
kinetic uptake rates, as proportional to
c
K+c
, where c is the relevant nutrient con-
centration, and K is a constant; such kinetics are called Monod kinetics. When two

424 7 Groundwater Flow
nutrients control growth, as in respiration, it is usual to take the growth rate as pro-
portional to the product of two Monod factors, thus (for example)
S =−r
0
X
c
1
K
1
+c
1
c
2
K
2
+c
2
, (7.183)
in which r
0
is a reaction rate constant, and X is the biomass density.
More complex models also consider the growth of the microbial population X
in terms of the nutrient supply, in particular in the form of a microbial mat which
becomes attached to the soil grains, and usually called a biofilm. In particular, the
concept of biodegradation, for example of oil spills in sea water, or of factory efflu-
ent in groundwater, commonly involves the development of bacterial colonies which
are able to efficiently use the offending contaminant to promote their own growth.
7.7.3 Non-dimensionalisation
It is commonly the case that the water table is at a depth of 10–20 metres, whereas a
plume may have spread (or we are concerned with whether it will spread) a distance
of order kilometres. Therefore these plumes generally have high aspect ratio, a cause
both of computational stress and analytical simplification. The latter may be offset
by the decreased lateral dispersion coefficient, as we now show.
Let us consider the scalar contaminant equation (7.179) in two dimensions, with
horizontal and vertical coordinates x and z, corresponding Darcy fluxes u and w,
and longitudinal (horizontal) and transverse (vertical) dispersivities D
and D
⊥
,
which we take to be constant. We suppose l and d are suitable horizontal and vertical
length scales, U is a horizontal Darcy flux scale (and therefore mass conservation
implies that hU/ l is a suitable vertical flux scale), Rl/U is then the convective time
scale, and we take S
0
to be a measure of the reaction rate term, and c
0
to be a typical
contaminant concentration. The units of concentration are mol l
−1
(moles per litre),
and the units of S
0
are mol l
−1
s
−1
.
We define dimensionless variables by writing
u =Uu
∗
,w=
hU
d
w
∗
,c=c
0
c
∗
,t=
Rl
U
t
∗
,
x =lx
∗
,z=dz
∗
, S =S
0
S;
(7.184)
substituting these into (7.179) (and forthwith dropping the asterisks), we obtain the
dimensionless equation
∂c
∂t
+u
∂c
∂x
+w
∂c
∂z
=
1
Pe
∂
2
c
∂x
2
+
1
Pe
⊥
∂
2
c
∂z
2
+ΛS, (7.185)
where
Pe
=
Ul
D
, Pe
⊥
=
Ud
2
D
⊥
l
,Λ=
S
0
l
Uc
0
. (7.186)
7.8 Three Specific Remediation Problems 425
The aspect ratio d/l and transverse dispersivity ratio D
⊥
/D
compete against
each other, and generally we might suppose Pe
⊥
∼Pe
; but also if D
∼d
p
U , then
we can expect that usually Pe
1, heralding the existence of thin boundary layers
in which dispersion is effective. The parameter Λ is the ratio of advection time
to reaction time, and will often be very large for microbially mediated reactions
of interest. These two observations cause numerical difficulties in solving (7.186),
but can aid analytical insight. In the following section, we discuss three specific
groundwater contamination problems of recent concern.
7.8 Three Specific Remediation Problems
Four Ashes Four Ashes is a site in the Midlands of England where a fifty year
old plume of phenol (C
6
H
6
0) and other contaminants, about 500 metres long, lies
within the saturated zone. The plume is thought to lie between depths of 10 m and
30 m at 130 m from the source, but is sinking as it moves west (perhaps because
of surface recharge), reaching depths 21–44 m at 350 m distance (this information
comes from two boreholes drilled at the site). Degradation is very slow: perhaps
only 5% of the total contaminant load has so far been degraded. This is thought
to be due to toxicity of the phenol within the plume, and to supply limitation of
electron acceptors (oxygen and nitrate) at the plume fringe.
