Fowler A. Mathematical Geoscience
Подождите немного. Документ загружается.

560 9 Magma Transport
while the momentum equation is
p
r
=η
s
u
rr
+
1
r
u
r
−
u
r
2
, (9.23)
whence p = p
s
is constant, and at r = a, a force balance implies −p
s
+2η
s
u
r
=
−p
l
, thus
C =−
(p
s
−p
l
)a
2
2η
s
. (9.24)
The constitutive law for p
s
−p
l
now follows from consideration of the kinematic
boundary condition for the solid flow which applies at the pore boundary. To relate
our cylindrical void to macroscopic quantities, we suppose it is encased in a grain
of dimension l (specifically, a cylinder of length l and radius l) so that φ = a
2
/l
2
.
The rate of melting is S (mass per unit volume per unit time). This corresponds to a
rate of removal (or ablation) at the pore boundary of
v =
l
2
S
2ρ
s
a
(9.25)
(because S/ρ
s
is the volume removed per unit volume per unit time, and this is equal
to 2πalv/πl
3
). Finally, the kinematic boundary condition for a is that
˙a =u|
a
+v, (9.26)
and in terms of macroscopic variables, this becomes (since
˙
φ =2a ˙a/l
2
)
˙
φ =
S
ρ
s
−
(p
s
−p
l
)φ
η
s
. (9.27)
The time derivative
˙
φ represents the derivative following the grain, i.e.
˙
φ = φ
t
+
u.∇φ.
(9.31) applies if the grain viscosity is constant, i.e., diffusion creep prevails. For
the more likely dislocation creep with a flow law given by
˙ε
ij
=Aτ
n−1
τ
ij
, (9.28)
and 2τ
2
=τ
ij
τ
ij
, we find that (9.24) is replaced by
u|
a
=
C
a
=−
Aa
n
n
|p
s
−p
l
|
n−1
(p
s
−p
l
). (9.29)
Note that this reduces to (9.24) when n =1, since then A =1/2η
s
. The correspond-
ing form of (9.27)is
˙
φ =
S
ρ
s
−
2Aφ
n
n
|p
s
−p
l
|
n−1
(p
s
−p
l
). (9.30)

9.4 Melt Transport in the Asthenosphere 561
A viscous prescription for the solid matrix would be
¯
σ
s
=−p
s
δ +2η
s
˙
ε
s
, (9.31)
where
˙
ε
s
is the solid strain rate. In (9.21), we effectively regain the mantle momen-
tum equation
∇.
¯
σ
s
=
ρ
s
(1 −φ) +ρ
l
φ
g
ˆ
k, (9.32)
because (as we shall find) φ 1, and we can suppose p
s
− p
l
<
∼
¯σ
s
.Themain
effect of partial melting on the circulation is in the buoyancy term in (9.32), where
1% partial melting (φ =0.01) is equivalent to a buoyancy temperature difference of
300 K (if the thermal expansion coefficient is α =3 ×10
−5
K
−1
).
Lastly, there is an energy equation for each phase. These equations are analogous
to the two momentum equations, with a huge interfacial heat transport term, which
consequently implies the two phases have locally equal temperatures. The other
equation then follows from the equation of total energy conservation. The simplest
form of this is
LS +ρ
s
c
s
(1 −φ)
dT
dt
s
+ρ
l
c
l
φ
dT
dt
l
+αT
φ
dp
l
dt
l
+(1 −φ)
dp
s
dt
s
=K∇
2
T, (9.33)
where L is the latent heat, and we take the specific heats c
s
and c
l
, and averaged
thermal conductivity K = K
s
(1 − φ) + K
l
φ to be constant; the time derivatives
d/dt
l
and d/dt
s
are equal to ∂/∂t + v.∇ and ∂/∂t + u.∇, respectively. Various
small dissipative terms have been neglected in (9.33).
