Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

19.17 Additional Problems 1029
PROBLEM 19.94
Write a mechanism for the following
transformation:
C
C
O
O
C
Ph
Ph
Ph
Ph
OH
OH
O
1. NaOH/H
2
O
100 ⬚C
2. H
2
SO
4
(>90%)
C
PROBLEM 19.95 A synthesis of pyridines involves the conden-
sation of an α,β-unsaturated ketone (1) with a methyl ketone
(2). This reaction gives a pyrilium salt (3), which then reacts
with ammonia to produce a 2,4,6-trisubstituted pyridine.
HClO
4
–
ClO
4
NH
3
N
R
1
R
2
R
O
R
1
R
2
R
+
+
O
R
O
R
2
R
1
12
3
4
CH
3
Propose mechanisms for the formation of pyrilium salt 3 from the
condensation of 1 and 2, and for the formation of the pyridine 4
from 3 and ammonia. Hints: The formation of 3 occurs best when
1.5–2.0 equivalents of 1 are used.The ketone 5 is a by-product of
the condensation; how can it be formed? Think “redox.”
O
5
R
1
R
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 19.96 Select the reaction “Aldol condensation”
and observe the reaction that is shown. The reaction stops at
the β-hydroxy aldehyde (the aldol). What conditions are
necessary to obtain the ultimate condensation product, the
α,β-unsaturated aldehyde? Stop the reaction at the first
intermediate (the enolate) and select the HOMO track.
The enolate has an anion shared between the oxygen and the
α carbon. How does the HOMO representation of the enolate
help us understand that an electrophile adds at the carbon
rather than at the oxygen?
PROBLEM 19.97 Observe the “Mixed aldol condensation”
animation. At the first transition state, the lithium coordinates
with the enolate and with the new carbonyl species (formalde-
hyde in this case). What is the size of the ring that is formed in
this transition state? What do you suppose is the shape of the
ring? How will the shape influence the orientation of more
highly substituted enolates and carbonyl electrophiles?
PROBLEM 19.98 The reaction titled “Michael addition” is also
known as 1,4-addition or conjugate addition. Select the LUMO
track of this reaction and notice the LUMO density of the
starting α,β-unsaturated ketone (enone). Is there a large differ-
ence between the LUMO density at the β carbon and the car-
bonyl carbon? Do you think that the nucleophile does 1,2- or
1,4-addition based on the orbital shown? What controls the
regioselectivity? Which do you think would be a faster process,
1,2- or 1,4-addition? Why?
PROBLEM 19.99 Observe the “Claisen condensation” and
watch the first step closely. This step is the deprotonation of
the α hydrogen. What is the orientation of the hydrogen that
is deprotonated with respect to the carbonyl? Why? Go back
and check the deprotonation step in the “Aldol condensation”
and see if the orientation of the α hydrogen matches your
prediction.

Special Topic
Reactions Controlled
by Orbital Symmetry
1030
20.1 Preview
20.2 Concerted Reactions
20.3 Electrocyclic Reactions
20.4 Cycloaddition Reactions
20.5 Sigmatropic Shift Reactions
20.6 The Cope Rearrangement
20.7 A Molecule with a Fluxional
Structure
20.8 How to Work Orbital
Symmetry Problems
20.9 Summary
20.10 Additional Problems
20
CONCERTED ACTIONS In the concerted reaction called a sigmatropic shift, one part of the
molecule flies about, eventually being caught at one speficic position within the same
molecule.
20.1 Preview 1031
The fascination of what’s difficult
Has dried the sap of my veins, and rent
Spontaneous joy and natural content
Out of my heart.
—WILLIAM BUTLER YEATS,
1
“THE FASCINATION OF WHAT’S DIFFICULT”
20.1 Preview
In Chapters 12–14, we explored the consequences of conjugation—the sideways
overlap of 2p orbitals. That study led us to the aromatic compounds, the great sta-
bility of which can be traced to an especially favorable arrangement of electrons in
low-lying bonding molecular orbitals. It can surely be no surprise to find that tran-
sition states can also benefit energetically through delocalization, and that most of
the effects, including aromaticity, that influence the energies of ground states are
important to transition states as well.