The overall reaction scheme which is considered to apply is that the phenol is
oxidised at the plume boundary by oxygenated groundwater, producing TIC (to-
tal inorganic carbon). Within the plume, phenol is fermented to produce hydrogen
amongst other things, which then reduces various oxides within the soil via mi-
crobial agency. A suitable set of reactions to describe the situation consists of the
following:
C
6
H
6
O +7O
2
+3H
2
O
r
1
→6HCO
−
3
+6H
+
,
C
6
H
6
O +5.6NO
−
3
+0.2H
2
O
r
2
→6HCO
−
3
+0.4H
+
+2.8N
2
,
C
6
H
6
O +5H
2
O
r
3
→3CH
3
COOH +2H
2
,
C
6
H
6
O +17H
2
O
r
4
→6HCO
−
3
+6H
+
+14H
2
,
H
2
+MnO
2(s)
+2H
+
r
5
→2H
2
O +Mn
2+
,
H
2
+2FeOOH
(s)
+4H
+
r
6
→4H
2
O +2Fe
2+
,
H
2
+SO
2−
4
+0.25H
+
r
7
→H
2
O +0.25HS
−
,
H
2
+CO
2−
3
+0.5H
+
r
8
→0.75H
2
O +0.25CH
4
,
(7.187)
and r
1
–r
8
are the reaction rates, defined using Monod kinetics. In terms of the reac-
tion rates, the source term in each reactant equation is then
S
j
=
l
s
jl
r
l
, (7.188)
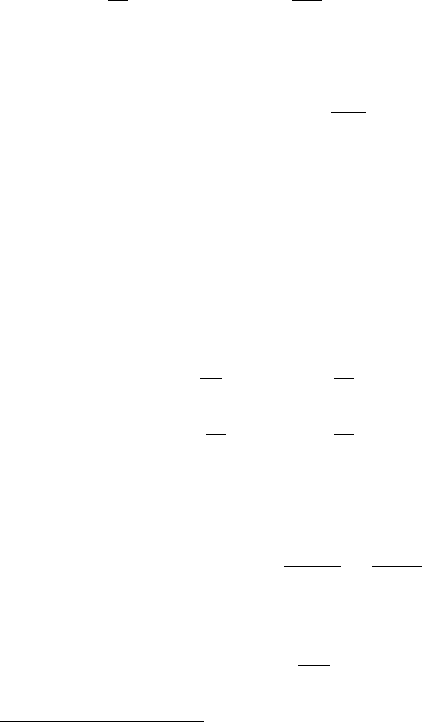
426 7 Groundwater Flow
where s
jl
is the (sign dependent) stoichiometric coefficient for reactant j in reac-
tion l.
The maximum (saturated) rates k
j
of the Monod rates r
j
are thought to range
from about 10
−13
mol l
−1
s
−1
to about 10
−10
mol l
−1
s
−1
. The background phenol
concentration p
0
is of the order of 10
−10
mol l
−1
, and the aqueous dissolved oxygen
level c
0
is of order 10
−4
mol l
−1
. The horizontal Darcy flux is estimated to be U =
10 m y
−1
. The dispersivities are taken in the form D =αU, and values of α
=1m
and α
⊥
=4 ×10
−4
m are assumed.
8
Also, we take l =500 m, d =20 m, and R =1.
With all of these values, we have
Pe
=
l
α
=500, Pe
⊥
=
d
2
α
⊥
l
=2000,Λ∼10
6
–10
9
, (7.189)
where the definition of Λ is based on p
0
, i.e.,
Λ =
S
0
l
Up
0
. (7.190)
As suggested, Pe
∼Pe
⊥
1, and the reaction rate parameter Λ is extremely large.
To get an idea of the solution behaviour when Pe 1 and Λ 1, we consider the
simpler reaction
C
6
H
6
O +σ O
2
r
→products, (7.191)
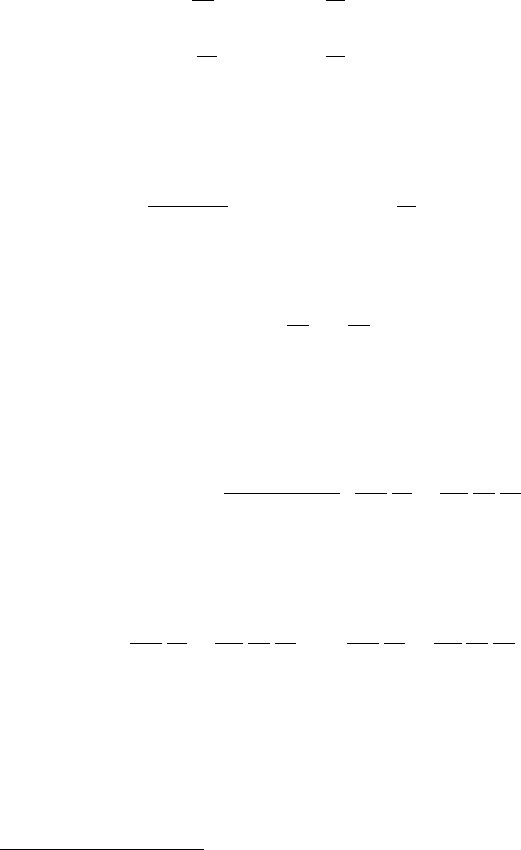
where σ is a suitable stoichiometric coefficient. The corresponding version of
(7.185)issimply
9
∂p
∂t
+∇.(pu) =
1
Pe
∇
2
p +ΛS,
∂c
∂t
+∇.(cu) =
1
Pe
∇
2
c +νΛS,
(7.192)
where p and c are the concentrations of the two species (phenol and oxygen), and
the reaction term is just
S =−
p
k
p
+p
c
k
c
+c
; (7.193)
the parameter ν is given by
ν =
σp
0
c
0
∼10
−6
. (7.194)
8
For both theoretical and experimental reasons, we expect α
∼ d
p
, the pore or grain size (Bear
1972, p. 609), with α
⊥
/α
∼0.01–0.05 (Sahimi 1995, p. 225). The large value of α
here suggests
flow in sub-parallel fractures with spacing on the order of a metre.