9.4.1 Summary
The equations we propose to describe the partial melt region are thus
T =T
0
+Γp
l
,
ρ
l
φ
t
+∇.(φv)
=S,
ρ
s
−φ
t
+∇.
(1 −φ)u
=−S,
v −u =−Π
0
φ[∇p
l
+ρ
l
g
ˆ
k],
0 =−∇(φp
l
) +∇.
(1 −φ)
¯
σ
s
−
ρ
l
φ +ρ
s
(1 −φ)
g
ˆ
k,
φ
t
+u.∇φ =
S
ρ
s
−
φ(p
s
−p
l
)
η
s
,
¯
σ
s
=−p
s
δ +2η
s
˙
ε
s
,
LS +(1 −φ)
ρ
s
c
s
dT
dt
s
−αT
dp
s
dt
s
+φ
ρ
l
c
l
dT
dt
l
−αT
dp
l
dt
l
=K∇
2
T.
(9.34)
Boundary conditions for these equations will be described in due course.
562 9 Magma Transport
9.4.2 Simplification
We anticipate that φ 1. Then we have, from (9.34)
3
,
∇.u ≈0, (9.35)
and
∇.
¯
σ
s
≈
ρ
l
φ +ρ
s
(1 −φ)
g
ˆ
k. (9.36)
Apart from the added buoyancy term, the solid matrix flow equations are contiguous
with those of the mantle outside the partial melt zone, and it is convenient to suppose
that u and p
s
can thus be determined independently of φ. In particular, p
s
is in any
case approximately lithostatic, and thus
∇p
s
≈−ρ
s
g
ˆ
k. (9.37)
In order to proceed, we want to non-dimensionalise these equations. We guess,
or anticipate, that the adiabatic terms are less important than the advective terms in
the energy equation, and that heat conduction is small. We also anticipate that v u
(melt velocity is rapid) but φv u (melt flux is small). Then in the energy equation,
LS ≈−ρ
s
c
s
Γdp
l
/dt
s
. The constitutive relation for the effective pressure p
s
−p
l
suggests that it is balanced by the melt rate, thus p
s
−p
l
∼η
s
S/ρ
s
φ. We anticipate
that this stress is less than lithostatic stress, and this in turn suggests p
l
≈p
s
. With
these assumptions we scale the variables as
x ∼d, u ∼u
m
, v ∼[v],t∼d/[v],φ∼[φ],S∼[S],
(9.38)
where d is a suitable length scale for the partial melt zone, and we write
p
l
=p
s
−[p]ψ, (9.39)
and put
p
s
=p
0
−ρ
s
gz, (9.40)
where z is the height above a convenient reference level.
Our choice of balances in the equations then suggests
ρ
l
[φ][v]
d
=[S],
[v]=Π
0
[φ](ρ
s
−ρ
l
)g,
[p]=
η
s
[S]
ρ
s
[φ]
,
[S]=
ρ
2
s
c
s
gu
m
Γ
L
,
(9.41)
9.4 Melt Transport in the Asthenosphere 563
Table 9.6 Assumed values of constants
Symbol Meaning Typical value
g gravity 10 m s
−2
ρ
s
matrix density 3 ×10
3
kg m
−3
ρ
l
melt density 2.5 ×10
3
kg m
−3
L latent heat 3 ×10
5
Jkg
−1
Γ Clapeyron slope 10
−7
KPa
−1
α thermal expansion coefficient 3 ×10
−5
K
−1
T
m
ambient melting temperature 1500 K
K thermal conductivity 4 W m
−1
K
−1
η
s
matrix viscosity 10
19
Pa s
b tortuosity 10
3
d
g
grain size 2 ×10
−3
m
η
l
melt viscosity 1 Pa s
c
s
, c
l
specific heats 10
3
Jkg
−1
K
−1
u
m
mantle ascent velocity 10
−9
ms
−1
(3 cm y
−1
)
d melt zone depth 10
4
m (10 km)
ρ ρ
s
−ρ
l
0.5 ×10
3
kg m
−3
and these determine the unknowns [φ], [v], [S] and [p]; explicitly
[φ]≡μ =
ρ
2
s
ρ
l
ρ
c
s
Γu
m
d
Π
0
L
1/2
, [v]=ρ
s
g
ρ c
s
Γu
m
dΠ
0
ρ
l
1/2
, (9.42)
where ρ =ρ
s
−ρ
l
. We can now examine whether the assumption that [φ][v]
u
m
[v] is valid.
