Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

20.5 Sigmatropic Shift Reactions 1049
This kind of process, in which starting material rearranges into itself, is called a
degenerate reaction (p. 1031). Isotopically labeled molecules reveal the degenerate
reaction and allow a determination of the kinetic activation parameters. The reac-
tion requires an activation energy of 36–38 kcal/mol. Other labels are possible, of
course,and substituted molecules will reveal the reaction as well as the more sophis-
ticated isotopic labels. In most substituted cases the reaction is no longer degener-
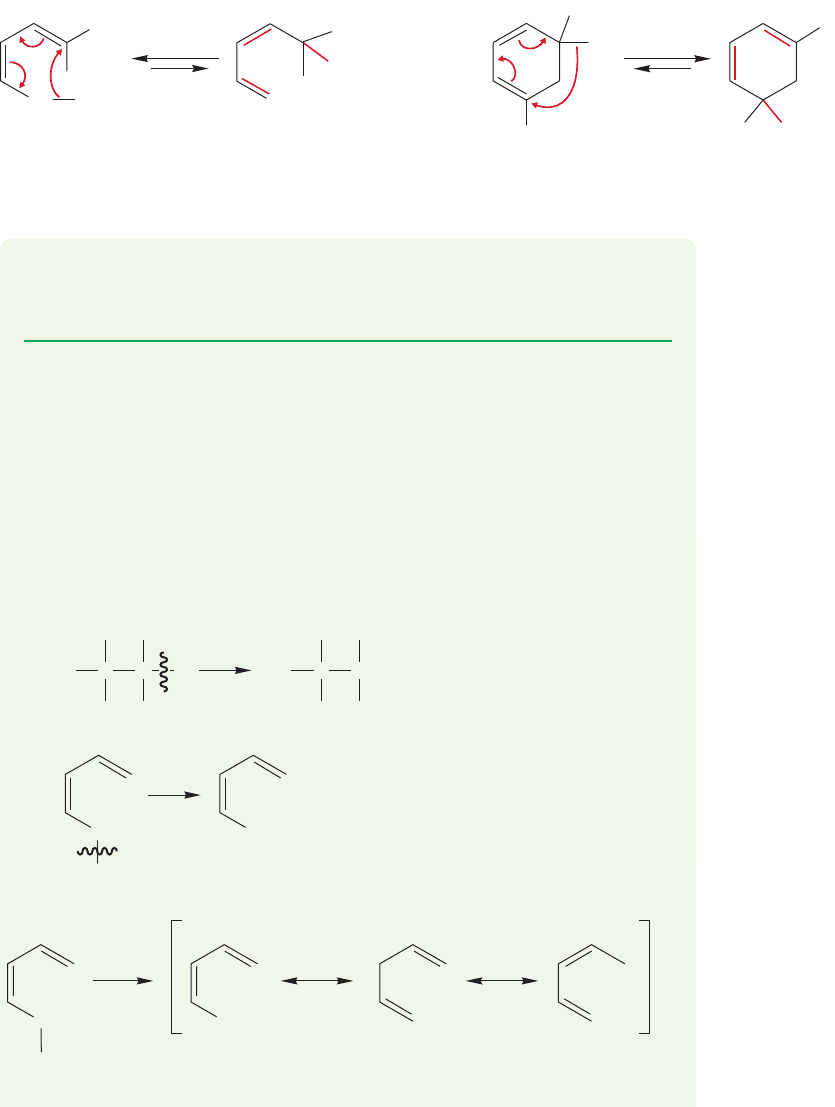
ate and the equilibrium constant K for the reaction can no longer equal 1 (Fig.20.31).
PROBLEM 20.11 Which of the molecules in each of the two reactions of Figure 20.31
will be favored at equilibrium? Explain your choice. Draw the product of heating
(Z)-3-methyl-1,3-pentadiene. Is this reaction degenerate or not?
WORKED PROBLEM 20.12 Estimate the bond dissociation energy (BDE) for the
migrating carbon–hydrogen bond in 1,3-pentadiene. Why is the observed activa-
tion energy of 36–38 kcal/mol for the [1,5] shift of hydrogen shown in Figure
20.30a so much lower than your answer?
ANSWER The carbon–hydrogen bond in ethane has a BDE of 101 kcal/mol
(Table 8.2). But the pentadienyl radical is resonance stabilized and that stabiliza-
tion will begin to be felt as the bond breaks, further decreasing the BDE. If allylic
resonance is worth about 13 kcal/mol (p. 475), we might expect the BDE to be
about 101 13 88 kcal/mol.
CH
3
CH
3
CH
3
CH
3
CH
3
H
H
H
H
H
H
CH
2
CH
2
CH
3
FIGURE 20.31 Substituted molecules also reveal the reaction, but the rearrangement is no longer degenerate,
because starting material and product are different.
H
H
H
C
BDE = 101 kcal/mol
BDE (first guess) = ~101 kcal/mol
H
H
H
C
H
H
C
H
H
H
C
.
.
.
.
H
CH
2
CH
2
HH
.
.
.
.
CH
2
H
CH
2
H
(continued)
1050 CHAPTER 20 Reactions Controlled by Orbital Symmetry
H
1
C
1
C
5
Yet the real BDE is much, much lower than this, about 37 kcal/mol. The reason
is that as the carbon–hydrogen bond begins to break, the new carbon–hydrogen
bond at C(1) is forming. It is not necessary to break the carbon–hydrogen bond
fully to make the very unstable hydrogen radical.
Instead, formation of the new bond is initiated prior to breaking of the
original bond. This bond formation decreases the energy required to
break the bond.
The deuterium labeling experiment of Figure 20.30b reveals that much is hap-
pening before the onset of high-temperature radical reactions. A hydrogen (deuterium,
in this case) atom is moving from one position in the molecule to another.This kind
of reaction has come to be known as a sigmatropic shift.
2
A sigmatropic shift is the
movement of a sigma bond from one atom to another. These reactions can involve
hydrogens, carbons, or heteroatoms.
How are sigmatropic shifts formally described? The first step is to identify the
bond that is broken in the reaction and the bond that is made. In other words, write
an arrow formalism. The bond that breaks in 1,3-pentadiene could be designated
the “1–1”bond. Remember that these numbers have nothing to do with the IUPAC
name. Next, note where the new bond is formed. In Figure 20.32a, the new bond is
[1,5],in (b) it is [2,3], and in (c) it is [3,3].The numbers denoting the shift are always
enclosed in brackets and separated by a comma.
C(1)
O
H
1
C(1)
O
H
1
C(5)
O
H
2
It must be admitted that the Woodward–Hoffmann theory is filled with jargon. There is nothing to do but
learn it.