9
We can take Pe
=Pe
⊥
by choosing d appropriately.
7.8 Three Specific Remediation Problems 427
The asymptotic structure of the solution of (7.192) is easily given. For Λ 1, the
reaction region is very thin, and will occur only at the fringe of the plume. Outside
the plume, p = 0, while inside the plume c = 0, so that S = 0 everywhere apart
from the reaction front, which is located at the plume boundary. Denoting the plume
by P and its boundary by ∂P, we thus have to solve
∂p
∂t
+∇.(pu) =
1
Pe
∇
2
p, x ∈P,
∂c
∂t
+∇.(cu) =
1
Pe
∇
2
c, x /∈P,
(7.195)
and p and c both satisfy p =c =0on∂P. The location of P has to be determined
(it is a free boundary), but this is simply done because of the precise conservation
law for c −νp, which follows from (7.192):
∂(c −νp)
∂t
+∇.
(c −νp)u
=
1
Pe
∇
2
(c −νp). (7.196)
Integrating this across the reaction front yields the extra condition
10
to determine
the front ∂P:
∂c
∂n
=ν
∂p
∂n
. (7.197)
Note that the normal derivative here has to be treated with some attention when
non-dimensionalised. If the plume fringe is at dimensionless position z = ζ(x,t),
then the dimensional normal component of the dispersive flux of oxygen (in terms
of dimensionless variables) is just
(D.∇c).n =
c
0
Ud
l(1 +δ
2
ζ
2
x
)
1/2
1
Pe
⊥
∂c
∂z
−
1
Pe
∂c
∂x
∂ζ
∂x
, (7.198)
where δ is the aspect ratio. There is a similar expression for ∂p/∂n. It follows from
this that more generally, the conservation boundary condition (7.197) can be ex-
pressed as
1
Pe
⊥
∂c
∂z
−
1
Pe
∂c
∂x
∂ζ
∂x
=ν
1
Pe
⊥
∂p
∂z
−
1
Pe
∂p
∂x
∂ζ
∂x
, (7.199)
and to interpret this as (7.197) requires the unit normal n to be suitably defined.
The problem is even easier when (as here) Pe 1. Then diffusive boundary lay-
ers of (dimensionless) thickness O(1/Pe
1/2
) adjoin the plume boundary, and away
from these, the reactants are simply advected with the flow.
In the present case, the parameter ν 1. This modifies the discussion as follows.
Reaction must still occur in thin regions, outside which the reactants obey (7.195),
10
In this particular case, it is even simpler, since we just integrate the convective-diffusion equa-
tion (7.196) with appropriate external boundary conditions, and ∂P is located by the curve where
c −νp =0.

428 7 Groundwater Flow
but there cannot be a front (in the sense of a moving boundary) at which c =
∂c
∂n
=0.
If, for example, we have the one-dimensional problem
c
t
+uc
x
=
1
Pe
c
xx
, (7.200)
with c = 1att =0 and as x →∞, and c =0atx =0, then if Pe ∼O(1), reaction
occurs near x =0, oxygen supply being sufficient to remove the phenol. If Pe 1,
then a front moves away from x =0 at speed u. Behind this front, c ≈ 1, ahead of
it c ≈ 0, and the front itself is purely diffusional, of width O{(t/Pe)
1/2
},havingan
error function profile for solution.
Rexco The site was a former coal carbonisation plant (between 1935 and 1970)
where ammonium liquor had been allowed to drain on site. The liquid drained
through the unsaturated zone of some 18 metres depth to the saturated zone, where
it is spreading as a plume of some 25 metres depth in a sandstone aquifer. At the
plume fringe, ammonium ions (NH
+
4
) ions are subject to oxidation, releasing nitro-
gen. Phenol is also present in front of the ammonium, which is highly retarded (with
a retardation factor of R = 5). Well extraction by a new factory on site is causing
the groundwater flow field to be time dependent, and this is an issue in modelling
studies.
The modelling scheme is similar to the Four Ashes site, but less is known of the
reaction rates. The reactions which are considered to be important for the ammo-
nium are
NH
+
4
+2O
2
r
1
→ NO
−
3
+H
2
O +2H
+
,
NO
−
3
+1.25CH
2
O +H
+
r
2
→ 0.5N
2
+1.25CO
2
+1.75H
2
O, (7.201)
NH
+
4
+0.6NO
−
3
r
3
→ 0.8N
2
+1.8H
2
O +0.4H
+
.