In this chapter, we will encounter reactions that were frustratingly difficult for
chemists to understand for many, many years. By now, you are used to seeing acid-
and base-catalyzed reactions,in which an intermediate is first formed and then pro-
duces product with the regeneration of the catalytic agent. Acid-catalyzed additions
to alkenes are classic examples and there are already many other such reactions in
your notes. There is one class of reactions, however, that is extraordinarily insensi-
tive to catalysis. In these reactions, bases and acids are largely without effect. Even
the presence of solvent seems of little relevance,because the reactions proceed as well
in the gas phase as in solution. What is one to make of such reactions? How does
one describe such a mechanism? We are used to speculating on the structure of an
intermediate and then using the postulated intermediate to predict the structures of
the transition states surrounding it. In these uncatalyzed reactions the starting ma-
terial and product are separated by a single transition state (a concerted reaction),
and there is very little with which to work in developing a mechanism. Indeed, one
may legitimately ask what “mechanism” means in this context. Such processes have
been called “no-mechanism” reactions. Some, in which starting material simply
rearranges into itself (a degenerate reaction), can even be called “no-mechanism,
no-reaction, reactions.” Figure 20.1 shows an arrow formalism for a typical no-
mechanism, no-reaction, reaction.
In 1965,R.B.Woodward (1917–1979) and Roald Hoffmann (b. 1937),both then
at Harvard, began to publish a series of papers that ventured a mechanistic descrip-
tion of no-mechanism reactions, and gathered a number of these seemingly differ-
ent processes under the heading of pericyclic reactions,concerted reactions that have
a cyclic transition state. Their crucial insight that bonding overlap must be main-
tained between orbitals during the course of a concerted, pericyclic reaction now
seems so simple that you may find it difficult to see why it eluded chemists for so
many years. All we can tell you is that simple things are sometimes very hard to see,
even for very smart people.Woodward–Hoffmann theory had been approached very
closely before without the crucial “aha!”, without the lightbulb over the head turn-
ing on. Perhaps what was lacking was the ability to combine a knowledge of theory
with an awareness of the chemical problem, which is precisely what the brilliant
experimentalist Woodward and the young theorist Hoffmann brought to the
1
William Butler Yeats (1865–1939) was an Irish poet who received the Nobel prize for literature in 1923.
1,5-Hexadiene
Still
1,5-Hexadiene
WEB 3D
FIGURE 20.1 A single-barrier,
one-transition-state,“no-mechanism,
no-reaction” reaction.The starting
material and the product are
indistinguishable.

1032 CHAPTER 20 Reactions Controlled by Orbital Symmetry
problem.Ultimately, these papers and their progeny resulted in the chemistry Nobel
prize in 1981 for Hoffmann [together with Kenichi Fukui (1918–1998) of Kyoto],
and would probably have given Woodward his second Nobel prize, had he lived long
enough.
The reaction of the chemical community to these papers ranged from enthu-
siastic admiration to “We knew all this trivial stuff already.”We would submit that
admiration was by far the more appropriate reaction, and suspect that behind much
of the carping was a secret smiting of many foreheads.Woodward–Hoffmann the-
ory is brilliant. It not only answered long-standing and difficult questions, but it
had remarkable “legs.” Its implications run far into organic and the rest of chem-
istry, and it makes what are called risky predictions. It is important to separate
theory that merely rationalizes—that which explains known phenomena—from
theory that demands new experiments.The success of Woodward–Hoffmann the-
ory can be partly judged from the flood of experiments it generated. Of course,
not all the experiments were incisive, and not all the interpretations were appro-
priate; the area entered a rococo phase quite early on. Still, some of the
work generated by the early Woodward–Hoffmann papers stands today as an
example of the combination of theory and experiment that characterizes the best
of our science.
In this chapter, we will see electrocyclic reactions in which rings open and
close, cycloaddition reactions in which two partners come together to make a new
cyclic compound, and sigmatropic shifts in which one part of a molecule flies
about coming to rest at one specific position and no other. Specificity is the hall-
mark of all these reactions; atoms move as if in lock step in one direction or anoth-
er, or move from one place to one specific new place, but no other. The chemical
world struggled mightily to understand these mysteriously stereospecific reactions,
and, thanks to the work by Woodward, Hoffmann, and several others, finally fig-
ured it out in a marvelously simple way. This chapter will show you some won-
derful chemistry.
