Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


122 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
3.4 Harmonic Approximation
3.4.1 Harmonic Entropy of Point Defects
Besides point defect formation energies, their formation entropies
are another important ingredient required for calculating equilibrium
defect concentrations. The defect formation entropy is traditionally
attributed to perturbations of atomic vibrations in the defect core as
well as around it. The electronic entropy and other entropy effects can
also make contributions, but their role has not yet been examined in
much detail. As far as the vibrational entropy is concerned, its calcula-
tions are commonly based on the harmonic approximation to atomic
vibrations.
[62, 63]
In the harmonic approximation, the potential energy of a statically
relaxed simulation block is approximated by a Taylor series up to the
second-order terms with respect to small atomic displacements from
equilibrium. For a system containing N atoms, a 3N 3N dynamical
matrix,
D
iajb
∂x
∂
ia
2
E
∂x
jb
,(11)
is constructed, where E is the potential energy, x
ia
is the displacement of
atom i in Cartesian direction a, and m
i
is the atomic mass. The normal
vibration frequencies of the block are determined as n
i
l
i
, where l
i
are positive eigenvalues of the dynamical matrix. The total number M of
normal modes depends on the boundary conditions. A block with fixed
boundary conditions in all three directions has M 3N normal modes,
while for a supercell M 3N 3 because three vibrational modes are
replaced by translations of the center of mass.
[63]
Knowing the normal
vibration frequencies, the free energy G associated with atomic vibrations
at temperature T can be found from the standard quantum-mechanical
expression:
G k
B
T
Σ
M
i1
ln
2sinh
, (12)
where h is Planck’s constant and k
B
is Boltzmann’s factor. The vibrational
entropy S is readily obtained from this expression as the temperature
derivative, S ∂G∂T. In the commonly used classical approximation
hn
i
2k
B
T
1
m
i
m
j
Ch_03.qxd 11/29/04 6:08 PM Page 122

ATOMISTIC COMPUTER SIMULATION OF DIFFUSION, MISHIN 123
(all hn
i
k
B
T), we have:
S k
B
Σ
M
i1
ln Mk
B
. (13)
The entropy s
f
v
of vacancy formation in an elemental crystal is determined
as the entropy of a relaxed simulation block with a single vacancy minus
the entropy of a perfect lattice block containing the same number of
atoms. Importantly, because the two entropies refer to the same number of
vibrational degrees of freedom, the factors k
B
T and Mk
B
in Eq. (13) cancel
out, and s
f
v
turns out to be a temperature-independent quantity that only
depends on the relevant vibration frequencies:
s
f
v
k
B
ln .
(14)
Here, n
i
and n
i
0
represent normal vibration frequencies in the defected and
perfect crystal, respectively. This expression holds true for any point
defect as long as the classical harmonic approximation is at work. In the
quantum-mechanical regime (low temperatures), the defect formation
entropy does depend on the temperature.
3.4.2 Effect of Boundary Conditions on
Point-Defect Entropy
Like with the defect formation energy, the defect formation entropy also
depends on the choice of boundary conditions. In particular, the constant-
volume and zero-pressure relaxation schemes result in two different defect
formation entropies, which we denote as (s
f
)
V
and (s
f
)
p
, respectively. We are
usually interested in (s
f
)
p
in the context of diffusion calculations. It can be
shown that both (s
f
)
V
and (s
f
)
p
tend to their infinite-system values linearly in
1N as we increase the number of atoms in the block.
[61]
However, in con-
trast to the defect formation energy (which converges to the same value
regardless of the boundary condition), the two extrapolated entropies are
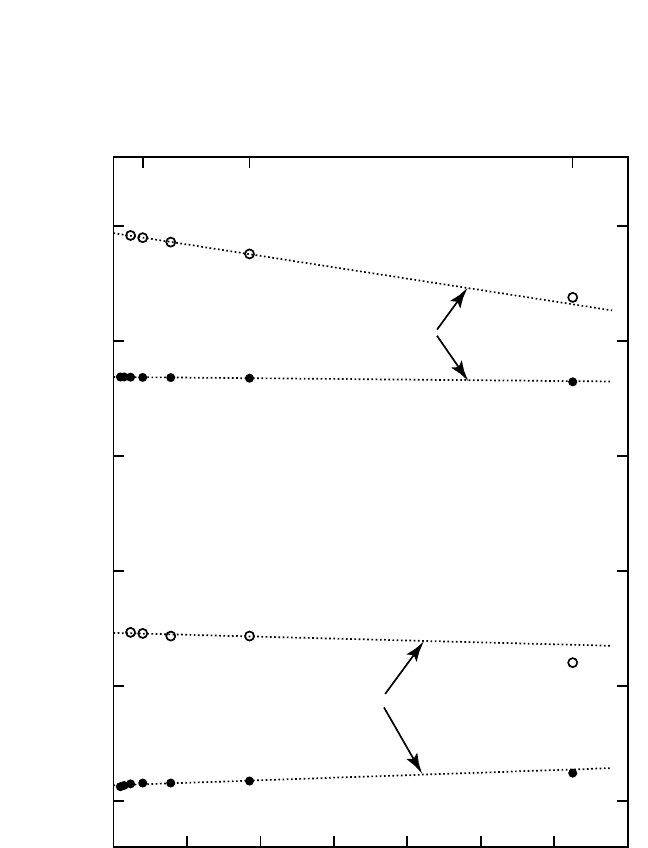
generally different. This fact is illustrated in Fig. 3.2 for a vacancy in copper.
Thus, even in an infinitely large system, the defect formation entropy
depends on the external conditions, and in particular is different under the
constant-volume and zero-pressure conditions. The difference between the
M
i1
n
i
M
i1
n
i
0
hn
i
k
B
T
Ch_03.qxd 11/29/04 6:08 PM Page 123
124 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
0.5
1.0
1.5
2.0
2.5
3.0
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5
32108500
Vacancy formation entropy / k
B
10
2
/N
N
V=const
p=0
LHA
LHA
FHA
FHA
Figure 3.2 Vacancy formation entropy in copper as a function of the inverse num-
ber of atoms (N) in a cubic simulation block under constant-volume (V const)
and zero-pressure (p 0) boundary conditions.
[61]
The vacancy formation entropy
in a macroscopic crystal is evaluated by a linear extrapolation to 1N
→ 0.
Calculations were performed with an embedded-atom potential in the full har-
monic approximation (FHA) and the local harmonic approximation (LHA).
Ch_03.qxd 11/29/04 6:08 PM Page 124