CH
2
H
H
1
1
2
3
4
5
5
1
[1,5]
Starting point
Other starting point
Terminus
Bond
broken
New bond
(a)
2
1
3
2
1
2
13
2
1
[2,3]
New bond
(b)
2
1
1
2
3
3
[3,3]
2
3
3
2
1
1
(c)
H
2
C
N
H
2
C
N
–
..
+
FIGURE 20.32 (a) In a [1,5]
sigmatropic shift of hydrogen, the
migrating hydrogen atom (atom 1)
moves to atom 5.The new sigma
bond is between atom 1 and atom 5,
hence the shift is [1,5]. (b) In the
ammonium ion shown the reaction
is a [2,3] sigmatropic shift, and in
(c) the shift is [3,3].
CONVENTION ALERT
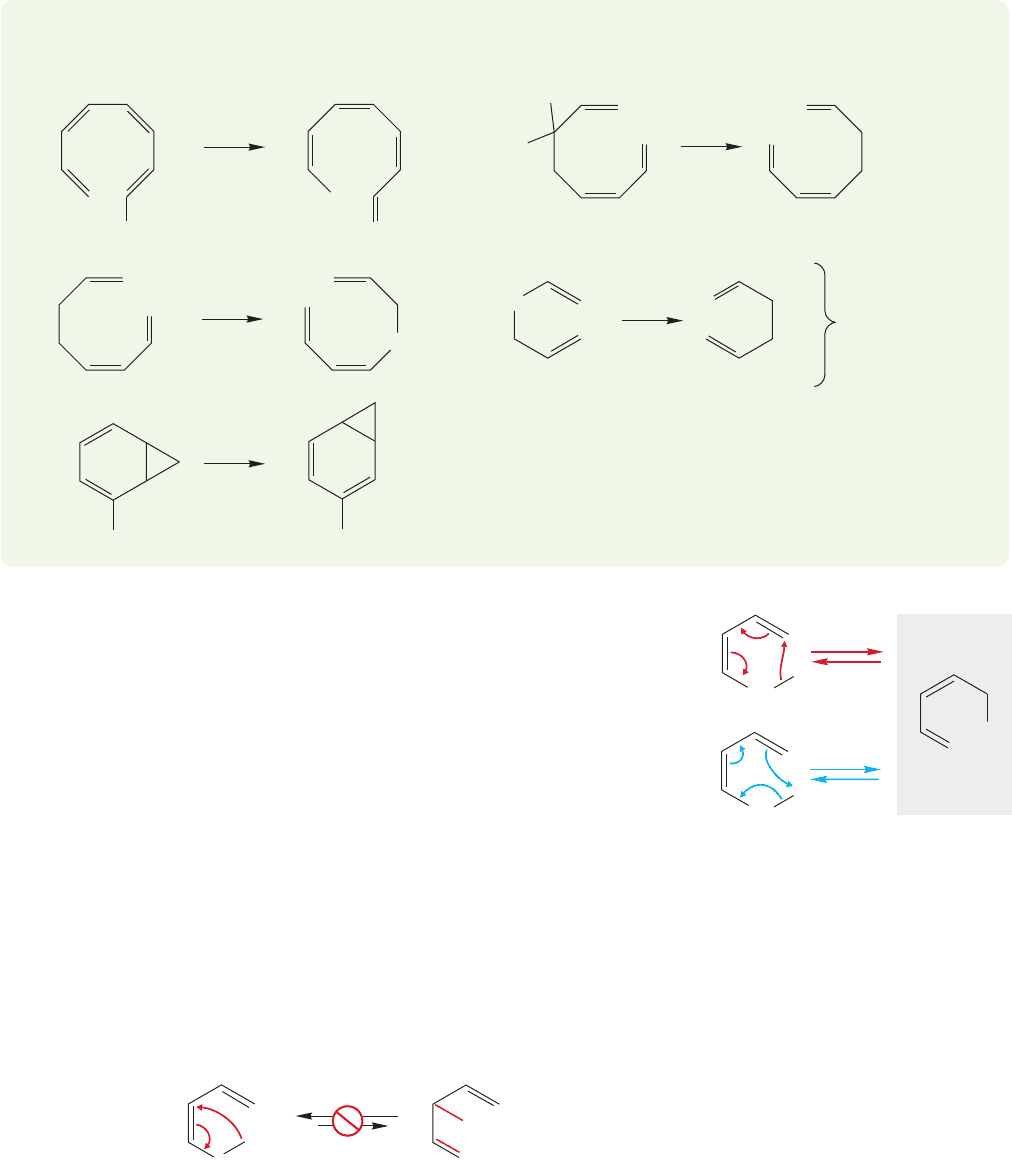
20.5 Sigmatropic Shift Reactions 1051
PROBLEM 20.13 Classify the following reactions as [x,y] sigmatropic shifts.
Which, if any, of the reactions are degenerate?
CH
2
CD
2
CD
2
D
2
C
CH
3
Parts (c) and (d)
are not [1, x ] shifts!
(a)
(b)
(c)
(d)
(e)
RR
CD
2
CD
2
CH
3
O
O
D
D
Sigmatropic shifts respond to attempted mechanistic experiments in true no-
mechanism fashion.Neither acids nor bases strongly affect the reaction, and the sol-
vent polarity, or even the presence of solvent, is usually unimportant. The reaction
proceeds quite nicely in the gas phase. It is simple to construct an arrow formalism
picture of the reaction, but this device does little more than point out the overall
change produced by the migration. In these figures,the arrow formalism is even more
of a formalism than usual.For example, the arrows of Figure 20.33 could run in either
direction,clockwise or counterclockwise.That is not true for a polar reaction in which
the convention is to run the arrows from nucleophile (the Lewis base) toward the
electrophile (Lewis acid).
It is already possible to identify one curious facet of this reaction, and a little
experimentation reveals others. The product of a [1,3] shift is absent. Why, if the
hydrogen is willing to travel to the 5-position, does it never stop off at the 3-posi-
tion? An arrow formalism can easily be written for a [1,3] shift (Fig. 20.34), and it
might reasonably be argued that the [1,3] shift, requiring a shorter path than the
[1,5] shift, should be easier. Why are [1,3] shifts not observed?
H
CH
2
[1,5]
[1,5]
CH
2
H
CH
2
H
FIGURE 20.33 Two arrow formalism
descriptions of the [1,5] shift of
hydrogen in (Z)-1,3-pentadiene.
