Fowler A. Mathematical Geoscience
Подождите немного. Документ загружается.

550 9 Magma Transport
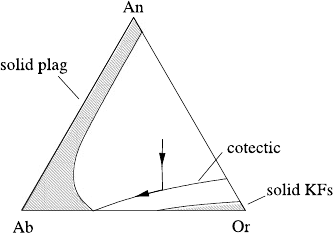
Fig. 9.8 Ternary phase
diagram in the feldspar
system
anorthite–albite–orthoclase.
The shaded regions represent
the stable fields for solid
plagioclase and K-feldspar,
respectively. After McBirney
(1984, p. 107)
must decrease more rapidly away from the melting point of pure diopside then the
liquidus. (In fact, essentially pure Di is produced.) As cooling proceeds, the resid-
ual melt is thus driven towards the cotectic, and subsequent crystallisation occurs
on the cotectic, with the plagioclase Ab–An concentration being determined by the
intersection of the cotectic temperature with the Ab–An solidus.
These general principles apply for more components. For example a four com-
ponent system would have a compositional tetrahedron to represent compositions.
Phases that do not form solid solutions would have eutectic points on the edges,
extending to cotectic lines on the surfaces, and cotectic surfaces in the interior. As
crystallisation proceeds, the residual melt would drive down temperature gradients
towards cotectic surfaces, then along these (with two phases crystallising) to cotec-
tic lines where two cotectic surfaces intersect, and three phases precipitate, and so
on. Mostly, one sticks with the easier ternary diagrams, though.
Let us now return to the feldspars. We recall that plagioclase is a solid solution of
albite and anorthite, Ab = NaAlSi
3
O
8
,An= CaAl
2
Si
2
O
8
, and the other principal
feldspar, orthoclase or potassium feldspar, Or = KAlSi
3
O
8
, forms a eutectic mix-
ture with Ab at elevated water vapour pressures (Fig. 9.5). Just as we considered
the crystallisation history of plagioclase-pyroxene melts, it is natural to wonder how
orthoclase-plagioclase melts crystallise, since we know that K-feldspar is a promi-
nent component of crustal rocks (Fig. 9.3).
We thus consider the three-component feldspar system Or–Ab–An, whose
ternary phase diagram is shown in Fig. 9.8. There is a single cotectic line dividing
plagioclase from K-feldspar. The diagram also shows (hatched) the regions of solid
phase stability (i.e., the projections of the solidus surfaces for Plag and Or). They
are well separated. For a liquid on the plagioclase side of the cotectic, plagioclase
will crystallise first, and then plagioclase and K-feldspar together.
Because the plagioclase is a solid solution, one can add silica to this diagram rel-
atively simply. The corresponding phase diagram for wet rock is shown in Fig. 9.9.
Now each two component sub-system has a eutectic, so there are three cotectic lines,
and these intersect at the cotectic point C. When the residual melt reaches the cotec-
tic point, then all three phases will crystallise. If we more properly allow Ab and An
as separate components, then each section of the four component phase tetrahedron
looks like Fig. 9.9, with the cotectic lines shifting somewhat as Ab/An varies. The
solid which crystallises at the cotectic point has a composition resembling granite

9.3 Phase Diagrams and Geochemistry 551
Fig. 9.9 ‘Ternary’ phase
diagram for the system
quartz–plagioclase–ortho-
clase. Because plagioclase is
a solid solution of albite and
anorthite, the base of the
triangle is really a side view
of the base of a tetrahedron,
the base being that shown in
Fig. 9.8. C denotes the
cotectic point, where all three
minerals crystallise
(but without the late stage hornblende and biotite of the discontinuous crystallisation
series).
9.3.3 Olivine
We have fought our way through from pyroxene to feldspar; we need to add olivine
to the story. Olivine (Mg,Fe)
2
SiO
4
is a solid solution of forsterite (Fo = Mg
2
SiO
4
)
and fayalite (Fa =Fe
2
SiO
4
), with a phase diagram like that of plagioclase, forsterite
having a higher melting temperature than fayalite.