ATOMISTIC COMPUTER SIMULATION OF DIFFUSION, MISHIN 125
two entropies can be evaluated from well-known thermodynamic rela-
tions,
[64]
which lead to:
[60, 61, 65, 66]
(s
f
)
p
(s
f
)
V
bB∆V, (15)
where ∆V is the relaxation volume of the defect formation, B is the bulk
modulus, and
b
p0
(16)
is the thermal expansion coefficient of the material at zero temperature
and pressure. The latter can be readily determined by independent com-
putations.
[61]
Notice that for defects whose formation is accompanied by a
significant volume effect, the difference (s
f
)
p
(s
f
)
V
can be quite large.
For example, it equals 1.7k
B
for a vacancy and 13.9k
B
for a self-interstitial
in copper.
[61]
To deduce the correct defect formation entropy, it is essential to per-
form the full (i.e., both atomic and volume) relaxation of the defected
block prior to the harmonic calculations. Alternatively, the volume can be
kept constant and (s
f
)
V
determined, but then (s
f
)
p
should be recovered
from Eq. (15). For point defects in Cu, the second scheme has proved to
be almost as accurate as the first.
[61]
3.4.3 Embedded Cluster Method
A major problem associated with harmonic calculations is their signifi-
cant computational cost. The calculation of the dynamical matrix for a simu-
lation block large enough to represent a defect still remains beyond the reach
of first-principles methods. Using interatomic potentials, the dynamical
matrix can be calculated relatively fast, but then the bottleneck of the compu-
tation shifts to its diagonalization. The diagonalization of a dynamical matrix
is a computationally demanding procedure which, for very large systems, is
associated with serious numerical problems. Because of this limitation, full
harmonic calculations become impractical beyond a certain block size (typi-
cally, N ≈ 10
3
). While for a single vacancy or other simple defect an accurate
formation entropy can be obtained with a few hundred atoms (Fig. 3.2), more
complex defects or defect clusters require more atoms. Anumber of approxi-
mate harmonic methods have been developed for dealing with large systems,
such as the embedded-cluster method,
[61, 67]
the local harmonic approxima-
tion,
[68]
the second-moment approximation,
[69–71]
and other methods.
[72]
∂V
∂T
1
V
Ch_03.qxd 11/29/04 6:08 PM Page 125