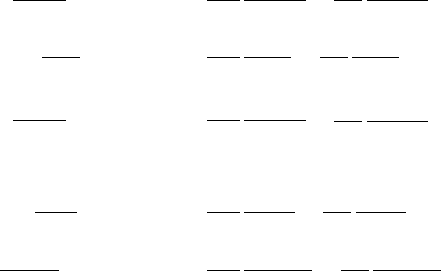
The kinetics (only) of these reactions is described by the five ordinary differ-
ential equations for the five reactants, ammonium NH
+
4
, oxygen O
2
, nitrate NO
−
3
,
hydrogen ion H
+
and organic carbon CH
2
O:
dc
NH
+
4
dt
=−r
1
−r
3
,
dc
O
2
dt
=−2r
1
,
dc
NO
−
3
dt
=r
1
−r
2
−0.6r
3
,
dc
H
+
dt
=2r
1
−r
2
+0.4r
3
,
dc
CH
2
O
dt
=−1.25r
2
.
(7.202)
7.8 Three Specific Remediation Problems 429
To estimate Péclet numbers, we take d = 25 m, l = 1000 m, R = 5, U =
100 m y
−1
. Values of dispersion coefficients are uncertain, so we choose for il-
lustration D
= 100 m
2
y
−1
, D
⊥
= 10 m
2
y
−1
, i.e., α
= 1 m (like Four Ashes)
and α
⊥
=0.1 m. The (unretarded) time scale l/U is then 10 years, and Pe
∼10
3
,
Pe
⊥
∼6. These values are not too reliable, and perhaps are consistent with a highly
fractured aquifer. For a homogeneous medium, we would expect much smaller α,
and thus much larger Pe.
It is possible to extend the Four Ashes discussion of the simple phenol/oxygen
reaction to the more complicated scheme above. Reaction rates are not well known.
Most of the reactants are present at the level of mmol l
−1
(millimole per litre). Spe-
cific estimates are
c
NH
+
4
∼12 mmol l
−1
,c
O
2
∼0.3 mmol l
−1
,c
NO
−
3
∼0.15 mmol l
−1
,
c
H
+
∼10
−4
mmol l
−1
,c
CH
2
O
∼1 mmol l
−1
.
(7.203)
If we use ˆc
NH
+
4
= 12 mmol l
−1
as a concentration scale in the definition of Λ in
(7.186) and arbitrarily pick a reaction rate scale of S
0
= 10
−10
mol l
−1
s
−1
(com-
parable to phenol degradation rate at Four Ashes), then we have Λ ∼O(1), and the
simple description for Four Ashes is inappropriate.
Despite this, it is thought that reactions are fast, and we will suppose that in fact
Λ 1. In particular, there are data from a borehole measurement which appear
to be consistent with the idea that there is a thin reaction zone at the upper plume
boundary. Oxygen (presumably) diffuses to this front from above, and ammonium
from below; in the reaction zone there is a huge spike of nitrate (see Fig. 7.9). We
wish to see whether the existence of this nitrate spike is consistent with the model,
assuming the measurement was realistic. (Figure 7.9 actually suggests the existence
of two fronts, with nitrate produced at the upper front diffusing down to the second
front, but we will focus only on the upper front.)
The dimensionless equations equivalent to (7.192) for the reaction scheme
(7.201)are
R
∂c
NH
+
4
∂t
+∇.(c
NH
+
4
u) =
1
Pe
⊥
∂
2
c
NH
+
4
∂z
2
+
1
Pe
∂
2
c
NH
+
4
∂x
2
−Λ(r
1
+r
3
),
∂c
O
2
∂t
+∇.(c
O
2
u) =
1
Pe
⊥
∂
2
c
O
2
∂z
2
+
1
Pe
∂
2
c
O
2
∂x
2
−2Λν
O
2
r
1
,
∂c
NO
−
3
∂t
+∇.(c
NO
−
3
u) =
1
Pe
⊥
∂
2
c
NO
−
3
∂z
2
+
1
Pe
∂
2
c
NO
−
3
∂x
2
+Λν
NO
−
3
(r
1
−r
2
−0.6r
3
),
∂c
H
+
∂t
+∇.(c
H
+
u) =
1
Pe
⊥
∂
2
c
H
+
∂z
2
+
1
Pe
∂
2
c
H
+
∂x
2
+Λν
H
+
(2r
1
−r
2
+0.4r
3
),
∂c
CH
2
O
∂t
+∇.(c
CH
2
O
u) =
1
Pe
⊥
∂
2
c
CH
2
O
∂z
2
+
1
Pe
∂
2
c
CH
2
O
∂x
2
−1.25Λν
CH
2
O
r
2
,
(7.204)