We can now explain what is meant by the discontinuous crystallisation series. We
illustrate this first by consideration of the Fo–SiO
2
system, whose phase diagram is
showninFig.9.10. If an Fo-rich liquid is cooled, it begins by crystallising Fo. On
cooling further, however, Fo can react with the liquid to form the pyroxene enstatite
En = Mg
2
Si
2
O
6
via
Mg
2
Si
2
O
4
+ SiO
2
Mg
2
Si
2
O
6
Fo En
. (9.6)
If the Fo liquidus dips underneath the En liquidus (as in Fig. 9.10), then the enstatite
is said to melt incongruently (since when it melts, it forms a liquid of a different
Fig. 9.10 Phase diagram for
Fo–SiO
2
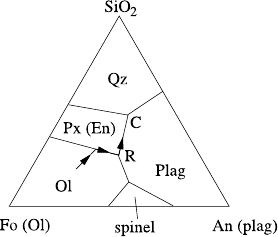
552 9 Magma Transport
Fig. 9.11
Forsterite–silica–plagioclase
system
composition), and the consequence on solidification is that when the melt composi-
tion reaches the reaction point R in Fig. 9.10, the Fo dissolves in the melt, and En is
precipitated. It is in this sense that the crystallisation is discontinuous.
If we now add anorthite to the system, then the phase diagram is as in Fig. 9.11.
The incongruent melting region extends to a pyroxene stability field, and in partic-
ular olivine and quartz cannot precipitate together; at least if the olivine is Mg-rich:
the pyroxene stability field disappears at high Fe in the Fo–Fa–SiO
2
system.
9.3.4 Summary
We now have a reasonable concept of how crystallisation of a magma may occur.
Olivine crystallises first, which enriches the melt in silica. As the melt cools, the
olivine reacts with it to form pyroxene. This drives the melt towards the feldspar
field, until also plagioclase (first calcium-rich, then sodium-rich) begins to crys-
tallise, followed by K-feldspar, and finally quartz. Or at least, this is one possible
sequence. It should be very clear from the degree of variability present in the dis-
cussion that there are many different possible crystallisation sequences. A part of
the story which we have not mentioned concerns the three minerals in Fig. 9.3 not
so far mentioned: nepheline, biotite and hornblende.
Nepheline (Ne = (Na,K)AlSiO
4
) is a silica-poor alkaline rock called a feldspath-
oid. One can think of it as a silica-poor feldspar, indeed one can think of it as a
reactant in forming albite or orthoclase:
(Na,K)AlSiO
4
+ 2SiO
2
(Na,K)AlSi
3
O
8
Ne Ab, Or
, (9.7)
and its behaviour can be understood by considering the phase diagram of the three-
component system SiO
2
–NaAlSiO
4
–KAlSiO
4
, which (since Ab and Or have com-
positions half way up the edges from the SiO
2
vertex) is a bit like that of Fig. 9.9
with an extra bit glued on the bottom. The nepheline stability field lies in this lower
part of the diagram. Nepheline is crystallised as an alternative to quartz, as suggested
by Fig. 9.3, and this can occur in a wide variety of conditions.
9.3 Phase Diagrams and Geochemistry 553
Hornblende and biotite are hydrous minerals; hornblende (Hb) is an amphibole,
and biotite (Bt) is a mica. They both have bewildering chemical formulae, but their
main characteristic is that they incorporate water. They are silica poor, and they are
increasingly stable at higher water vapour pressure (unlike the anhydrous minerals
we have been discussing, whose solidus and liquidus temperatures decrease dramat-
ically with increasing water vapour pressure, amphiboles and micas have the oppo-
site behaviour). Thus as the anhydrous minerals crystallise, water is concentrated
in the residual melt, which lowers the liquidus of, say, pyroxene, while raising that
of amphibole. Eventually they become equal and amphibole will precipitate. Essen-
tially this sequence can be understood in terms of a suitable ternary phase diagram
of the system En–Hb–H
2
O, for example, via the migration of a cooling path to-
wards a cotectic dividing the enstatite and hornblende fields. In this way, one can
add amphibole and mica to the story of late stage crystallisation through the action
of water.