We use the values of the constants in Table 9.6. From these we find
μ =[φ]∼0.24 ×10
−2
, [v]∼4.8 ×10
−8
ms
−1
(1.5my
−1
),
[S]∼3 ×10
−11
kg m
−3
s
−1
,
(9.43)
and thus [φ][v]∼1.2 ×10
−10
ms
−1
, u
m
∼ 10
−9
ms
−1
, [v]∼4.8 ×10
−8
ms
−1
,
vindicating our assumptions.
Dimensionless equations for the partial melt variables are then
φ
t
+∇.(φv) =S,
v −δu =φ[
ˆ
k +ν∇ψ],
(1 +r)[φ
t
+δu.∇φ]=S −φψ,
S +(1 −μφ)
(δ
−1
∂
t
+u.∇)(−z −˜νψ) +λ
s
θw
+
1
St
(1 −λ
l
θ)(∂
t
+v.∇)(−z −˜νψ) =−ε∇
2
ψ,
(9.44)

564 9 Magma Transport
where w =u.
ˆ
k, and the parameters are defined by
St =
L
c
l
T
,T=ρ
s
gdΓ,
δ =
u
m
[v]
,ε=
K[p]
ρ
2
s
c
s
gu
m
d
2
,
ν =
[p]
ρgd
, ˆν =
[p]
ρ
s
gd
,
λ
s
=
αT
m
ρ
s
c
s
Γ
,λ
l
=
αT
m
ρ
l
c
l
Γ
,r=
ρ
ρ
l
,
(9.45)
and also θ = T/T
m
. Using Table 9.6,wehaveT ∼ 30 K, [p]∼4 ×10
7
Pa, and
thus
St ∼10,δ∼0.02,ε∼1.2 ×10
−3
,ν∼0.8,
˜ν ∼0.12,λ
s,l
∼0.15,r∼0.2.
(9.46)
On this basis, we neglect relatively small terms proportional to μ, λ
s
, St
−1
in the
energy equation (the last two mainly for simplicity):
φ
t
+δ∇.(φu) +∇.
φ
2
{
ˆ
k +ν∇ψ}
=S,
(1 +r)[φ
t
+δu.∇φ]+φψ =S,
δ
−1
∂
∂t
+u.∇
(z +˜νψ) −ε∇
2
ψ =S.
(9.47)
Further simplification results from the equation for conservation of mass of solid
(9.34)
3
, which can be written in the dimensionless form
∇.u =μ∇.(φu) +
μ
δ
(1 +r)φ
t
−
S
1 +r
. (9.48)
Neglecting terms of O(μ),(9.47)
1
can be written as
φ
t
+δu.∇φ +∇.
φ
2
{
ˆ
k +ν∇ψ}
=S, (9.49)
and combining this with (9.47)
2
gives
2φφ
z
+ν∇.
φ
2
∇ψ
=φψ +rφ
t
, (9.50)
where we also neglect the advective derivative of φ,ofO(δr).
Finally, combination of (9.49) with (9.47)
3
yields
δ
−1
∂
∂t
+u.∇
(z +˜νψ −δφ) −∇.