126 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
In the embedded-cluster method, only some of the free atoms, namely
those forming a cluster centered on the defect core, are treated as
dynamic; all other atoms are treated as static. Thus, the defect is created
and statically relaxed in a large simulation block, but the dynamical
matrix is only constructed and diagonalized for a relatively small cluster
embracing the defect. The defect formation entropy is then computed
using the dynamic atoms only. The elasticity theory analysis, as well as
atomistic simulations, show that the defect entropy S
*
delivered by the
embedded-cluster method equals approximately:
[61]
S
*
S
c
S
el
. (17)
Here, S
c
is the so-called defect core entropy, S
el
is the entropy associ-
ated with the elastic strain field around the defect, N
*
is the number of
atoms in the cluster, and N is the total number of atoms in the simula-
tion block. Given that the true formation entropy of the defect is
s
f
S
c
S
el
, we see that the cluster entropy S
*
is incomplete: it
includes only a part of the elastic strain entropy S
el
in proportion to
N
*
N. By calculating S
*
for several N
*
N values and extrapolating this
function to N
*
N → 0, we can determine the defect core entropy S
c
. The
latter turns out to be a well-defined physical quantity that does not
practically depend on the boundary conditions and characterizes the
degree of local distortions within the defect core. For example, in cop-
per S
c
1.73k
B
for a vacancy and 5.75k
B
for an interstitial.
[61]
On the
other hand, the linear extrapolation to N
*
N → 1 should give us s
f
. In
practice, the accuracy of the obtained s
f
value is not very high because
Eq. (17) relies on strong approximations, including a spherical symme-
try of the elastic field around the defect.
Amore accurate scheme, called the “elastically corrected” embedded-
cluster method, has been proposed.
[61]
In this method, the cluster entropy
S
*
is augmented by a quasi-continuum term S
q
such that the sum of S
*
and
S
q
equals the true formation energy of the defect:
s
f
S
*
S
q
. (18)
The quasi-continuum term represents the entropy associated with the elas-
tic strain field outside the cluster. The calculation of S
q
is based on the
thermodynamic fact that the entropy density of a continuum (entropy per
unit volume) equals bp, where p is the local hydrostatic pressure
(negative of hydrostatic stress). This fact follows from the standard
N
*
N
Ch_03.qxd 11/29/04 6:08 PM Page 126

ATOMISTIC COMPUTER SIMULATION OF DIFFUSION, MISHIN 127
theromodynamic relation:
[64]
T
bV, (19)
where S is the system entropy and V is its volume. Based on this relation,
S
q
is represented by the sum:
S
q
b
Σ
i
p
i
Ω
i
, (20)
where p
i
and Ω
i
are hydrostatic pressures and atomic volumes associated
with individual lattice sites. The summation is performed over all atoms
outside the cluster. The product p
i
Ω
i
can be readily calculated within the
EAM scheme, as well as with any other semi-empirical potential. We
emphasize that Eqs. (18) and (20) are more general than Eq. (17). They
are not restricted to any symmetry and only require that the lattice strains
beyond the cluster be within the linear elasticity range. This general char-
acter makes the method applicable to point defects in GBs and disloca-
tions, to defect clusters, and to other complex situations.
This method again appeals to the idea that, in a large simulation block,
most atoms belong to elastically strained lattice regions whose direct
atomistic treatment is computationally inefficient. Instead, properties of
such regions (entropy, in this case) can be well represented in a continuum
approximation, while the highly distorted regions comprising the defect
cores are treated atomistically. We can notice a parallel with similar ideas
currently emerging in the area of multiscale modeling of materials.
Because the calculation of S
q
from Eq. (20) is a fast linear-N procedure,
the quasi-continuum term comes at almost no extra cost in comparison
with the cluster term S
*
. This computational advantage makes the method
applicable to very large systems, including those involving multiple
length scales. Simple tests
[61]
have demonstrated a high accuracy and
excellent computational efficiency of the method.
3.4.4 Local Harmonic Approximation
An even greater acceleration of entropy calculations can be achieved
by adopting the so-called local harmonic approximation (LHA).
[68]
In this
approximation, the off-diagonal terms of the dynamical matrix, which are
responsible for the coupling of vibrations of different atoms, are neglected:
D
iajb
0, i j. This reduces the dynamical matrix to a block-diagonal
form consisting of 3 3 blocks, each representing the local dynamical
∂S
∂p
Ch_03.qxd 11/29/04 6:08 PM Page 127