9.3.5 Melting
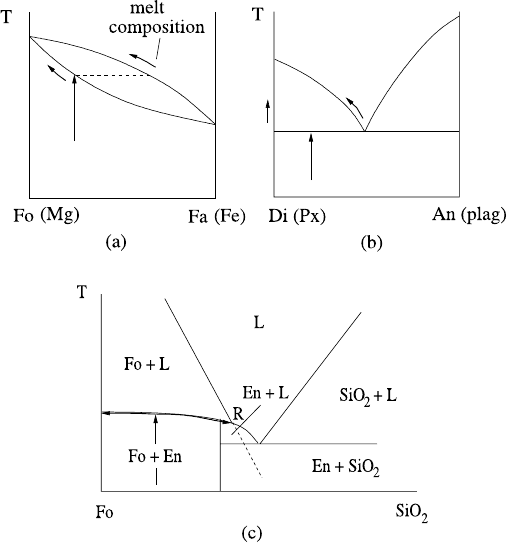
Mostly we have discussed crystallisation. What happens on melting a mantle rock?
On the face of it, it is just the opposite of crystallisation. If we warm a solid solu-
tion to the solidus, it will form a melt with an enriched composition on the liquidus
(Fig. 9.12a). Subsequent heating drives the solid composition up the solidus and the
melt composition up the liquidus, becoming less enriched. For a eutectic mixture
(Fig. 9.12b) such as diopside–anorthite, if we heat a diopside-rich rock to the eutec-
tic temperature, then the melt will be of eutectic composition, and the solid will be
driven towards diopside. Only when all the anorthite is melted will the temperature
be able to rise along the diopside liquidus.
It is easy to generalise this to more complicated rocks. The first melt will be of
cotectic composition, containing all the phases present. As melting proceeds, the
melt will remain of this composition until one of the phases has completely melted;
subsequently the melt composition will migrate up cotectic lines. This simple story
is slightly soiled by the possibility of reactions between minerals, for example in the
incongruent melting of enstatite through the reaction of forsterite with silica ((9.6)
and Fig. 9.10). An olivine-pyroxene rock will begin to melt as shown in Fig. 9.12c;
the pyroxene will melt to form melt of peritectic composition at the point R.
There are two complications. One is geochemical: the phase diagrams are com-
plicated! However, the basic picture that we have is that silicate rocks are com-
pounds formed by reactions of metal oxides with silica, and to some extent, the
different oxide-silica phase diagrams can be glued together. Figure 9.13 shows the
‘basalt tetrahedron’ which glues together three tetrahedra (corresponding to alkali
basalt, olivine tholeiite and quartz basalt) comprising nepheline, diopside, silica and
olivine, with the intermediate reactive products albite and hypersthene. The only
obvious ingredient missing is potassium, which can be glued on via the residual
system nepheline–kalsilite–silica (NaAlSiO
4
–KAlSiO
4
–SiO
2
), which also contains
the intermediate reaction products Ab = NaAlSi
3
O
9
and Or = KAlSi
3
O
8
, and is
554 9 Magma Transport
Fig. 9.12 Melting of (a) a solid solution; (b) a eutectic solid; (c) an incongruent system
closely associated with felsic rocks such as granite. Oceanic basalts contain very
little potassium, and Fig. 9.13 is a reasonable synopsis.
The second complication is this: physics. Our entire discussion assumes melting
and crystallisation occurs at equilibrium. This is unlikely to be the case! As melting
proceeds, equilibrium requires solid-state diffusion to be effective in allowing solid
phase concentrations to remain on the solidus, at least for solid solutions; whether
this can be so remains to be seen.
But the more obvious point is that as soon as partial melting occurs, the buoy-
ant melt wants to move. As soon as partially molten rock is permeable, melt will
rise and be replaced by melt from below. In general, changing pressure will alter
the location of cotectic points, so that the replacement melt will have a different
composition (and temperature) than its predecessor. We can suppose local melting
or refreezing will occur in order to keep the liquid at the liquidus, but one can no
longer assume that the melt rate is purely controlled by the rise velocity of adiabatic
mantle, nor that the melt composition is that of the local cotectic point. In order to
be able to consider the effects of melt transport on melt composition, we need to be
able to describe the physics of magma transport, and this will commence in the fol-
lowing section. Before doing so, we make some geochemically informed comments
on various types of differentiation in the Earth.