ESSENTIAL SKILLS AND DETAILS
1. To be able to understand the course of “no-mechanism” reactions, you need only
remember that in any concerted (one barrier, one transition state) reaction, a bonding
overlap must be maintained between orbitals.The rest is all detail.
2. The opening and closing reactions of rings—electrocyclic reactions in the jargon of this
chapter—are best understood through an analysis of the HOMO (p. 133) of the open-
chain partner. Ring closing involves only bonding interactions for allowed reactions.
3. Cycloaddition reactions require an analysis of the interactions of the HOMO and
LUMO (p. 133) of the two reacting species. Allowed reactions have two bonding
interactions between the reacting partners; “forbidden” reactions do not.
4. In sigmatropic shift reactions, one portion of a molecule detaches from one atom
and reattaches at another position of the same molecule.The transition state is best
described by an atom (or group of atoms) moving across a polyenyl radical. Once
again, an analysis of the symmetry of the HOMO of the polyenyl radical is the key
to understanding. A bonding interaction must be achieved at the arrival point for
the reaction to be allowed.
20.2 Concerted Reactions
Woodward and Hoffmann provided insights into concerted reactions.As noted on page
389, a reaction is concerted if starting material goes directly to product over a single
20.2 Concerted Reactions 1033
transition state.Such reactions are “single-barrier”processes.The S
N
2 and Diels–Alder
reactions are nice examples of concerted reactions you know well (Fig. 20.2).
Examples
RXNu
..
–
RX+Nu
S
N
2
Diels–Alder
..
–
Energy
Reaction progress
Transition
state
Products
Starting
material
FIGURE 20.2 The S
N
2 and
Diels–Alder reactions are concerted
processes.
H
2
O
+
Addition of HBr
S
N
1
I
–
Br
–
Br
–
Br
–
HBr
Intermediate
Intermediate
Energy
Reaction progress
Transition
state 2
Transition
state 1
Intermediate
Starting
material
Products
+
I Br
Br
H
FIGURE 20.3 The S
N
1 reaction and
HBr addition to an alkene are
nonconcerted reactions.
Energy
Reaction progress
Transition
state
Transition
state
FIGURE 20.4 Any nonconcerted
reaction can be separated into a
series of concerted processes.
Of course,many reactions involve intermediates, and in these reactions more than
one transition state is traversed on the way from starting material to product. As
noted previously, these are called nonconcerted or stepwise reactions.Typical exam-
ples are the S
N
1 reaction or the polar addition of HBr to an alkene, two reactions
that go through the same intermediate, the tert-butyl cation (Fig. 20.3).
It is important to recognize that nonconcerted reactions are made up of series
of single-barrier, concerted reactions, each of which could be analyzed indepen-
dently of whatever other reactions followed or preceded (Fig. 20.4).
1034 CHAPTER 20 Reactions Controlled by Orbital Symmetry
COOCH
3
CH
3
OOC
HH
A cis 3,4-disubstituted
cyclobutene
COOCH
3
H
CH
3
OOC
Δ
H
A cis,trans 1,3-butadiene
cistrans
FIGURE 20.5 The thermal opening
of a cyclobutene to a 1,3-butadiene.
The cis disubstituted cyclobutene
rearranges to the cis,trans butadiene.
Woodward and Hoffmann offered explanations of why some reactions are con-
certed and others not. They found a way to analyze reactions by determining the
pathways required to maintain bonding interactions between orbital lobes as the reac-
tion progressed. They used orbital symmetry and the aromaticity of the transition
state in their analysis. The following sections take up some examples in which
Woodward–Hoffmann theory is particularly useful.
20.3 Electrocyclic Reactions
In 1957, a young German chemist named Emanuel Vogel (b. 1927) was finding
his way through the labyrinth of the German academic system, working on the
experiments necessary for an entrance ticket for a position at a German univer-
sity: a super Ph.D. degree called the “Habilitation.” His topic included the ther-
mal rearrangements of cyclobutenes. Although cyclobutene had been studied by
R. Willstätter (1872–1942, Nobel prize in 1915) as early as 1905, and although
the rearrangement to 1,3-butadiene was noted by the American chemist John D.