CH
2
H
1
2
3
4
Not observed
5
[1,3]
Δ
CH
2
H
1
FIGURE 20.34 An arrow formalism
for the [1,3] shift of hydrogen that
does not occur.
A second strange aspect of sigmatopic shifts appears in photochemical experi-
ments. As you already know, it is possible to deliver energy to a molecule in more
than one way. One way is to heat up the starting material.However, conjugated mol-
ecules absorb light (p. 529), light quanta contain energy, and this gives us another
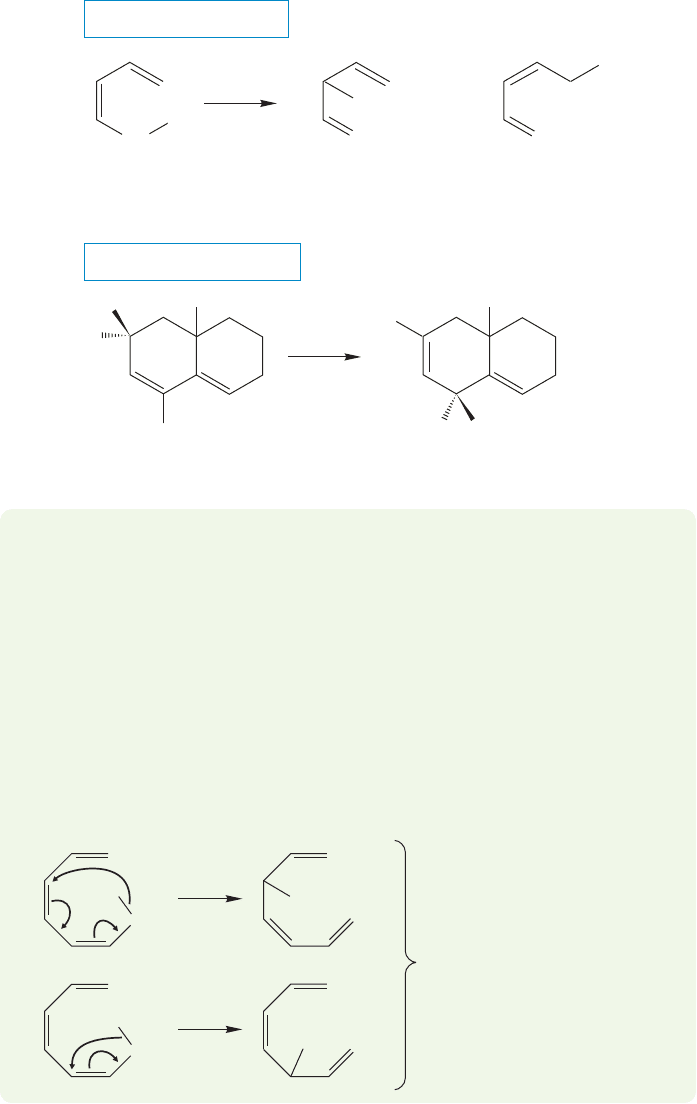
1052 CHAPTER 20 Reactions Controlled by Orbital Symmetry
CH
2
CH
2
H
H
Product of
a [1,3] shift
Product of
a [1,5] shift
[1,3] H
shift
not
h
ν
2
3
1
1
1
3
5
4
5
CH
2
H
CH
3
CH
3
H
H
1
1
2
3
CH
3
CH
3
H
H
hν
THE GENERAL CASE
A SPECIFIC EXAMPLE
1
FIGURE 20.35 Photolysis of
1,3-dienes induces [1,3] shifts.
way to transmit energy to the molecule. Curiously, when 1,3-pentadienes are irra-
diated, the products of the reaction include the molecules formed through [1,3]
shifts, but not those of [1,5] shifts (Fig. 20.35). The reactions are sometimes com-
plicated, because other thermal or photochemical reactions often take place, but if
we just concentrate on the products of shifts, this strange dependence on the method
of energy delivery appears.
WORKED PROBLEM 20.14 Perhaps it could be argued that the loss of conjugation
in the product of the [1,3] shift results in an energetic favoring of the [1,5]
process. Design a substituted 1,3-pentadiene that would test this (incorrect)
surmise.
ANSWER This problem may be hard because you are not used to thinking about
designing reactions.The hypothesis is that the [1,5] shift is preferred to the [1,3]
shift because it preserves the conjugation present in a 1,3-diene. In order to test
this surmise,we need to design a molecule in which both the [1,5] and [1,3] shifts
preserve the same amount of conjugation.The deuterium label is needed to reveal
the [1,5] or [1,3] nature of the reaction. Of course a [1,7] shift might also occur
in this reaction.
These are the same molecule
except for the isotopic label
CD
2
CD
2
CD
2
D
D
[1,5]
CD
2
D
D
[1,3]
20.5 Sigmatropic Shift Reactions 1053
In summary, thermal energy induces [1,5] shifts and photochemistry favors [1,3]
shifts. So, any mechanism must provide an explanation for these observations.
Let’s start our analysis by examining the early stages of the [1,5] sigmatropic
shift. As the reaction begins, a carbon–hydrogen bond of the methyl group begins
to stretch (Fig. 20.36a). If this stretching were continued to its limit, it would pro-
duce two radicals, a hydrogen atom and the pentadienyl radical (Fig. 20.36a). We
know this cleavage to give radical intermediates does not happen because the prod-
ucts of recombination of these radicals are not found (Fig. 20.36b).
H
.
CH
2
H
H
.
.
CH
2
CH
2
break the
bond
Radical dimer, not formed
A pair of
radicals
stretch the
bond
CH
2
CH
2
CH
2
2
(a)
(b)
FIGURE 20.36 (a) In the
earliest stages of the
reaction, a carbon–hydrogen
bond begins to stretch.
Ultimately a pair of radicals
would result. (b) We know
this bond breaking does not
take place because the
radical dimer is not found.
We should stop right here to question the assumption that the carbon–hydrogen
bond is breaking in homolytic (radical) fashion. Why not write a heterolytic cleav-
age to give a pair of ions rather than radicals (Fig. 20.37)?
The stability of ions depends greatly on solvation. It is inconceivable that a het-
erolytic bond breaking would not be highly influenced by solvent polarity. As we
have just seen, a change in solvent polarity affects the rate of this reaction only very
slightly. Only homolytic bond breaking is consistent with the lack of a solvent effect.