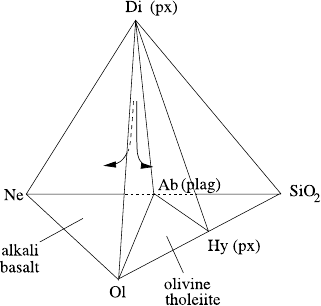
9.3 Phase Diagrams and Geochemistry 555
Fig. 9.13 Olivine–pyroxene–quartz–nepheline system. Because albite (a plagioclase) has a com-
position intermediate to nepheline and silica, and similarly hypersthene (a pyroxene) is intermedi-
ate to olivine and silica, the system acts like three separate subsystems glued together. In particu-
lar, similar compositions close to the olivine–pyroxene–plagioclase system can evolve into olivine
tholeiites, enriched in silica, or the silica-poor alkali basalts, as indicated by the arrowed curves
9.3.6 Continental Crust
The average composition of continental crust is andesitic, while the upper mantle is
essentially peridotite (see Table 9.1). The differentiation of the light crust from the
heavier mantle occurred near the beginning of Earth’s history, and can be understood
in various ways. If the Earth was covered by a magma ocean, as has been suggested,
then as it crystallised, one would expect the heavier crystals to sink towards the base.
With olivine and then pyroxene crystallising first, we would obtain an olivine rich
mantle and a more silicic ‘crust’.
Instead, or as well, subsequent partial melting of a silica-poor (relative to cotec-
tic) convecting solid mantle would cause silica-enriched melt to be erupted. This
provides a means to establish a chemical discontinuity (the Mohorovi
ˇ
ci
´
c discon-
tinuity, or ‘Moho’) above which lies the crust which is (presumably) enriched in
minerals lying in the cotectic direction from a primitive mantle composition. So it is
not difficult to see how to form the crust. Crust is still forming, in the sense that one
has continental volcanism, but this is offset by the return of continental crust to the
mantle via erosion, sedimentation and subsequent subduction of ocean floor sedi-
ments. It may be the case that crust production and removal has produced a steady
state, but this is not really known. The break-up of the crust to form the continents
is presumably entirely associated with active plate tectonics.
9.3.7 MORB, OIB, CFB
Tables 9.4 and 9.5 show the major oxide and silicate chemistry associated with
some mid-ocean ridge basalts (MORB), ocean island basalts (OIB), continental
556 9 Magma Transport
Table 9.4 Oxide assemblages of different basalts
Basalt SiO
2
Al
2
O
3
Fe
2
O
3
/FeO MgO CaO Na
2
OK
2
O Ba (ppm)
MORB 50 16 11 8 11 3 < 110
OIB 49 14 11 8 10 2 < 1 125
CFB 48 15 11 7 10 3 < 1 280
AlkB 46 16 11 10 9 4 < 148
Table 9.5 Mineral
assemblages of different
basalts
Basalt Plag Px Ol Ne
MORB 55 29 11 –
OIB 48 43 – –
CFB 55 30 8 –
AlkB 50 14 24 5
flood basalts (CFB), and alkali basalts. It can be seen that the overall oxide and sil-
ica content is similar, suggesting similar source rocks, but the crystallisation history
has produced different mineral assemblages. In principle, it is easy to understand
how this can be so, since different temperature-pressure histories will cause such
variations due to the dependence of the phase diagrams on both variables.
Table 9.4 also shows the concentration (in ppm, parts per million by weight, or
µg/g) of one of the trace elements, Ba (barium). Trace elements are those present in
minute quantities in mantle rocks, and there are many of them (rubidium Rb, tho-
rium Th, uranium U, etc.). These are all incompatible elements, in the sense that the
atoms do not fit well into the crystalline silicate structure, and melt preferentially in
the mantle; they are mostly found in the continental crust for this reason. It is no-
ticeable that MORB is depleted (relative to OIB) of Ba, and this is also true of other
incompatible elements. Together with the analysis of radioactive isotope ratios, this
has been taken to infer that the sources of OIB and MORB have been chemically
separated for a period of the order of 2 Ga (2 ×10
9
y), and this in turn has been
interpreted as evidence for some form of layered convection. Since OIB presum-
ably comes from mantle plumes from the lower mantle, while MORB comes from
subducting plate driven recirculation of the upper mantle, this is not too shocking,
and the phase change at 670 km, perhaps with a density increase of several per cent,
may help to maintain a separate circulation in the lower mantle.