φ
2
{
ˆ
k +ν∇ψ}
=ε∇
2
ψ. (9.51)
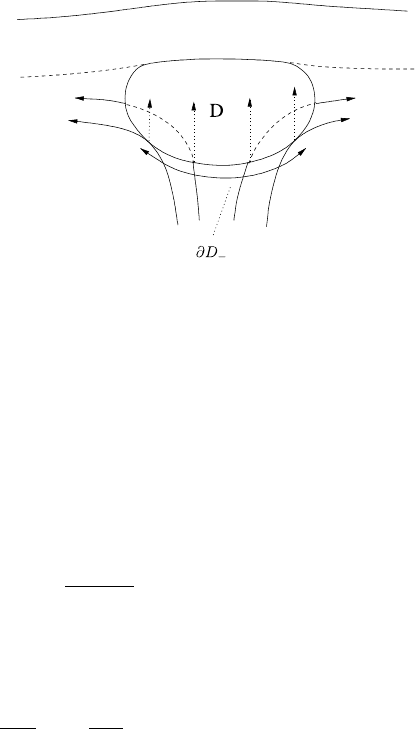
9.4 Melt Transport in the Asthenosphere 565
Fig. 9.14 Schematic
illustration of the domain D.
The lower part of the
boundary ∂D
−
is indicated;
on this we would expect to
prescribe φ =0
A comment on the parameters ε, ν and ˜ν is in order. From (9.45), these are
proportional to [p], which in turn is proportional (via (9.41)) to η
s
. We took
η
s
= 10
19
Pa s in Table 9.6, reflecting the typical inferred asthenospheric viscos-
ity (e.g., from postglacial rebound studies). The consequent inferred grain stress is
then large (400 bars), which for typical dislocation creep laws would suggest effec-
tive viscosities about three to four orders of magnitude lower. Such lower viscosities
are not inconsistent with global asthenospheric values, since melting occurs locally.
In turn they would suggest lower values of [p], which in turn would reduce the low-
ering of viscosity via nonlinear creep. A more consistent way to guess grain stress
values is to use the nonlinear closure law (9.30), which implies, for n =1,
[p]=n
[S]
2Aρ
s
[φ]
1/n
. (9.52)
If we identify η
s
=1/2Aτ
n−1
, where τ is a typical asthenospheric shear stress, say
10 bars, then (9.52) can be written as
[p]
n
τ
=n
[p]
1
τ
1/n
, (9.53)
where [p]
n
is the pressure scale for flow exponent n.Forn = 3.5, for example,
[p]
3.5
≈100 bars, and ν, ˜ν and ε would be a factor of four lower. It is therefore not
unreasonable to think of ν as being small also, and we will adopt this strategy in our
analysis below.
9.4.3 Boundary Conditions
Equations (9.47)
3,4
are to be solved for φ and ψ in a domain which we can expect
to resemble that shown in Fig. 9.14.
Both (9.50) and (9.51) are hyperbolic equations for φ if ψ is known. It is there-
fore natural to suppose that we should prescribe φ on those parts of the boundary

566 9 Magma Transport
where the characteristics enter the domain. The physically appropriate condition
would seem to be
φ =0on∂D
−
, (9.54)
where ∂D
−
denotes that part of the boundary ∂D of D on which u.n < 0, where n
is the outward normal to D, and this appears consistent with the choice of apparent
characteristics for (9.51), if this is written in the form (using (9.50))
δ
−1
∂
∂t
+u.∇
(z +˜νψ −δφ) =φψ +rφ
t
+ε∇
2
ψ. (9.55)
If this is an appropriate condition for φ, then the elliptic (9.47)
3
suggests that
ψ should be prescribed on ∂D, except where φ = 0 and the elliptic term for ψ is
degenerate. Since the melting temperature is T
0
+Γp
l
in the partially molten region,
while it is T
0
+ Γp
s
in the cold mantle, it seems we should ensure continuity of
temperature by having p
l
=p
s
on ∂D,i.e.ψ =0on∂D (φ =0). The two boundary
conditions can be combined in the form
φψ =0on∂D. (9.56)
While this is plausible, it is by no means certain, and one might expect the non-
degenerate elliptic ψ term in (9.55) to require ψ to be prescribed everywhere. This
is an example of a model where some functional analysis would actually be useful.