Roberts (b. 1918) in 1949, only Vogel was alert enough to notice the startling
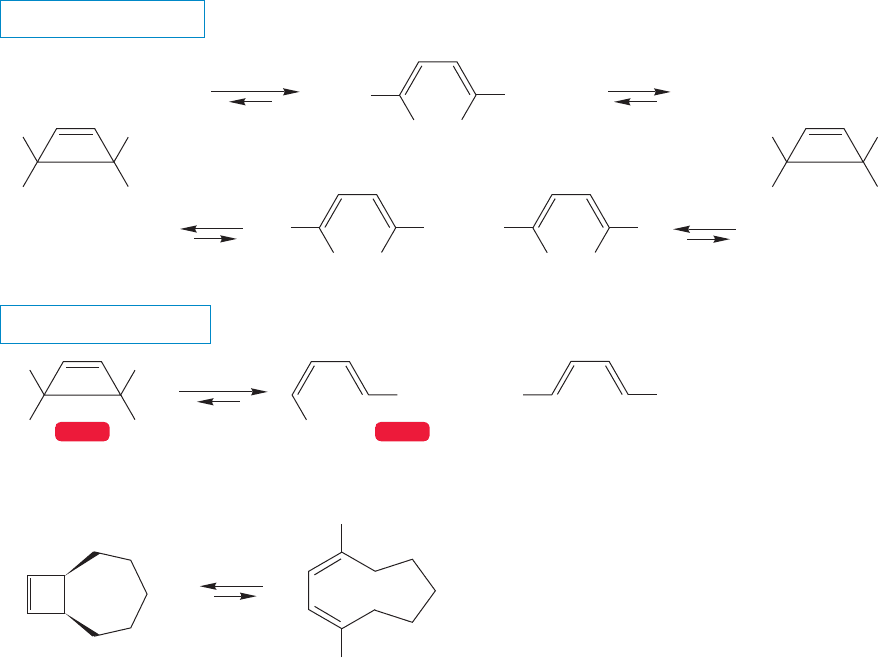
stereospecificity of the thermal reaction. For example, the cis 3,4-disubstituted
cyclobutene shown in Figure 20.5 rearranges only to the cis,trans 1,3-butadiene
and no other stereoisomer.
The arrow formalism of Figure 20.5 is easy to write, but the stereochemical
outcome of the reaction is anything but obviously predictable. It’s worthwhile to
see exactly why. There are three possible stereoisomers of the product (Fig. 20.6),
and a naive observer would certainly be forgiven for assuming that thermodynamic
considerations would dictate the formation of the most energetically favorable
isomer, the trans,trans diene, in which the large ester groups are as far apart as
possible. Notice that the opening of the cyclobutenes must initially give the
dienes in their s-cis conformations rather than in the lower energy s-trans arrange-
ments. Of course,the s-cis molecules will rapidly rotate to the lower energy s-trans
conformations.
E = COOCH
3
E = entgegen
E
cis,cis or (Z,Z)
E
HH
H
cis,trans or (Z,E)
Thermodynamic stability
E
HE
H
trans,trans or (E,E)
H
EE
E
E
H
H
H
E
H
E
H
H
E
E
s-cis
Forms
s-trans
Forms
rotate rotate rotate
FIGURE 20.6 The three possible
stereoisomers of the diene product
for the reaction shown in Figure 20.5.
Only one, the cis,trans diene of
intermediate thermodynamic
stability, is formed in the reaction.
WEB 3D
20.3 Electrocyclic Reactions 1035
As Vogel first found, and as others showed later for many other systems, forma-
tion of the trans,trans diene is not the actual result at all; nor is the reaction stereo-
random, as only a single stereoisomer is formed. Vogel well understood that this
reaction was rather desperately trying to tell us something. In the intervening years,
many similar reactions were found and many a seminar hour was spent in a fruit-
less search for the explanation. What the reaction had to say to us waited for the
papers of Woodward and Hoffmann.