9.3.8 Granite
As indicated by Table 9.1 and Fig. 9.3, granite is a highly silicic rock with very little
magnesium or iron. It primarily consists of feldspar (orthoclase), quartz, plagioclase
and mica, and most simply can be viewed as the crystallised end product of a magma
which is itself the differentiated melt of more basic magma. That is to say, complete
9.4 Melt Transport in the Asthenosphere 557
melting of a mean mantle composition followed by crystallisation (and settling out)
of olivines and pyroxenes would leave a residual melt of potentially granitic type.
However, there are two (related) problems with this idea. The first is that as the
silica content of a magma increases, the viscosity increases enormously. For ex-
ample, plagioclase magma viscosity changes from about 2 Pa s to 10
5
Pa s as the
composition changes from anorthite (43% SiO
2
) to albite (69% SiO
2
). This raises
the physical question of whether granitic magma can separate from its earlier form-
ing crystals at all before freezing. For example, one does not find granite dikes,
presumably for this reason (they would freeze before they could form).
The second problem is that granites are often emplaced as enormous batholiths
with linear dimensions of order 50 km, which are abundantly in evidence at the
Earth’s surface. Dartmoor in Devon, Southwest England, is a granite of some 40 km
diameter and about 290 Ma age, and batholiths of this size litter the Earth. Such
batholiths are far too large to be explained by differentiation of more basic magma,
and must have formed by anatexis—the melting of crustal rock by (for example) an
upwelling mantle plume. Such batholiths may then have solidified in situ, if they
were too viscous to rise as diapirs or via crack formation through the crust. Their
exposure at the Earth’s surface then owes its occurrence to the effects of crustal
erosion, together with the fact that granite is hard rock and difficult to erode. The
erosion of 40 km of continental crust over 300 Ma indicates a surface erosion rate
of 0.13 mm a
−1
, which is comparable to the average inferred rate measured from
fluvial sedimentary discharge, of 0.03 mm a
−1
.
In the following sections we will examine some of the physical processes asso-
ciated with generation and transport of magma.
9.4 Melt Transport in the Asthenosphere
As mantle rock rises at mid-ocean ridges or in hot spots such as that underneath
Hawaii, it begins to melt. The melt should first form at the cotectic temperature,
yielding a cotectic melt which is lighter than the surrounding rock. For thermody-
namic reasons, it is thought that this melt forms at three-grain junctions, forming a
connected tubular network (see Fig. 9.2) which is therefore permeable, and the melt
will tend to rise.
The cotectic temperature will depend on pressure, and the cotectic fluid compo-
sition will also, but we begin by supposing that the liquid composition is constant.
We then assume that the cotectic temperature is given by
T =T
0
+Γp
l
, (9.8)
where p
l
is the pressure in the connected liquid phase, and T is the absolute temper-
ature. (This allows for the possibility of differential stress in the solid grains, since
the Clapeyron relation (9.8) must actually relate melting temperature to the normal
interfacial stress, which in the liquid can be taken to be simply the pressure.)
Let us suppose the solid and liquid densities are ρ
s
and ρ
l
, and are constant (at
least in the sense of the Boussinesq approximation). If the solid and liquid have
558 9 Magma Transport
velocities u and v, and the volumetric melt fraction is φ, then conservation of mass
of solid and liquid take the form
ρ
l
φ
t
+∇.(φv)
=S,
ρ
s
−φ
t
+∇.
(1 −φ)u
=−S,
(9.9)
where S is the rate of melting (which is not known apriori).