128 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
matrix D
i
L
with elements D
iaib
. The problem thus reduces to diagonalizing
the local dynamical matrix for each atom, or, equivalently, calculating its
determinant D
i
L
. For example, the classical entropy expression, Eq. (13),
in LHA becomes:
S 3k
B
Σ
N
i1
ln 3Nk
B
. (21)
In the second-moment method proposed by Sutton,
[69, 70]
the summa-
tions over the density of vibrational states are expressed through the local
second moments, m
i
∑
a
D
iaia
. Aslight modification of this method was
proposed by Foiles.
[71]
Although the derivation of the basic equations in
both cases proceeds along different lines in comparison with the LHA,
this boils down to neglecting even the diagonal elements of the local
dynamical matrices. This obviously means a more drastic approximation
than LHA.
An attractive feature of LHA and the second-moment methods,
besides their computational efficiency, is the feasibility of finding the
thermodynamic equilibrium of a system at a finite temperature by mini-
mizing its free energy with respect to all atomic displacements. This idea
has been implemented in the free-energy minimization method by LeSar
et al.
[68]
and through temperature-dependent interatomic forces by Sutton.
[69, 70]
Due to the relatively simple form of the free energy, analytical expressions
can be derived for its derivatives with respect to individual atomic coor-
dinates (thermodynamic forces), which enables the use of gradient methods
for minimizing the free energy. Unfortunately, the crude approximations
underpinning LHAand the second-moment methods inevitably result in a
significant loss of accuracy compared with the full harmonic scheme.
[71–73]
Taking a Cu vacancy as an example, LHA gives the vacancy formation
entropy (at zero pressure) a factor of two lower than the full harmonic
value (Fig. 3.2). Thus, when the free energy is minimized within LHA, the
optimized atomic coordinates can differ significantly from their true equi-
librium positions.
3.4.5 Quasi-Harmonic Approximation
For a perfect crystal, the full harmonic free energy can be readily min-
imized with respect to the crystal volume, thus giving the thermal expansion
factor at a chosen temperature. This minimization is easy to implement
due to its one-dimensional character. Indeed, the dynamical matrix and
thus the free energy can be computed for several volumes, and the free
hD
i
L
16
k
B
T
Ch_03.qxd 11/29/04 6:08 PM Page 128

ATOMISTIC COMPUTER SIMULATION OF DIFFUSION, MISHIN 129
energy minimum can be located by a polynomial interpolation. This
scheme is often referred to as the quasi-harmonic approximation
[71, 72]
to
emphasize that the anharmonicity-caused thermal expansion is evaluated
through harmonic calculations. Unfortunately, an extension of this
scheme to a simulation block containing a defect is problematic. Since
thermal expansion is then nonuniform, the free energy should be mini-
mized with respect to all atomic positions, a problem too ambitious for
today’s computers. As a rough approximation, a statically relaxed simula-
tion block can be simply expanded uniformly by the thermal expansion
factor of the perfect lattice, followed by the construction of the dynamical
matrix and calculation of the defect formation entropy.
[71]
This way of
including the thermal expansion into the defect formation entropy has
proved to work well in some cases
[71, 74]
but fails in other cases where some
of the eigenvalues of the dynamical matrix after the expansion become neg-
ative. Such failures are not surprising since the neglect of the local thermal
expansion in the defect core introduces an uncontrollable approximation.
There are methods of thermodynamic calculations beyond the har-
monic approximation. Perhaps the most common of them are various
thermodynamic integration schemes
[56, 72]
and the adiabatic switching
method,
[75, 76]
which can be implemented within either Monte Carlo or MD
simulations. Such methods have been applied to calculate the formation
entropy of point defects in metals
[71, 76]
and intermetallic compounds.
[77]
Although very powerful, such methods require extremely extensive com-
putations for achieving a reasonable accuracy. They are also useful as a
reference for assessing the applicability range of harmonic methods. As
expected, deviations between harmonic and “exact” methods are observed
at high temperatures, often already above half of the melting temperature.
The onset temperature and degree of such deviations depend on the par-
ticular defect type, the atomic interaction model, and other factors.
[72]
3.5 Equilibrium Defect Concentrations
3.5.1 Elemental Solids
The knowledge of the formation energies and entropies of point
defects is required for calculating their equilibrium concentrations. For
example, the equilibrium vacancy concentration (number of vacancies per
lattice site) in an elemental solid at temperature T is given by:
c
v
exp
e
f
v
k
B
T
Ts
f
v
. (22)
Ch_03.qxd 11/29/04 6:08 PM Page 129