As the methyl carbon–hydrogen bond begins to stretch (Fig. 20.36a), some-
thing must happen that prevents the formation of the pair of radicals. As we have
already concluded in Problem 20.12, the developing hydrogen atom is intercept-
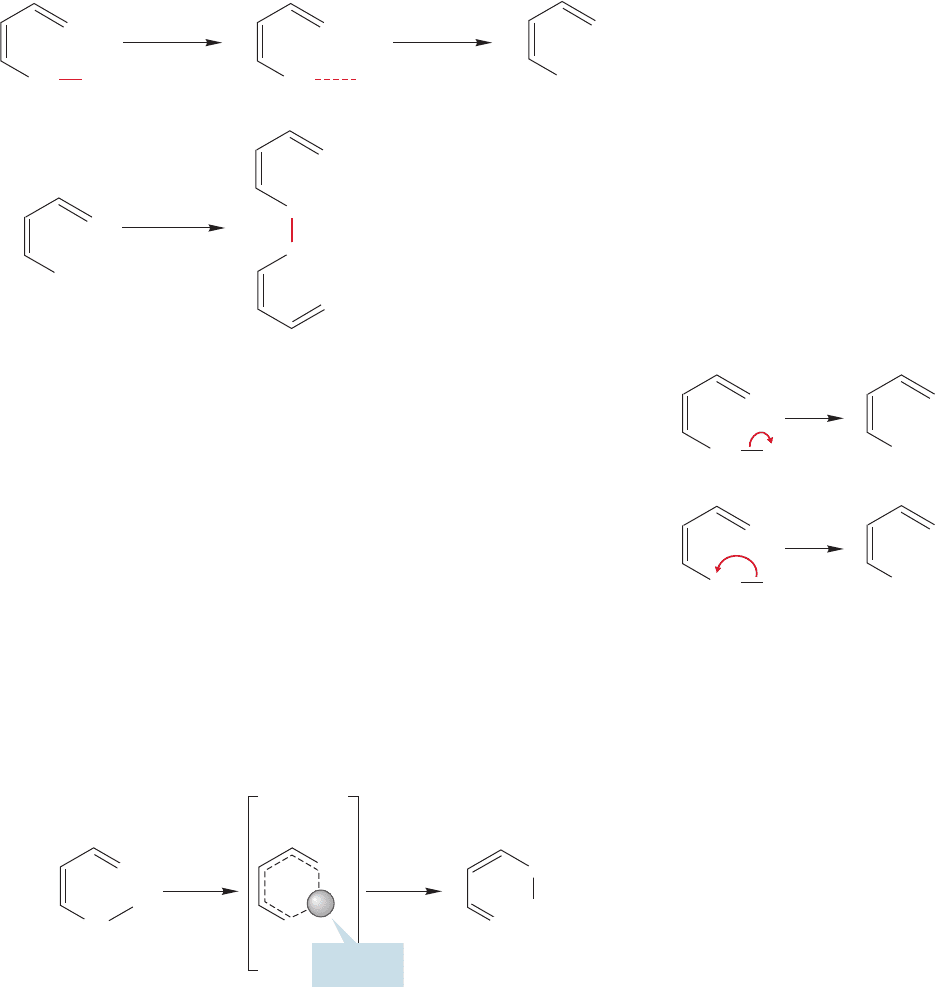
ed by reattachment at atom 5.In orbital terms, we would say that as the
bond breaks, the developing 1s orbital on hydrogen begins to overlap with the p
orbital on the carbon at position 5. Now we have a first crude model for the tran-
sition state for this sigmatropic shift (Fig. 20.38). Notice that the transition state
is cyclic.
C(1)
O
H
H
+
+
H
CH
2
CH
2
–
..
H
CH
2
H
CH
2
+
–
..
or
FIGURE 20.37 There are two possible
ways to break the carbon–hydrogen
bond heterolytically to give a pair of
ions. Either way depends strongly on
solvent polarity. Because there is
essentially no dependence of the rate
of this reaction on solvent polarity,
this mechanistic possibility is
excluded.
H
Transition
state
CH
2
CH
2
1
1
1
1
H
CH
2
1
1
5
5
CH
2
CH
2
5
Hydrogen
1s orbital
CH
2
H
FIGURE 20.38 Radical formation can be thwarted by partial bonding of the migrating
hydrogen to the orbitals on carbon atoms 1 and 5 in the transition state. As the
bond stretches, the hydrogen 1s orbital begins to overlap with an orbital on C(5).
C(1)
O
H
1054 CHAPTER 20 Reactions Controlled by Orbital Symmetry
This model leaves unanswered the serious questions of mechanism: Why does
the migrating hydrogen reattach at the 5-position in thermal reactions and at the
3-position in photochemical reactions? We have answered neither the questions of
regiochemistry ([1,5] or [1,3]) nor of dependence on the mode of energy input (ther-
mal or photochemical).
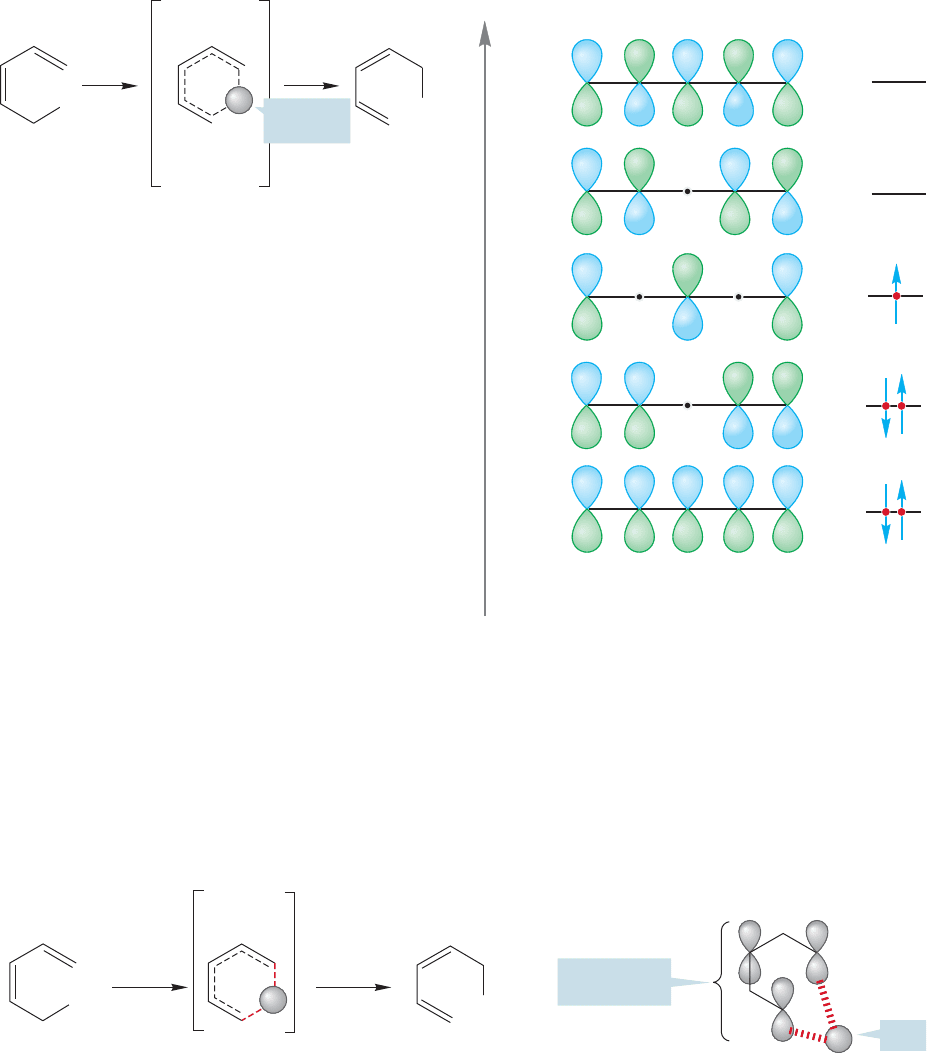
Let’s look more closely at our transition state model. In essence, it describes a
hydrogen atom moving across the π system of a pentadienyl radical (Fig. 20.39).