It is customary to suppose that the fluid motion is described by Darcy’s law, thus
φ(v −u) =−
k
η
l
[∇p
l
+ρ
l
g
ˆ
k], (9.10)
where k is permeability, η
l
is liquid viscosity, g is gravity, and
ˆ
k is a unit vector di-
rected vertically upwards. Because of the tubular nature of the liquid phase network,
it is appropriate to take
k =
d
2
g
φ
2
b
, (9.11)
where d
g
is grain size, and b is a constant taking a value in the range b ≈100–1000.
We define a permeability coefficient
Π
0
=
d
2
g
η
l
b
. (9.12)
Before proceeding, we step back for a moment. We can write (9.10)intheform
0 =−φ∇p
l
−ρ
l
φg
ˆ
k −
η
l
b
d
2
g
(v −u). (9.13)
More generally, the averaged momentum equations for two-phase flow (with no
inertia terms) are
0 =∇.(φ
¯
σ
l
) −ρ
l
φg
ˆ
k +M,
0 =∇.
(1 −φ)
¯
σ
s
−ρ
s
(1 −φ)g
ˆ
k −M,
(9.14)
where
¯
σ
l
and
¯
σ
s
are the locally phase-averaged liquid and solid stress tensors, and
M is the interfacial momentum source, defined as
M =−
σ
l
.∇X =−σ
s
.∇X, (9.15)
where X is the phase function, X(x) =1ifx is in the liquid phase, X =0ifx is in
the solid phase. The two expressions in (9.15) are equal, because
f.∇X =f
n
, (9.16)
where f
n
is the interfacial average of the normal component of f, where n points
from solid to liquid, and because (σ
s
−σ
l
).n =0 at the interface (due to equal and

9.4 Melt Transport in the Asthenosphere 559
opposite reactions). It is reasonable to suppose that the liquid pressure does not vary
on the microscale, so that, if σ =−p
l
δ +τ
l
, then, since also ∇X =∇φ,
M =p
l
∇φ +M
, (9.17)
where
M
=−τ
l
.∇X (9.18)
is the interactive drag. Then (9.14) becomes
0 =−φ∇p
l
+∇[φ
¯
τ
l
]−ρ
l
φg
ˆ
k +M
,
0 =∇.
(1 −φ)
¯
σ
s
−ρ
s
(1 −φ)g
ˆ
k −p
l
∇φ −M
.
(9.19)
Darcy’s law follows from the assumption that
¯
τ
l
=0 (which is reasonable given the
long time scale and low melt viscosity), and
M
=−
η
l
b
d
2
g
(v −u), (9.20)
which is the drag due to viscous intergranular pore flow.
The second momentum equation describes the bulk motion of the solid matrix.
Note that alternatively we may consider the equation of total momentum conserva-
tion,
0 =−∇(φp
l
) +∇.
(1 −φ)
¯
σ
s
−
ρ
l
φ +ρ
s
(1 −φ)
gk. (9.21)
In order to solve this, we need to prescribe a constitutive relation for the solid stress.
First, note that in general the solid pressure will not be equal to the liquid pres-
sure. Indeed, we can expect solid pressure to approach lithostatic values, but liquid
pressure to approach (lesser) hydrostatic values, and the resultant tendency must
be to drive melt upwards (and thus solid downwards). However, the resultant solid
compaction is unlike the settling of a suspension since the grains are in contact. Dif-
ferential pressure between solid and liquid will cause viscous deformation on the
long time scales of relevance here. Just as we did for the liquid, it seems reasonable
to propose for the solid phase that σ
s
=−p
s
δ +τ
s
, where p
s
is the locally-averaged
pressure, so that only the deviatoric stress τ
s
varies at the granular microscale.
Similarly, just as Darcy’s law (i.e., (9.20)) can be posited on the basis of a local
Poiseuille flow in the cylindrical pores, so a constitutive equation to relate p
s
to p
l
can be posited on the basis of a model of closure of a cylindrical void in a viscous
medium.
To be specific, let us consider a circular void of radius r =a in an infinite viscous
medium; the void pressure is p
l
and the far field medium pressure is p
s
. Suppose
the grain has (constant) viscosity η
s
. Then mass conservation of the grain implies
that the radial velocity in the grain is
u =
C
r
, (9.22)