A further difficulty is that to say the melting temperature in the cold mantle is
T
0
+ Γp
s
carries in itself no meaning, since equilibrium melting temperature is
defined through thermodynamic equilibrium at the interface. In addition, we should
add that (9.56) has not been systematically derived from any physical principle. We
now attempt to resolve this latter issue.
9.4.4 Thermodynamic Equilibrium
At the microscopic interface between partially molten and cold mantle ∂D,were-
quire that the jump in temperature, pressure and Gibbs free energy be zero:
[T ]
m
c
=[p]
m
c
=[G]
m
c
=0. (9.57)
The first of these is standard, the second is a force balance (it should properly be a
balance of normal stress, but we omit deviatoric stresses for simplicity), while the
third is the condition of thermodynamic equilibrium, and it is this condition which
we need to prescribe, instead (perhaps) of (9.56).
Consider first a reference state in which p
s
= p
l
in the partial melt region. The
Gibbs free energy of each separate phase depends on temperature and pressure, and
we can write for the partial melt side of ∂D, G =G
m
, where
G
m
=φ(h
l
−TS
l
) +(1 −φ)(h
s
−TS
s
), (9.58)
9.4 Melt Transport in the Asthenosphere 567
in which h
k
and S
k
are the specific enthalpies and entropies of each phase. In this
reference state, p =p
s
on the cold side, and therefore on this side G =G
c
, where
G
c
=h
s
−TS
s
. (9.59)
The enthalpies and entropies are evaluated at the interfacial temperature T and in-
terfacial pressure p =p
s
=p
l
. The jump in G across the boundary is
G =[G]
m
c
=φ[h −TS], (9.60)
where h =h
l
−h
s
, S =S
l
−S
s
, and this is zero since the latent heat L =h =
TS; []
m
c
represents the jump across ∂D from cold to partially molten.
Now suppose we change p
s
, p
l
and φ away from the reference state, by amounts
δp
s
,δp
l
and δφ. On the cold side, the force balance condition implies
δp
c
=δp
s
−δ
φ(p
s
−p
l
)
, (9.61)
and thus
δG
c
= v
s
δp
c
−S
s
δT
= v
s
δp
s
−S
s
δT −v
s
δ
φ(p
s
−p
l
)
, (9.62)
whereas on the molten side
δG
m
= δφ[h −TS]+φ(v
l
δp
l
−S
l
δT )
+(1 −φ)(v
s
δp
s
−S
s
δT ). (9.63)
Using the fact that δG
c
=δG
m
and h =TS, we get
−v
s
δ
φ(p
s
−p
l
)
=φ
(v
l
δp
l
−S
l
δT ) −(v
s
δp
s
−S
s
δT )
. (9.64)
Now the right hand side is φ[δG
l
− δG
s
], where G
l
and G
s
are the local spe-
cific Gibbs free energies of liquid and solid within the mush. We are assuming
that thermodynamic equilibrium prevails locally at the pore-grain interface so that
δG
l
= δG
s
(which is in fact equivalent to the Clapeyron relation T = T
0
+ Γp
l
);
therefore the assumption of macroscopic thermodynamic equilibrium [G]
m
c
=0im-
plies δ[φ(p
s
−p
l
)]=0, and since φ(p
s
−p
l
) =0 in the reference state, the condi-
tion we prescribe is
φ(p
s
−p
l
) =0on∂D. (9.65)
This condition is identical to (9.56). It is deduced from the principle of macroscopic
thermodynamic equilibrium, rather than being an ad hoc proposition. It remains to
be seen whether it is sufficient for the determination of φ and ψ if the domain is
known.