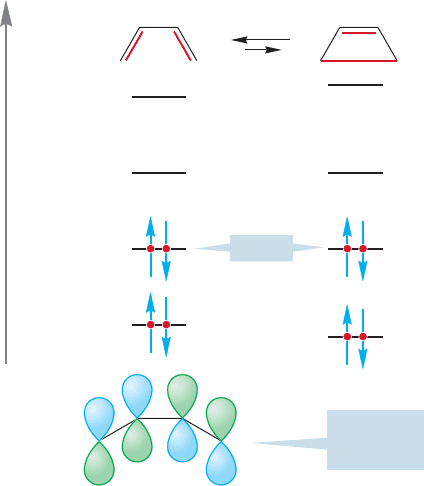
Remarkably, it was discovered that the stereochemical outcome of the reac-
tion was dependent on the energy source. Heat gave one stereochemical result
and light another. Figure 20.7 sums up the results of the thermal and photochem-
ical interconversions of cyclobutenes and butadienes. In the thermal reaction, a cis
3,4-disubstituted cyclobutene yields the cis,trans diene. By contrast, in the photo-
chemical process the same cis cyclobutene yields a pair of molecules,the cis,cis diene
and the trans,trans diene.
THE GENERAL CASE
SPECIFIC EXAMPLES
R +
R
Δ
Δ
R
HH
RH
trans,cis
trans,trans cis,cis
cis
H
H
H
H
CH
3
H
3
C
cis
cis cis,cis
HR
RH
trans
RH
HH
R
Δ
hν
CH
3
+
cis,trans trans,trans
(0.005%)
(99.995%)
CH
3
H
RR
H
CH
3
H
3
C
hν
hν
WEB 3DWEB 3D
FIGURE 20.7 The stereochemical outcomes of the thermal and photochemical reactions of
cyclobutenes and butadienes are different.
Notice that the cis disubstituted cyclobutene can be converted into the trans
disubstituted cyclobutene through a thermal reaction and a photochemical reaction
(Fig. 20.7). Regardless of the starting material, the thermal reaction favors the more
stable 1,3-butadiene. The photochemical process, depending on the wavelength of
light used, favors the less stable cyclobutene.
1036 CHAPTER 20 Reactions Controlled by Orbital Symmetry
Δ
Energy
Φ
2
=
Φ
1
Φ
2
Φ
3
Φ
4
σ
π
π*
σ*
HOMO
HOMO
butadiene
FIGURE 20.8 The molecular orbitals involved in the
interconversion of cyclobutene and butadiene.
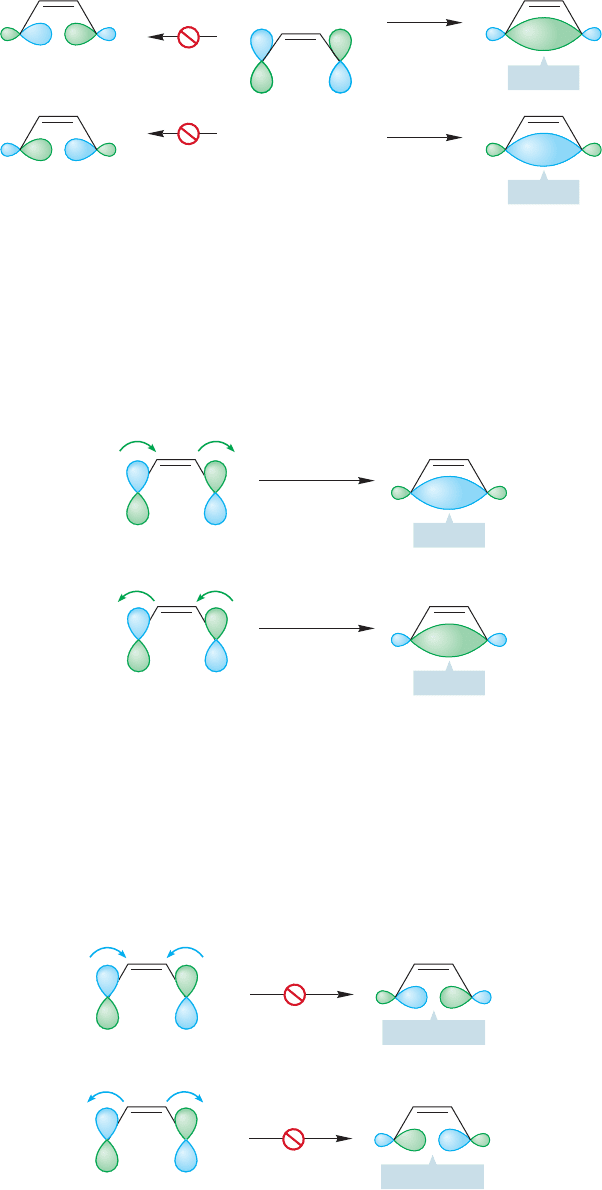
Let’s start our analysis by thinking about the thermal opening of cyclobutene
to 1,3-butadiene. Here is a general practical hint. It is usually easier to analyze
these thermal or photochemical processes, called electrocyclic reactions,by exam-
ining the open-chain polyene partner, regardless of which way the reaction actual-
ly runs. Figure 20.8 shows the molecular orbitals involved in the cyclobutene to