130 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
A similar equation holds for self-interstitials and other simple defects,
except that a pre-exponential factor z should be introduced to reflect the
entropy arising from the multiplicity of possible orientations of the defect.
For example, a self-interstitial in fcc metals typically exists as a split
dumbbell centered on a lattice site. The dumbbell possesses an additional,
“rotational” entropy k
B
ln z, z being the number of its symmetrically
equivalent orientations. Thus, the concentration of a general point defect
can be written as:
c z exp
. (23)
Factor z is often eliminated by including the term k
B
ln z into the defect
formation entropy s
f
. Another way of writing Eq. (23) is:
c exp
, (24)
where
g
f
e
f
Ts
f
(25)
is the free energy of defect formation. At this point, we assume that the
external pressure is zero. The effect of high pressures on defect concen-
trations is discussed in Sec. 3.5.3.
3.5.2 Non-Stoichiometric Compounds
The situation is more complex in ordered compounds due to the inher-
ent multiplicity of point defects.
[39, 78–80]
Vacancies can occupy different
sublattices and thus have different formation energies and entropies.
Compounds are also able to support antistructural atoms, or antisites
(atoms occupying the wrong sublattice), and in some cases also intersti-
tials. Since all point defects should be in equilibrium with each other, the
vacancy concentration cannot be determined separately without considering
all other defects. In other words, even if we are only interested in the
vacancy concentration, we still have to solve the problem of global
dynamic equilibrium in the entire defect system.
As an example, consider an equiatomic intermetallic compound AB.
We assume that the structure of this compound consists of two sublattices
g
f
k
B
T
e
f
Ts
f
k
B
T
Ch_03.qxd 11/29/04 6:08 PM Page 130

ATOMISTIC COMPUTER SIMULATION OF DIFFUSION, MISHIN 131
a and b, which in the perfectly ordered state are occupied by atoms Aand
B, respectively. The compound can support four types of point defects:
vacancy on sublattice a (V
a
), vacancy on sublattice b (V
b
), antisite on
sublattice a (B
a
), and antisite on sublattice b (A
b
). Interstitial atoms are
neglected for simplicity. The creation of each point defect slightly changes
the chemical composition of the compound. The latter, therefore, depends
on all four defect concentrations and can deviate from the exact stoichio-
metric composition. Statistical models describing the defect equilibrium
in compounds normally treat point defects as a lattice gas of noninteract-
ing particles.
[39, 78–80]
In our case, we deal with a four-component gas. The
particles of the gas can only interact through defect reactions, which are
similar to chemical reactions in a real gas. For example, an exchange of a
regular atom B with a neighboring vacancy V
a
is represented by the defect
reaction:
V
a
∆ B
a
V
b
. (26)
The equilibrium in the defect gas can be described by writing the mass
action law for three independent reactions.
[80]
The fourth equation
expresses the constraint that the defect concentrations should match a par-
ticular chemical composition of the compound. These four simultaneous
equations are solved numerically for four equilibrium defect concentra-
tions. That way, the defect concentrations can be computed as functions
of the chemical composition and temperature. The input data for this cal-
culation are the free energies of the chosen defect reactions. These can be
expressed by linear combinations of the respective formation free energies
g
f
of the defects involved in the reactions.
Instead of dealing with individual point defects, it is more convenient
to introduce hypothetical composition-conserving defect complexes.
[39, 78]
For example, the formation of a divacancy V
a
V
b
or an exchange defect
B
a
A
b
does not change the chemical composition of the compound.
Such complexes are assumed to be totally dissociated, so that interactions
between their constituent defects can be neglected. The advantage of deal-
ing with composition-conserving complexes is that all reference constants
involved in the energies and entropies of individual defects always cancel
out when combined into a complex energy or entropy.
[78]
This allows
direct comparison of results obtained by different calculation methods, for
example by EAM and first-principles calculations.
The language of composition-conserving complexes also has certain
conceptual advantages that have been well discussed in the literature.
[39]
Point defects can be thought of as appearing and disappearing in the form
of such complexes. Furthermore, the complexes can be viewed as ele-
mentary excitations of thermal disorder that follow Boltzmann’s distribution.
Ch_03.qxd 11/29/04 6:08 PM Page 131