Pentadienyl
radical
(HOMO)
Energy
π Molecular orbitals of the pentadienyl system
(for the radical, the HOMO is Φ
3
)
Φ
5
Φ
4
Φ
3
Φ
2
Φ
1
H
Transition
state
11
1
2
3
4
H
1
1
5
5
5
.
H
.
Hydrogen
1s orbital
FIGURE 20.39 A model for the
transition state shows a hydrogen 1s
orbital migrating across a pentadienyl
radical, for which the π molecular
orbitals are shown.
The hydrogen atom is simple to describe—it merely consists of a proton and a sin-
gle electron in a 1s atomic orbital.The pentadienyl molecular orbital system isn’t dif-
ficult either.There are five π molecular orbitals and these can be constructed by taking
combinations of five carbon 2p orbitals. We assume again that it is the highest energy
electrons, those most loosely held, that are the most important in the reaction.These
are the electrons in the HOMO. If we accept this assumption, we can elaborate our
picture of the transition state for the reaction to give Figure 20.40. Notice that the
transition state for a [1,5] shift involves six electrons (4n 2). They come from the
sigma bond that is broken and the two double bonds in the π system.
H
HOMO, Φ
3
of
pentadienyl
H
1s
H
Transition
state
H
.
.
H
FIGURE 20.40 An elaboration of our model for the transition state, showing a crude outline of the interaction of the
hydrogen 1s orbital with the lobes of the HOMO for pentadienyl,
3
.£
20.5 Sigmatropic Shift Reactions 1055
Φ
3
of Pentadienyl (HOMO)
Positive overlap
is possible at
C(5)—the orbital
phases are correct!
1s
H
3
2
1
1
5
4
FIGURE 20.41 Now we can elaborate
our transition state model by filling
in the lobes of the HOMO of
pentadienyl,
3
.£
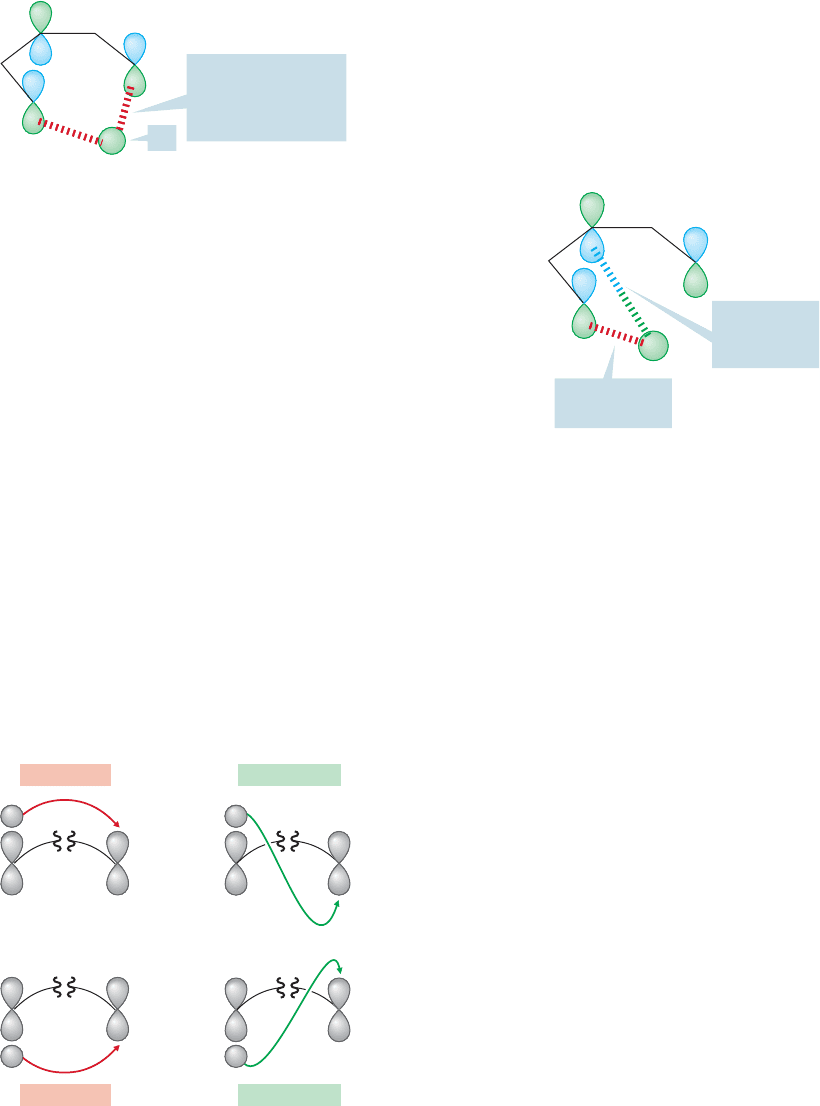
Notice a very important thing about the transition state outlined in Figure 20.40.
In the starting pentadiene, the bond made between the 1s orbital of hydrogen and
the hybrid orbital on carbon atom 1 must of course be made between lobes of the
same phase, which is represented by color coding (Fig. 20.41). As this bond stretches,
the phases of the lobes do not change: The 1s orbital is always fully or partially bonded
to a lobe of the same phase (Fig. 20.41).