568 9 Magma Transport
9.4.5 Stefan Condition
The location of ∂D is not known apriori. Its determination is effected from a second
thermal boundary condition, which represents the energy balance across ∂D.This
is known as the Stefan condition, and takes the form
ρ
l
Lφ(v −V).n =
K
∂T
∂n
m
c
, (9.66)
where V is the velocity of ∂D. Scaling T −T
0
with T =ρ
s
gdΓ and V with [v]
leads to the dimensionless version of (9.66),
˜νφ(v
n
−V
n
) =−ε
∂
∂n
(z +˜νψ) −
∂T
∂n
on ∂D. (9.67)
In particular the part of the boundary where φ =0 is determined by the condition
∂T
∂n
=
∂
∂n
(z +˜νψ), (9.68)
and to leading order (neglecting ˜ν), this is independent of the partial melt dynamics.
9.4.6 Steady State Solution, One Dimension
Our confidence in the prescription of the boundary conditions will be enhanced by
showing that solutions can be found which satisfy them. One way of doing this is
to find explicit approximate solutions. To this end, we seek asymptotic solutions
for (9.50) and (9.55) based on the assumptions that ε, δ and ˜ν are small, but that
ν =O(1).
Specifically, we will consider steady state, one-dimensional solutions, which will
be appropriate if u =wk. In this case, the equations reduce to
2φφ
z
+ν
φ
2
ψ
z
z
=φψ,
w[1 +˜νψ
z
−δφ
z
]=φψ +εψ
zz
,
(9.69)
with anticipated boundary conditions
φ =0onz =0,ψ=0onz =1. (9.70)
In what follows we will suppose that w is constant.
9.4.7 Outer Solution
We put ε, ˜ν and δ to zero. Then φψ ≈w, and a first integral of (9.69)
1
is
φ
2
+νφ
2
ψ
z
=w(z +C), (9.71)

9.4 Melt Transport in the Asthenosphere 569
where C is constant. With ψ =w/φ, it follows that
νwφ
z
=φ
2
−w(z +C), (9.72)
which is a Riccati equation. It turns out that in order to obtain a coherent boundary
layer solution at z = 1, we need the solution of (9.72) which tends to infinity at
z =1. The solution of (9.72) which does this is
φ =ν
1/3
w
2/3
Ai(ζ
1
)Bi
(ζ ) −Bi(ζ
1
)Ai
(ζ )
Bi(ζ
1
)Ai(ζ ) −Ai(ζ
1
)Bi(ζ )
, (9.73)
where
ζ =
z +C
(ν
2
w)
1/3
,ζ
1
=
1 +C
(ν
2
w)
1/3
. (9.74)
Direct consideration of (9.72) shows that there is a unique choice of φ
0
= φ|
z→0
such that φ →∞as z →1, providing C is not too small.
9.4.8 Boundary Layer at z =0
Near z =0, we put z =δZ, so that (9.69) becomes
2δφφ
Z
+ν
φ
2
ψ
Z
Z
=δ
2
φψ,
w
1 +
˜ν
δ
ψ
Z
−φ
Z
=φψ +
ε
δ
2
ψ
ZZ
.
(9.75)
Correct to O(δ),wehaveφ
2
ψ
Z
= constant =0 to match to the outer solution ψ ∼
w/φ
0
as Z →∞, thus ψ =w/φ
0
+O(δ), and to leading order (9.75)
2
is
w(1 −φ
Z
) =
wφ
φ
0
, (9.76)
with solution
φ =φ
0
1 −e
−Z/φ
0
. (9.77)
This assumes that φ
0
> 0, which is certainly the case if C>0. This boundary layer
has been called the compaction layer.
9.4.9 Boundary Layer at z =1
As z →1, φ ∼νw/(1 −z), ψ ∼(1 −z)/ν, and thus we put
z =1 −δ
1/2
Z, φ =Φ/δ
1/2
,ψ=δ
1/2
Ψ ; (9.78)