butadiene interconversion. The four π molecular orbitals of 1,3-butadiene are
converted into the σ, σ*, π, and π* orbitals of cyclobutene. Now comes a most
important point. The HOMO of the system controls the course of electrocyclic reac-
tions. This assumption is not ultimately necessary, but it makes predicting the
products of these reactions very easy—and it works! It derives from the aro-
maticity of the transition state, and it can be defended in the following way. It
is the electrons of highest energy, the valence electrons located in the highest
energy orbital, that control the course of atomic reactions, and it should be no
surprise that the same is true for molecular reactions. To do a complete analy-
sis of any reaction, atomic or molecular, we really should follow the changes in
energy of all electrons. That process is difficult or impossible in all but a very
few simple reactions. Accordingly, theorists have sought out simplifying
assumptions, one of which says that the highest energy electrons, those most
loosely held, are most important in the reaction. These are the electrons in the
HOMO. The HOMO of butadiene is
2
, and we assume that it is the con-
trolling molecular orbital. Now ask how
2
must move as 1,3-butadiene
becomes cyclobutene. Don’t worry that we are analyzing the reaction in the
counterthermodynamic sense. If we know about the path in one direction, we
know about the path in the other direction as well. In order to create the ring-
forming bond between the butadiene end carbons, the p orbitals on the end car-
bons must rotate 90°, and this rotation must be in a fashion that creates a bond (σ)
£
£
20.3 Electrocyclic Reactions 1037
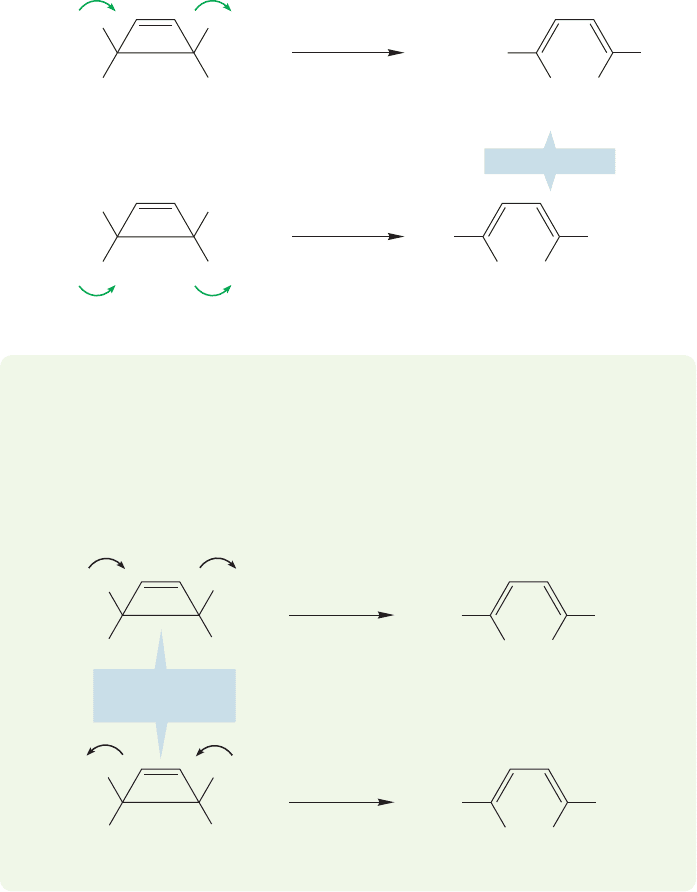
between the atoms, not an antibond (σ*) (Fig. 20.9).There are four possible rota-
tions we can consider: both p orbitals clockwise, both counterclockwise, left one
clockwise and right counterclockwise, and left one counterclockwise and right
one clockwise.