In this way, we know the relative symmetries of some of the lobes in the transi-
tion state for the reaction. We also know the symmetry (from Fig. 20.39, or by fig-
uring it out) of Φ
3
, the HOMO of pentadienyl. So, we can elaborate the picture of
the transition state to include the symmetries of all the orbital lobes. The symme-
try of the lobes allows the [1,5] shift to take place as shown.
Notice that the process through which the hydrogen detaches from carbon 1 and
reattaches to carbon 5 from the same side allows the maintenance of bonding
(same sign) lobal interactions at all times.This reaction is “orbital symmetry allowed.”
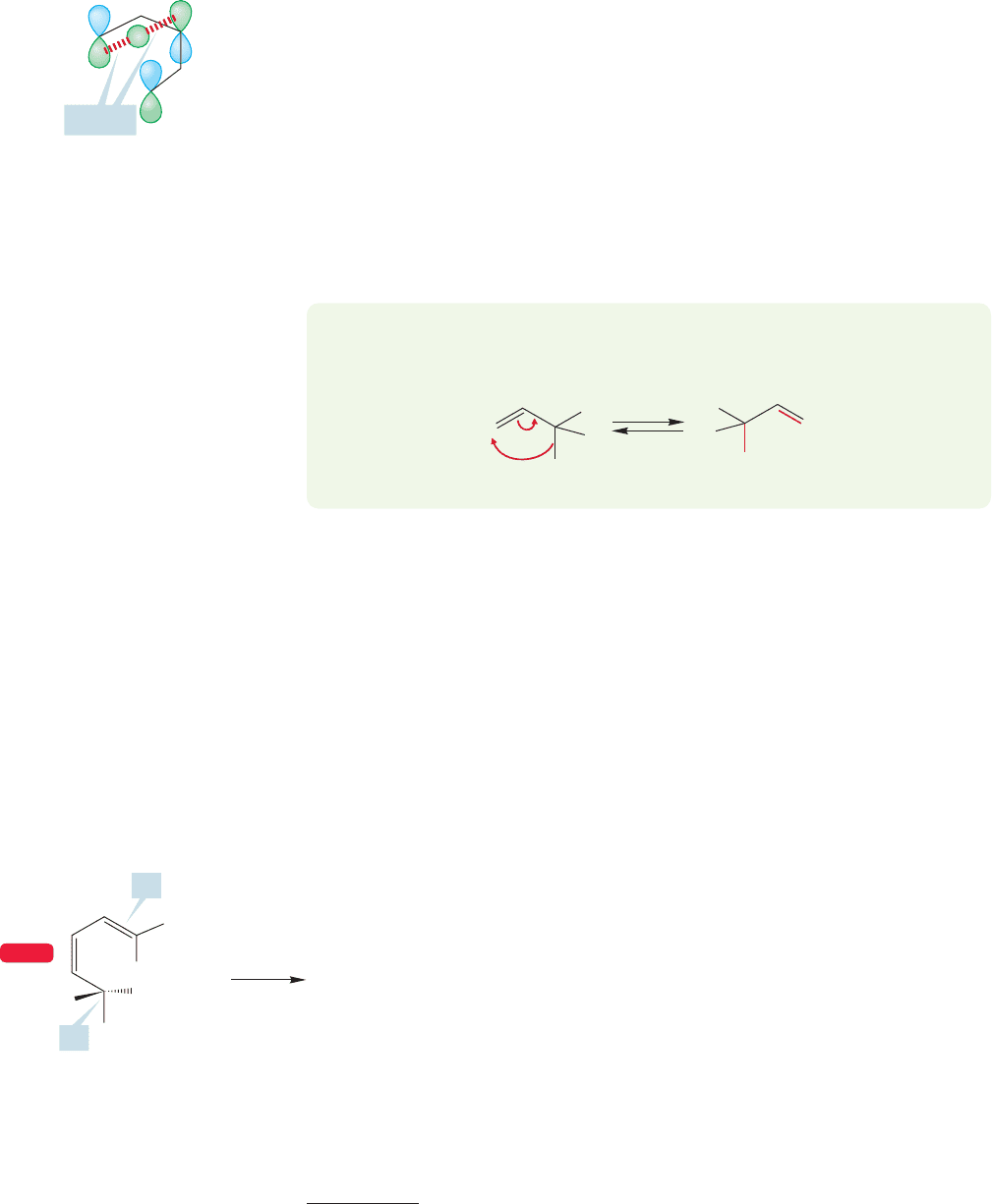
Now look at the [1,3] shift (Fig. 20.42). Notice that it involves only four electrons
(4n) during the reaction: two electrons in the sigma bond that breaks and two in
the π system. To do the analogous [1,3] shift, keeping the shifting hydrogen on the
same side of the molecule requires the overlap of lobes of different symmetry—the
formation of an antibond. This reaction is “orbital symmetry forbidden”!
More important jargon enters here: The motions we have been describing in
which the migrating atom or group of atoms leaves and reattaches from the same
side of the π system are called suprafacial. If the migrating atom or group of atoms
leaves from one side of the π system and arrives at the other, the process is called
antarafacial (Fig. 20.43). In the [1,5] hydrogen shift, the typical sigmatropic shift
involving 4n 2 electrons, suprafacial motion is allowed by the symmetries of the
orbitals; in a [1,3] shift of a hydrogen, a typical 4n electron process,it is not (Figs.20.41
and 20.42).
H
3
2
1
1
5
4
Overlap is
antibonding
here
Overlap is
bonding here
FIGURE 20.42 By contrast, the [1,3]
shift, which is a four-electron
process, involves the formation of an
antibond if the hydrogen reattaches
to carbon 3 from the same side.
Bottom to bottom
Top to top
Bottom to top
Top to bottom
Suprafacial Antarafacial
Suprafacial Antarafacial
FIGURE 20.43 Suprafacial motion
involves bond breaking and bond
making on the same side of the π
system. Antarafacial motion involves
bond breaking on one side and bond
making on the other.
1056 CHAPTER 20 Reactions Controlled by Orbital Symmetry
Why not migrate in a different fashion? Why can’t the hydrogen depart from
one side of the π system and reattach at the other? This antarafacial migration
would result in an orbital symmetry–allowed [1,3] migration (Fig. 20.44). The
problem is that the 1s orbital is small and cannot effectively span the distance
required for an antarafacial migration.There is nothing electronically disallowed
or forbidden about the antarafacial [1,3] migration, but there is an insuperable
steric problem.
The [1,5] sigmatropic shift is intramolecular and the maintenance of bonding
interactions explains why [1,5] hydrogen shifts are possible and [1,3] hydrogen shifts
are exceedingly rare. But we have not yet been able to deal with the dependence of
the course of this reaction on the mode of energy input—thermal or photochemical.
PROBLEM 20.15 Do you expect the [1,3] shift shown below to occur when
propene is heated? Explain carefully, using a molecular orbital argument.
H
Φ
3
of Pentadienyl
(HOMO)
1
1
2
3
4
5
Bonding
FIGURE 20.44 The antarafacial [1,3]
shift is allowed by orbital symmetry,
but the small size of the 1s orbital
makes the stretch impossible.
H
H
H
1
1
2
3
[1,3]
H
H
H
Δ
H
2
C
CH
2
Now comes a most important point. Our model not only rationalizes known
experimental data, but includes the roots of a critical prediction. The Woodward–
Hoffmann explanation predicts that the [1,5] shift occur in a suprafacial fashion.
That is the only way in which bonding interactions can be maintained throughout
the migration of a hydrogen atom from carbon 1 to carbon 5. There is nothing so
far in the available experimental data that allows us to tell whether the observed shifts
are suprafacial, antarafacial, or a mixture of both modes.
Let’s set about finding an experiment to test the requirement of the theory for
suprafacial [1,5] motion. What will we know at the end of the experiment? If we
find that the [1,5] shift is indeed strictly suprafacial, the theory will be supported
(not proved!), and we will certainly feel better about the mechanistic hypothesis. If
we find antarafacial motion, or both suprafacial and antarafacial motions, the the-
ory will be proved (yes, proved) wrong.There is no way our hypothesis can accom-
modate antarafacial motion; there is an absolute demand for suprafaciality,
which nicely illustrates the precarious life of a theory. It can always be dis-
proved by the next experiment, and it can never become free of this state
of affairs.
The crucial test was designed by a German chemist, W. R Roth
(1930–1997).
3
It is the first of a series of beautiful experiments you will
encounter in this chapter. Roth and his co-workers spent some years in
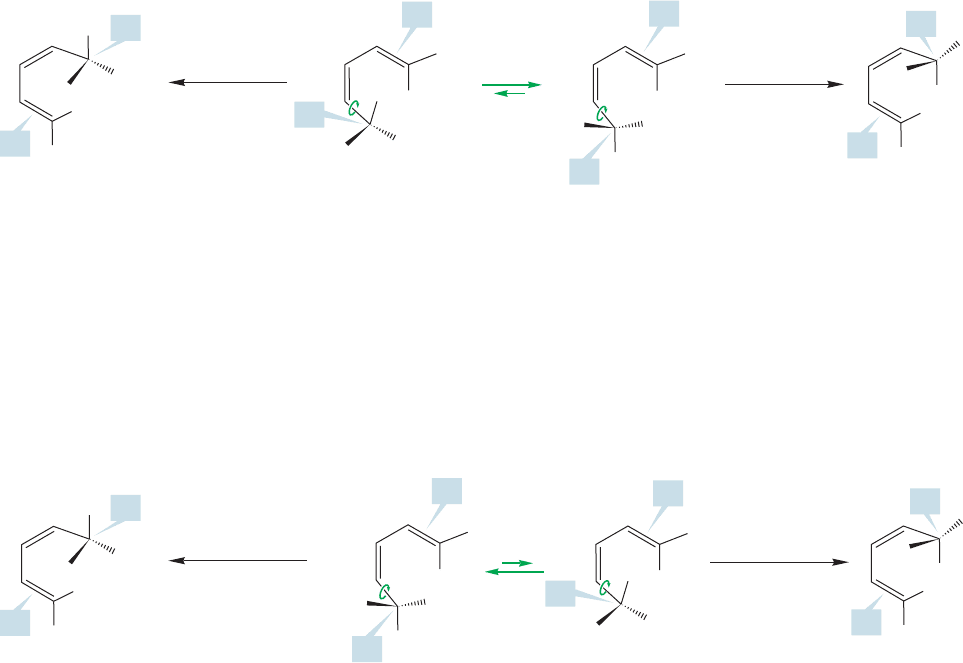
developing a synthesis of the labeled molecule shown in Figure 20.45.
Notice that the molecule has the (S) configuration at carbon 1,and the (E)
stereochemistry at the double bond.
Now let’s work out the possible products from suprafacial and antarafacial
[1,5] shifts in this molecule. The situation is a little complicated because there
C(4)
O
C(5)
?
D
H
Roth’s diene
CH
3
CH
3
CH
3
CH
2
Δ
(
S
)
(
E
)
1
1
2
3
4
5
WEB 3D
FIGURE 20.45 The 1,3-diene used
by Roth and his co-workers to see
whether the [1,5] shift proceeded
in a strictly suprafacial manner.
3
No typo here. There is no period after the R. Roth claimed that he was advised by his postdoctoral advisor
at Yale, William Doering, to take a middle initial in order not to be lost among the myriad “W. Roth’s” in
German chemical indexes. Others with common last names, and who were at Yale at the time, rejected sim-
ilar advice.
20.5 Sigmatropic Shift Reactions 1057
CH
3
CH
2
CH
3
CH
3
D
H
suprafacial
[1,5]
(
E
)
suprafacial
[1,5]
D
CH
3
H
CH
3
CH
3
CH
2
(
S
)
(
E
)
H
CH
2
CH
3
H
3
C
D
CH
3
(
S
)
(
E
)
(
R
)
CH
2
CH
3
CH
3
CH
3
D
H
(
Z
)
(
S
)
FIGURE 20.46 The predicted products formed by suprafacial migration in the two
rotational isomers of Roth’s diene.
are two conformational isomers of this molecule that can undergo the reaction,
shown in Figure 20.46. In one, the methyl group is aimed at the
double bond. In the other, it is the ethyl group that is directed toward the
double bond. In suprafacial migration of hydrogen in one confor-
mation, the product has the (R) configuration at C(5) and the (E) stereochem-
istry at the new double bond located between C(1) and C(2). Another possible
suprafacial migration starts from the second conformation. Here it is the oppo-
site stereochemistries that result. The configuration of C(5) is (S) and the new
double bond is (Z).
C(4)
O
C(5)
C(4)
O
C(5)
In antarafacial migrations, the hydrogen leaves from one side and is delivered
from the other. Figure 20.47 shows the results of the two possible antarafacial migra-
tions. Note that the products are different from each other and also different from
those predicted from suprafacial migration.