Now look at the symmetry of the end orbitals of
2
, the controlling HOMO
of butadiene. There are two ways to make a bond: in one the blue lobes overlap, in
the other it is the green lobes that overlap (Fig. 20.10). In each case we have creat-
ed a bonding interaction. In this example, the blue/blue overlap requires that the
two ends of the molecule rotate in a clockwise fashion. The green/green overlap
requires that the two ends rotate counterclockwise.
£
Antibonding
not ok
rotate
Antibonding
not ok
rotate
rotate
Bonding
Bonding
End lobes of
the HOMO, Φ
2
rotate
FIGURE 20.9 In the interconversion
of cyclobutene and butadiene, the
orbitals at the end of the diene must
rotate so as to form a bond, not an
antibond.
conrotation
(both lobes
clockwise)
conrotation
(both lobes
counter-
clockwise)
Bonding
Bonding
counter-
clockwise
counter-
clockwise
clockwiseclockwise
FIGURE 20.10 Formation of a
bonding interaction requires what is
called conrotation of the ends of the
diene.There are two, equivalent
conrotatory modes.
disrotation
disrotation
Antibonding!
Antibonding!
counter-
clockwise
counter-
clockwise
clockwise
clockwise
FIGURE 20.11 Disrotation creates an
antibonding interaction between the
lobes at the ends of the diene.
Neither of the two possible
disrotatory modes can be a favorable
process for butadiene.
These two modes of rotation,in which the two ends rotate in the same direction,
are called conrotatory motions, and the whole process is known as conrotation.
There is another rotatory mode possible, called disrotation, in which the two
ends of the molecule rotate in different directions. Figure 20.11 shows the two pos-
sible disrotatory motions. In both, an antibond, not a bond, is created between the
two end carbons. Disrotation cannot be a favorable process in this case.
An electrocyclic reaction is as easy to analyze as that. Identify the HOMO, and
then see whether conrotatory or disrotatory motion is demanded of the end carbons
by the lobes of that molecular orbital. All electrocyclic reactions can be understood
in this same simple way. The theory tells us that the thermal interconversion of
cyclobutene and 1,3-butadiene must take place in a conrotatory way. For the
cyclobutene studied by Vogel, conrotation requires the stereochemical relationship
that he observed. The cis 3,4-disubstituted cyclobutene can only open in conrota-
tory fashion, and conrotation forces the formation of the cis,trans diene. Note that
there are always two possible conrotatory modes (Fig. 20.12), either one giving the
same product in this case.
1038 CHAPTER 20 Reactions Controlled by Orbital Symmetry
Same molecule!
COOCH
3
CH
3
OOC
HH
COOCH
3
H
trans,cis
CH
3
OOC
conrotation
H
CH
3
OOC
HH
COOCH
3
H
cis,trans
CH
3
OOC
conrotation
H
COOCH
3
FIGURE 20.12 For the cis
disubstituted cyclobutene studied
by Vogel, both conrotatory modes
demand the formation of the diene
with one cis and one trans double
bond.
WORKED PROBLEM 20.1 Show through an equivalent analysis that a mixture of
the cis,cis and trans,trans dienes should be formed in the thermal opening of trans
3,4-disubstituted cyclobutenes.
ANSWER Because the opening takes place thermally, it must be conrotatory.
There are two conrotatory modes for the trans compound. One leads to the cis,cis
(Z,Z) isomer and the other to the trans,trans (E,E) isomer.
XH
HX
XX
cis,cis (Z,Z)
H
conrotation
H
XH
HX
X
H
trans,trans (E,E)
H
conrotation
Δ
Δ
X
trans Substituted
cyclobutene
Now what about the photochemical reaction? Look again at the molecular
orbitals of 1,3-butadiene (Fig. 20.8). Recall that absorption of a photon pro-
motes an electron from the HOMO to the LUMO (p. 528), in this case from