CH
3
D
H
CH
2
CH
3
CH
3
antarafacial
[1,5]
antarafacial
[1,5]
H
CH
2
CH
3
H
3
C
D
CH
3
(
Z
)
(
S
)
D
CH
3
H
CH
3
CH
3
CH
2
(
S
)
(
E
)
(
E
)
(
R
)
CH
3
CH
2
CH
3
CH
3
D
H
(
E
)
(
S
)
FIGURE 20.47 The possible products of antarafacial migration in Roth’s diene.
Roth and his co-workers had the experimental skill necessary to sort out the
products and found that only the products of suprafacial migration were formed.
The theory is beautifully confirmed by this experiment.
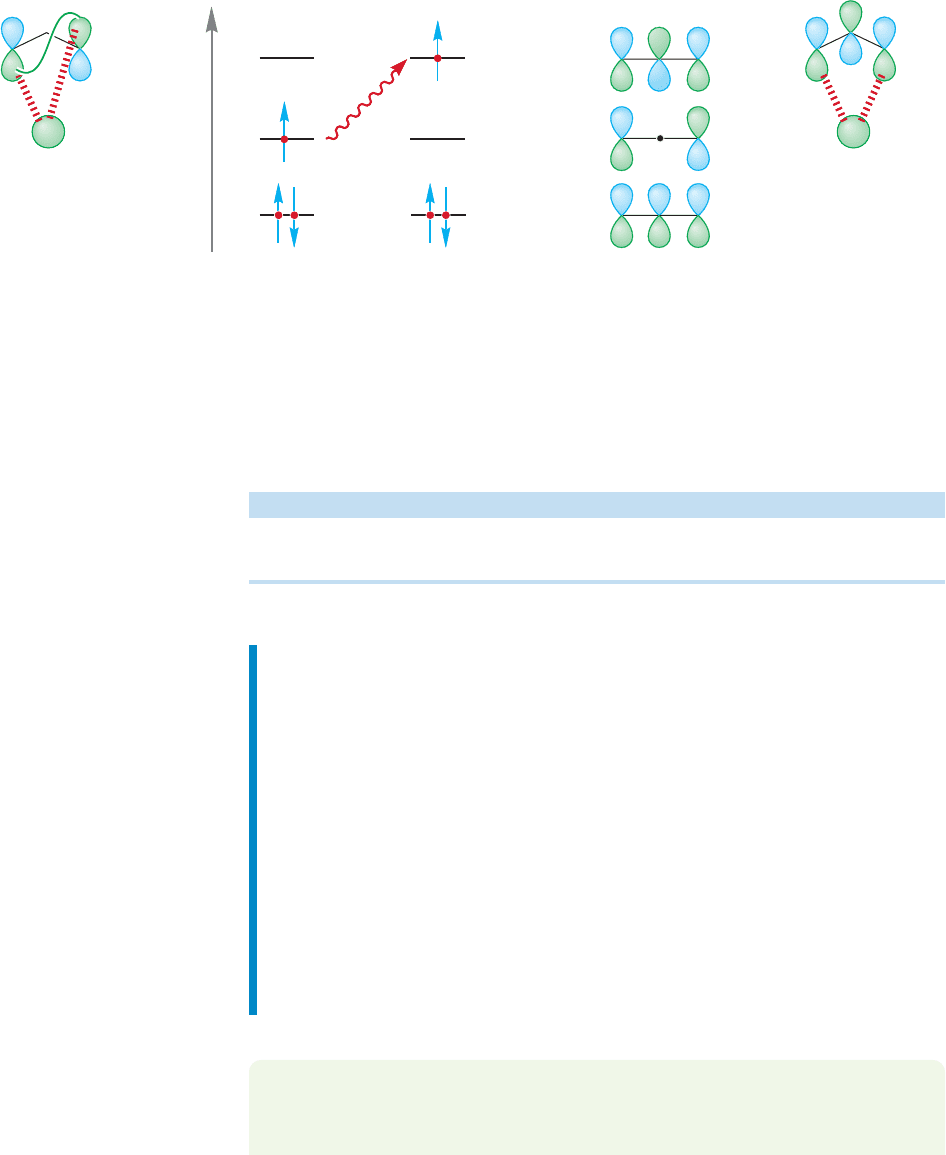
At last we come to the remaining question. Why should the source of ener-
gy make a difference in the mode of migration? Remember, the common, ther-
mally induced shifts are [1,5], whereas the photochemical shifts are [1,3]. A
molecule absorbs a photon of light and an electron is promoted from the
HOMO to the LUMO, producing a new HOMO and changing the symmetry
of the lobes at the ends of the π system. What happens when the symmetry of
the HOMO changes?
1058 CHAPTER 20 Reactions Controlled by Orbital Symmetry
We can now put together Table 20.3 that compares the types of sigmatropic shifts
that are possible and links the stereochemical outcome to the number of electrons
involved in the process and the type of energy used to drive the reaction.
Photochemical
HOMO
Antarafacial motion
is required for
thermal reaction
Suprafacial motion
is required for
photochemical reaction
Φ
3
Φ
2
Φ
1
Energy
HH
hν
Φ
3
Φ
2
FIGURE 20.48 Suprafacial motion of a hydrogen is possible in
3
, the photochemical HOMO for this reaction.£
Figure 20.48 shows the situation for the simplest [1,3] shift in propene.
Absorption of a photon by propene promotes an electron,shown below in
3
of allyl.
Migration can now occur in a suprafacial fashion, and the steric barrier to thermal,
necessarily antarafacial [1,3] shifts is gone. The hydrogen can migrate in an easy
suprafacial manner.
£
Summary
We now understand three seemingly magical concerted reactions. Each is con-
trolled by orbital symmetry. Electrocyclic reactions occur in a conrotatory or
disrotatory fashion depending on the number of electrons in the system and
whether heat or light is used to drive them.The cycloaddition reactions occur
with orbital overlap between the reacting partners and the symmetry of the
HOMO and the LUMO determines the outcome of the reaction. Sigmatropic
shifts are perhaps the most challenging to understand. As long as you keep
in mind the necessity to form bonds and not antibonds, analysis of the
HOMO gives you the positions available for reattachment of a migrating
hydrogen or group. One can determine whether the reaction is allowed in a
suprafacial or antarafacial fashion. Add to this analysis the idea that hydro-
gen has only a small 1s orbital, and therefore any short antarafacial shifts are
generally impossible.
TABLE 20.3 Rules for Allowed Sigmatropic Reactions
Reaction Number of Electrons Thermal Photochemical
Typical 4n reactions 4n Antarafacial Suprafacial
Typical 4n 2 reactions 4n 2 Suprafacial Antarafacial
(continued)
PROBLEM 20.16 Provide mechanisms for the reactions shown on the next page.
Hint: Reactions (a) and (b) each require more than one sigmatropic shift.
